Abstract
The impact of red and blue light-emitting diodes (LEDs) irradiation on the sugar metabolism and γ-aminobutyric acid in postharvest table grapes stored at 4 °C for 21 days after harvest were explored. Grape clusters were exposed to red and blue light irradiation at the same intensity (500 lx). The findings revealed that red and blue light treatments enhanced total soluble solids (TSS) and total acidity (TA) content. Furthermore, red-light irradiation retained higher phenolic compound during storage than blue light and control. Red-light radiation sustained higher levels of phenolic biosynthesis-related enzymes, including phenylalanine ammonia-lyase (PAL), tyrosine ammonia-lyase (TAL), cinnamate 4-hydroxylase (C4H), and p-coumarate ligase (4CL), than blue light irradiation and control. The results showed that red light increased glucose, fructose, and sucrose levels, which were 32.19, 33.18, and 2.95 mg g−1, compared with those in blue light and control at the end of storage and enhancing the sucrose synthase synthesis (SS-synthesis) and acid invertase (AI), while blue light enhanced sucrose synthase cleavage (SS-cleavage). In addition, red light irradiation increased glutamic acid decarboxylase (GAD) activity while red and blue light irradiation inhibited γ-aminobutyric acid transaminase (GABA-T) activity by 22.14 and 20.71 U g−1 at 21 days of storage, compared to control, leading to γ-aminobutyric acid (GABA) accumulation. These findings suggest that red and blue light will help in maintaining the quality of grape during postharvest storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Table grape is highly perishable after harvest due to significant water losses induced by rachis, which results in browning, weight loss, and fruit softness. Also, the amount of sugar in grape berries is one of the key factors determining its quality (Balic et al., 2012). Its metabolism has a substantial influence on the quality of its produce. Sugars are produced by photosynthesis in the leaves and accumulate in the fruit via many physiological mechanisms (Falguera et al., 2013). The sweetness of grape berries is influenced by the buildup of soluble sugars throughout fruit growth. Sugar catabolism in postharvest grape berries alters the composition and amount of soluble sugars, altering fruit flavor, an essential requirement for customer acceptance (Lu et al., 2018). Global interest is rising in finding a more efficient, non-hazardous, and cost-effective alternative strategy to chemical treatments for improving and sustaining the quality of grapes during postharvest storage.
Chemical additives such as sulfur dioxide (Xue & Yi, 2017), spermine (Harindra Champa et al., 2015), nitric oxide (Song et al., 2022), chitosan (Lo’ay & Dawood, 2017), and salicylic acid (Nia et al., 2022) used in grapes to extend postharvest shelf life may endanger consumers health. As a newly emerging technique in postharvest fruit and vegetable storage, the application of light-emitting diodes (LEDs) has gained popularity in fruit preservation because of their cost-effectiveness, non-thermal sterilizing, ease of operation, high efficiency, and relatively cold emitting surfaces (Nassarawa et al., 2020). LEDs radiation is also a feasible postharvest treatment alternative for improving nutritional content and preventing senescence in fruit and vegetable during storage (Khan et al., 2022). LEDs light has been used as a postharvest treatment to stimulate the production of total phenolic and total flavonoid content in tomatoes (Castagna et al., 2014), grapes (Barut Gök, 2021), blueberry (Xu & Liu, 2017), okra (Wilawan et al., 2019), pear (Falguera et al., 2014), pakchoi (Xijia et al., 2022), watermelon (Pendyala et al., 2020), fresh-cut pineapple (Bhavya et al., 2021), tomatoes (Jagadeesh et al., 2011), fresh-cut apple (Gómez et al., 2015), fresh-cut endive (Hägele et al., 2016), and strawberries (Avalos-Llano et al., 2020). Red-light irradiation increased the accumulation of soluble sugars in postharvest citrus fruit by modifying the sucrose metabolism enzyme activity (Yuan et al., 2017). Blue light at 10 °C for 8 days extended the shelf life and increase soluble sugar content in Chinese bayberry (Shi et al., 2016). During the storage of lime fruit, red light treatment increased the concentration of soluble sugars and maintained the flavor of lime (Kaewsuksaeng et al., 2011). Similarly, blueberry fruit treated with red and blue light during postharvest storage increase the soluble sugar content (Xu et al., 2016). During 35 days of storage at 13 °C, UV-C LED treatment significantly increased sugar content and increased GAD activity while inhibited GABA-T activity in tomato fruit (Yan et al., 2021). In postharvest tomatoes, blue light irradiation was more effective than red-light irradiation in promoting GABA accumulation (Dhakal & Baek, 2014a; Xu et al., 2014a).
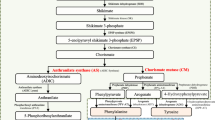
To our best knowledge, no comprehensive study of the influence of red and blue light radiation on sugar metabolism and the aminobutyric acid shunt pathway in postharvest grape clusters has been conducted. To provide a better understanding of the impact of red and blue light irradiation on sucrose metabolism and the aminobutyric acid shunt pathway during postharvest storage of grape clusters, the current study investigates the influence of continuous LEDs light on specific wavelengths 660 nm (red) and 450 nm (blue) on sugar content and the aminobutyric acid shunt pathway in postharvest grape clusters during storage. In addition, the phenolic compounds and phenolic biosynthesis-related enzymes in grape clusters were studied.
Materials and Methods
Fruit Sample Collection and LEDs Light Treatments
Table grape clusters (cv. Kyoho) were picked from an orchard in Zhejiang Province, Hangzhou, China, and immediately transferred to an agro-product postharvest facility for pre-cooling to dissipate field heat. Thirty-six grape clusters without any defect were chosen and divided into three groups at random (12 grape clusters in each group). The lighting system panel with a length of 63 cm and a width of 52.5 cm and contained eight LEDs bar in each panel consisting of red or blue LEDs were assembled on refrigerator chambers. One refrigeration was made to contain 12 grape clusters; grape clusters were placed under continuous red light irradiation (Systion, Inc., Saitama Portugal; wavelength 660 nm; power 13 W; length 60 cm and width 3 cm) at a distance of 20 cm from the red light emission, while another 12 grape clusters were kept in the second refrigeration under continuous blue light irradiation (Systion, Inc., Saitama Portugal; wavelength 450 nm; power 6 W; length 60 cm and width 3 cm) at a distance of 20 cm from the blue light emission. During the light illumination, all the grapes clusters in both treatments were placed between the two sets of the panel to avoid the shadow distance between the bottom and upper parts of the bunches and to obtain the same illumination energy. The control group was stored in darkness without any light supply. The light intensity which was applied in both the red and blue lights was set to 500 lx and measured with a lux-meter (PM 6612L). All grape samples were stored at 4 °C throughout the whole storage period. The temperature and humidity within the incubators were 5.5 ± 1 °C and 50.5 ± 4 %, respectively. On days 0, 7, 14, and 21, three clusters of berries from each treatment were removed, mixed, and 10 berries in three replicates were randomly sampled for titratable acidity (TA) and total soluble sugars (TSS). Grapes from early harvest were used as 0 day. Subsequent the quality analyses, the remaining berries from each of the three replicates were randomly sampled, mixed, and instantly refrigerated with liquid nitrogen and kept at −80 °C for later use.
Fruit Quality Measurement
Total Soluble Solids and Total Acidity Content in Berries
The total soluble solids and total acidity in berries were determined on fresh squeezed grape juice (3 replicates from 10 berries) using a pocket refractometer meter (PAL-BXIACID F5; Atago, Japan), and the results were expressed as percentage.
Chemical Analyses
Determination of Bioactive Compound
The frozen grape tissue (5 g) (3 replicates from 10 berries) was homogenized in 9 mL of 95 % (v/v) methanol. After 24 h of incubation at 25 °C in the dark, the homogenate was centrifuged at 13,000 × g for 5 min at 4 °C. The extract was used to determine the total phenolic, total flavonoid, and total anthocyanin content using the method of Farhadi et al. (2016). UV-5800PC spectrophotometers were used to measure the absorbance of the following supernatant at 280 nm (phenolic) and 325 nm (flavonoid). Phenolic and flavonoid content was calculated using gallic acid (phenolic) and rutin (flavonoid) as a standard and results were expressed as milligrams per gram fresh weight. The anthocyanin content was determined using a pH differential method. The result was expressed as milligrams per gram cyanidin 3-glucoside fresh weight.
Determination of Phenolic Biosynthesis-Related Enzymes
The frozen grape tissue (5 g) (3 replicates from 10 berries) was extracted in 0.3 mmol L−1 sodium phosphate buffer (pH 8.0) containing 30 mM β-mercaptoethanol for the PAL and TAL assays. For the C4H and 4CL tests, 5 g of frozen grape tissue was extracted with 5 mL of 0.3 M Tris–HCl buffer (pH 7.0) containing 0.2 M dithiothreitol and 25 % glycerol. The reaction mixture was centrifuged for 20 min at 12,000 × g, and the supernatant was collected for enzymatic activity determination. PAL and TAL activity was measured based on the previous method of Zeng et al. (2015). The method defined by Li et al. (2019) was used to conduct the C4H and 4-CL activities. The result was expressed as units per gram protein fresh-weight basis.
Determination of Soluble Sugars
The glucose, fructose, and sucrose contents were determined in grape samples. For extraction, 50 mL of distilled water was added to 5 g of frozen grape tissue (3 replicates from 10 berries). The mixture was extracted in a water bath for 20 min at 80 °C; after cooling at room temperature, 20 mL of 3% sulfosalicylic acid and 30 mL of distilled water were added to the mixture. The mixture was then allowed to cool for 15 min at room temperature. Ten milliliters of the reaction mixture was transferred to a 10-mL tube and allowed to settle for 15 min before filtering through a 0.45-m filter and proceed with ion chromatography analyses. The sugars extracted were analyzed using an ion chromatography Dionex CarboPac PA10 column (4 mm × 250 mm) to separate soluble sugars. The mobile phases were 120 mM NaOH (A) and H2O (B) at a flow rate of 1.0 mL min−1. The injection volume was 10 µL, and the column temperature was 25 °C. Glucose, fructose, and sucrose were used to make the calibration standard curves. The result was expressed as milligrams per gram fresh-weight basis.
Determination of SS-Cleavage, SS-Synthesis, and Acid Invertase
The frozen grape samples (5 g) (3 replicates from 10 berries) were homogenized in 10 mL of 50 mmol L−1 sodium phosphate buffer (pH 7.8) containing 10 mM MgCl2, 2.0 mL L−1 β-mercaptoethanol, 2.0 g L−1 PVPP, and 2.0 mL L−1 Triton X-100. The reaction mixture was centrifuged for 15 min at 12,000 × g at 4 °C. The extract was used for testing. The SS-cleavage, SS-synthesis, and acid invertase assays were performed using a detection kit in accordance with the manufacturer’s instructions (Nanjing Herb Source Bio-Technology Co., Ltd., China). The result was expressed as units per gram fresh-weight basis.
Determination of GABA Content
(5 g) of frozen grape samples (3 replicates from 10 berries) was homogenized in 9 mL of PBS (pH 7.4). The reaction mixture was centrifuged for 20 min at 4 °C at 3000 × g. The extract was used for GABA determination. The UV 5800PC spectrophotometer was used to measure absorbance at 450 nm. GABA was determined using a plant GABA ELISA assay kit (Nanjing Herb Source Bio-Technology). The GABA content was determined using a GABA standard curve and expressed in micromoles of GABA equivalent per gram of fresh weight (µmol g−1).
To determine the GAD and GABA-T activities, frozen grape tissues (5 g) (3 replicates from 10 berries) were extracted in 9 mL of PBS (pH 7.4). The supernatants were used for the GAD and GABA-T tests after centrifugation at 5000 × g for 15 min at 4 °C. The activity of GAD and GABA-T assays was measured according to manufacturer’s instructions using the plant GABA-T ELISA assay kit and the plant GAD ELISA assay kit (Nanjing Herb Source Bio-Technology). The results was expressed as units per gram fresh-weight basis.
Statistical Analyses
The results were processed using IBM SPSS (version 25). The data were analyzed using one-way analysis of variance (ANOVA), and the values were presented as the mean ± standard deviation of three replications. The multiple comparison of the means between the treated samples and the control was determined using Duncan’s multiple ranges (P < 0.05).
Results
Impact of Red and Blue Light Emission on TSS and TA
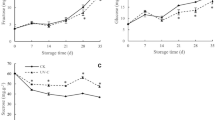
During the postharvest storage period, the TSS content of grape samples increased slightly from 16.30% to 16.63%, 16.93%, and 17.45% in both treatments (Fig. 1a). However, treated grape samples had slightly higher soluble solids than the control samples. The results showed that red light irradiation maintained a significant higher increase in TSS from 7 to 21 days of storage. TA content of grape samples in both treatments decreased. TA declined from 0.69% to 0.59%, 0.62%, and 0.63%. The TA content of berries treated with red light retained a slightly higher level on the 7–21 days of storage than blue light and control berries (Fig. 1b).
Impact of Red and Blue Light Radiation on Phenolic Compounds
The total phenolic content of treated and control grape samples gradually increased (Fig. 2a). The red light treatment resulted in the greatest increase in phenolic content in grape samples. On day 21, the total phenolic content of the control fruit was 3.01 mg g−1, which was significantly (P < 0.05) lower than that of the red and blue light treatments (3.58 mg g−1 and 3.22 mg g−1) at the end of storage. Red light treatment exhibited higher total flavonoid content of grape samples during the whole storage (Fig. 2b). The flavonoid content of red and blue light was 1.09 mg g−1 and 1.03 mg g−1 higher than control fruit (0.97 mg g−1) at the storage end. A significant (P < 0.05) difference in the total phenolic and total flavonoid content of grape samples between the treatments and the control was observed. Figure 2c depicts changes in total anthocyanin content in grape samples. The overall anthocyanin content of both control and treated grapes increased during storage. Grape samples exposed to red light had significantly (P < 0.05) higher total anthocyanin levels than blue light and control fruit from 7 to 21 days. The anthocyanin content of grape samples treated with red light was 2.58 mg g−1 at the end of storage, which was greater than the blue light and control.
Impact of red and light exposure on a total phenolic content, b total flavonoid content, and c total anthocyanin content of grape berries stored at 4 °C. The standard deviation is represented by the data shown (SD, n = 3). Different letters reflect significant differences (P < 0.05) between the treatments
Impact of Red and Blue Light Radiation on Phenolic Biosynthesis-Related Enzymes
The result of PAL activity is presented in Fig. 3a; the PAL activity in grape samples increased in both treatments. However, after 7 days of storage, red light radiation has retained higher PAL activity. In comparison to the control, red and blue light radiation had higher PAL activity (30.10 U g−1 and 29.24 U g−1) at the end of storage. In this experiment, TAL activity increased within 14 days of storage in all the treatments, and then declined at the end of storage closest to the initial storage level of around 27.44, 29.69, and 30. 51 U g−1 (Fig. 3b). Red light treatment had the highest TAL activity than blue light and control during the whole storage. During storage, the C4H and 4CL activity decreased progressively as storage time prolonged (Fig. 3c, d). However, C4H activity in grape berries treated with red light was significantly (P < 0.05) higher than blue light and control throughout the entire storage (Fig. 3c). C4H activity in red light treatment was 38.52 U g−1 higher than the control and blue light treatment at the end of storage. A similar pattern to C4H activity was observed for 4CL activity (Fig. 3d).
Impact of Red and Blue Light Radiation on Soluble Sugars
Changes in glucose, fructose, and sucrose showed a similar pattern in both treatments (Fig. 4). The content of glucose, fructose, and sucrose increased in both control and red and blue light-treated grapes (Fig. 4a–c). The content of glucose and fructose at the end of storage in the control berries was 29.31 and 30.72 mg g−1, respectively, whereas the content under red and blue light was (30.64 and 32.19 mg g−1) (glucose) and (32.36 and 33.18 mg g−1) (fructose), respectively, which was significantly (P < 0.05) higher than the control berries. There was virtually little sucrose in the grape samples (Fig. 3c). The sucrose content of grape samples stored under red light treatments were significantly (P < 0.05) higher than blue light and control berries.
Impact of Red and Blue Light Radiation on Sucrose Metabolism-Related Enzyme Activities
Figure 5a shows that SS-synthesis activity increased throughout the storage time in all treatments. The activities of SS-synthesis in blue light treatment was significantly (P < 0.05) higher than the red light treatment and control. This trend did not change throughout the storage. After 21 days of storage, blue light treatment resulted in 0.31 U g−1 higher than red light and control samples. Changes in the SS-cleavage activity during storage are shown in Fig. 5b. SS-cleavage activity decreased in both treatments. The SS-cleavage in red light treatment was maintained slightly higher than the blue light and control samples during the whole storage. Compared to blue light and control fruit, red light treatment resulted in 0.24 g U g−1 slightly higher in SS-cleavage activity in grape berries at the end of storage. There were no significant (P > 0.05) differences between the treated and control samples. The acid invertase activities continued to increase during storage (Fig. 5c). Compared with the control and blue light treatment, acid invertase was significantly (P < 0.05) higher in grape berries exposed to red light. At the end of storage, acid invertase activity in red and blue light treatments was 0.24 g U g−1 and 0.22 g U g−1 higher than in control samples.
Impact of Red and Blue Light Radiation on GABA Content, GAD, and GABA-T Activities
As demonstrated in Fig. 6a, the content of GABA increased in both treatments during the whole storage period as the storage time increased. Grape berries exposed to blue light treatment exhibited higher GABA content than red light treatment and control berries. Blue light treatment had the highest GABA content (36.94 µmol g−1) at the storage end, followed by red light treatment (34.89 µmol g−1). As exhibited in Fig. 6b, the GAD activity showed a similar pattern with GABA content; the GAD activity presented an increasing trend in both the treatments throughout the storage. During the whole storage period, the red light treatment significantly (P < 0.05) retained higher GAD activity than blue light and control, and the red light-treated sample had the most increased GAD activity (72.81 U g−1) at the end of storage. As depicted in Fig. 6c, GABA-T activity increased continuously in all treatments. It was, however, lower in treated grape berries than in control berries. GABA-T activity in the control fruit was significantly (P < 0.05) higher (23.31 U g−1) than in the treated samples at the end of the storage period.
Discussion
The amount of sugar in fruit is one of the key factors determining its quality. The changes in sugar content and composition in the fruit are also affected by respiration. Moreover, abiotic stress affects sugar metabolism in fruit as well. Here, we present a non-chemical technique for determining the influence of light quality, intensity, and storage duration on the quality of post-harvested table grapes. LED light controls the physiological metabolism in horticultural crops, which influences color and quality (Nassarawa et al., 2020, 2022a, b). LEDs light irradiation of harvested horticultural crops at the postharvest stage has become a more prevalent and efficient method of extending their shelf life and maintaining their visual quality (Nassarawa et al., 2020). TSS and TA are considered the most reliable measures of fruit flavor (Sheng et al., 2018). Increasing TSS levels in fruit suggest that the ripening process is speeding up and longer fruit shelf life, and lower TSS, on the other hand, indicates slower ripening. We found that red and blue light treatments, as shown in Fig. 1a, retained a higher TSS content than berries under control, with the slight highest recorded under red light treatment. These findings revealed that red and blue light could regulate total soluble solids and preserve grape berries’ quality during the storage period. Similar outcomes were reported in tomatoes (Dhakal & Baek, 2014b), peaches (Gong et al., 2015), Chinese bayberries (Shi et al., 2016), and grape (Nassarawa et al. 2022a). During storage, TA decreased, but both treatments delayed this decline (Fig. 1b). Red and blue light treatment alleviated the decrease in TA during storage, further suggesting the role of red and blue light in delaying fruit maturation and ripening. This outcome was in line with previous research on grape berries (Azuma et al., 2019).
Phenolic compounds are health-promoting secondary metabolites found in fruit and vegetable that contribute to antioxidant activity. Light acts as stress that promotes the biosynthesis of these compounds (Nassarawa et al., 2020). The phenylpropane route produces phenolics and flavonoids, which are essential secondary metabolites in grapes. Chalcone-flavanone isomerase (CHI), flavanone-3-hydroxylase (F3H), and chalcone synthase (CHS) catalyze the conversion of phenylalanine to phenolic content, which are then converted into flavonoids by the catalysis of PAL, TAL, 4CL, and C4H (Nassarawa et al., 2020). Previous studies have shown that red lights have been demonstrated to enhance the synthesis of phenolic compound in grapes by improving phenolic biosynthesis-related enzymes (Pinto et al., 2016). Similarly, our current study found that red light treatments retained higher total phenolic content compared with blue light and control, suggesting that red light activated the phenylpropanoid and flavonoid biosynthesis pathways (Fig. 2a). Also, it has been reported that red light radiation increased the phenolic content of some fruits and vegetables, including grape (Falguera et al., 2013), red prickly pear (Ortega-Hernández et al., 2018), and strawberries (Xu et al., 2014b). Our analysis showed that at constant intensity (500 lx), red light irradiation had the higher flavonoid content in grape berries than those in blue light and control, as shown in Fig. 2b. Our findings were similar to Shen et al. (2013) in satsuma mandarin, mushroom (Jiang et al., 2010), blueberries (Wang et al., 2009), and grape (Nassarawa et al., 2022a, b), who found that red light irradiation increased flavonoid content. Shao et al. (2020) also noted that red light treatment for 20 days (500 μmol m−2 s−1) at 23 °C had significant stimulating impacts on the increase of total flavonoid content in lettuce. We believe that flavonoid biosynthesis is a complicated process that can be inclined by various factors, including storage conditions and postharvest treatments, which may influence total flavonoid content changes during storage.
Anthocyanins are a group of flavonoids found naturally in plants liable for fruit color (Azuma et al., 2019). Environmental conditions cause the proliferation of anthocyanin by major groups of higher plants (Xu et al., 2014a). Our present study found that red light irradiation has higher anthocyanin content during storage in postharvest grape than those in blue light treatment and control (Fig. 2c). This demonstrated that red light could be a viable method for increasing anthocyanin accumulation and maintaining a postharvest grape product of high quality after harvest. Similar findings showed that red light treatment increases the anthocyanin content in red cabbage (Wu et al., 2017). Also, red light radiation enhanced the accumulation of anthocyanin in postharvested grape at 3 days (Azuma et al., 2012). Similar findings were obtained by Choi et al. (2015), who found that the irradiation of red-light in treated grape was significantly higher than those in control. To study more about the impact of red and blue light irradiation on phenolic biosynthesis-related enzymes, the enzymes involved in the phenylpropanoid pathway were also investigated. The phenylpropanoid pathway is made up of two enzymes called PAL and TAL. PAL catalyzes l-phenylalanine to trans-cinnamic acid conversion. TAL works similarly to PAL as it transforms l-tyrosine to ammonia and p-coumaric acid (Shing et al., 2016). PAL and TAL were also exposed to be the first essential enzymes in the phenolic metabolism catalyst for converting phenylalanine to cinnamic acid. Cinnamic acid degrades into phenolic compound (Li et al., 2014). Thus, in our current study, PAL and TAL were enhanced by red and blue light during postharvest storage, with the highest recorded under red light treatment (Fig. 3a, b). The results showed that red light treatment was more efficient in enhancing the activities of PAL and TAL, which suggested that red light could potentially improve and sustain PAL and TAL activities. These findings demonstrate that an increase in phenolic compounds was associated with an increase in PAL and TAL activities. These results were positively interrelated to phenolic compound, and Pinto et al. (2016) found a similar relationship between PAL, TAL, and phenolic compound accumulation in grape berries. Similar findings have been reported by Pombo et al. (2011), who noted that red light improved the activities of PAL and TAL in treated strawberries. Similarly, table grape treated with red and blue light yielded similar results, in which red light stimulated the expression of phenolic biosynthesis-related enzymes (Crupi et al., 2013). Study shows that red light treatment enhanced the activities of PAL and TAL, demonstrating that PAL and TAL are active in response to phenolic compound treatments (Pombo et al., 2011). C4H and 4-CL are also essential regulators in the phenylpropanoid pathway, contributing to anthocyanin synthesis (Li et al., 2014). The mediating activities of anthocyanin metabolic enzymes can control anthocyanin accumulation. In our current study, C4H and 4-CL decreased during storage (Fig. 3c, d). We found that red light exposure was more effective than blue light exposure in enhancing C4H and 4-CL activities. The findings were consistent with those of the previous study that red light treatment enhanced enzyme activities in strawberry fruit (Xu et al., 2014b). We hypothesized that our findings showed that red and blue light treatment can regulate phenolic biosynthesis-related enzymes, resulting in an increase in phenolic compound in grape berries during storage.
During the postharvest storage of fruit, soluble sugars play an important role in cell signaling and resistance to external stress factors (Sun et al., 2014). Previous research has shown that glucose, fructose, and sucrose are the three main sugar components in grape fruit and sucrose stimulates the activity of certain antioxidant enzymes that eliminate reactive oxygen species (ROS) (Li et al., 2017). In our current study, red light treatment had the highest concentrations of glucose, fructose, and sucrose in grape berries compared with those in blue light (Fig. 4a–c). Our findings showed that irradiation with red and blue light at an intensity of 500 lx efficiently regulated and promoted the conversion of sucrose to hexose in vacuoles. Li et al. (2017) reported that red light treatment enhanced the activity of sucrose metabolism-related enzyme activities, leading to a higher level of soluble sugar in tomato fruit. Similar findings were also reported in Chinese bayberry fruit (Shi et al., 2016) and lime (Kaewsuksaeng et al., 2011). The reversible conversion of sucrose to fructose, glucose, and uridine diphosphate glucose (UDPG) can be catalyzed by SS-synthesis and SS-cleavage. The primary purpose of sucrose synthesis is to produce sucrose. Sucrose phosphate synthase (SPS) is an enzyme involved in the irreversible conversion of UDPG and 6-phosphate-fructose to sucrose. Our recent study showed that SS-synthesis was higher under the blue light, while SS-cleavage and acid invertase activities were higher under red light treatments than those in control (Fig. 5a–c); this was concurrent with high soluble sugar levels in treated berries. Similar results were also observed in Chinese bayberry fruit (Shi et al., 2016) and grape (Rodyoung et al., 2016). We hypothesized that higher enzyme activity in sucrose synthesis contributed to maintaining the quality of grape berries during postharvest storage.
In higher plants, GABA is mostly metabolized through a short mechanism called GABA shunt. GAD and GABA-T are two important enzymes in the GABA shunt. The GABA-T pathway converts glutamate to succinic semialdehyde after being catalyzed by GAD (Khan et al., 2021). Our findings revealed that blue-light treatment increased GABA content than red-light and control fruit during storage at 4 °C (Fig. 6a). However, the higher GAD activity was observed in red light compared to blue-light and control fruit (Fig. 6b). Nevertheless, control sample had the highest GABA-T activity than treated samples (Fig. 6c). Compared with control fruit, treated samples inhibited the GABA-T activity. Based on these findings, we hypothesize that red and blue light treatment at 4 °C can impact the GABA shunt pathway by promoting GAD activity and inhibiting GABA-T activity, resulting in GABA accumulation, which could be an adaptive mechanism for protecting against senescence from grape berries. Also, higher GABA content and GAD activity in grape berries during 4 °C storage, which leads to phenolic compound accumulation, could be due to inhibition of GABA-T, which is responsible for supplying skeleton carbon in prolyl endopeptidase (PEP) form and is linked to Er4P, which may act as a starter of the phenylpropanoid pathway. Yan et al. (2021) reported that UV-C LED exposure increased GABA content and GAD activity while inhibiting GABA-T activity in tomato fruit. The increase in the GABA content in tomato fruit stored under blue-light treatment was reported by Dhakal and Baek (2014b).
Conclusion
The current study investigates the positive influences of red and blue light radiation on postharvest grape berries quality during storage. The quality characteristic was maintained by red or blue light treatment during storage. Blue light treatment had less influence on phenolic compound during storage than red light treatment. Red light treatment at 500 lx was more efficient in enhancing phenolic biosynthesis-related enzymes than blue light treatment. The red light treatment was also efficient in stimulating soluble sugars and maintaining higher SS-cleavage and acid invertase in grape berries, while blue light treatment increased SS-synthesis during storage. Our results also showed that treated samples increased GAD activity by inhibiting GABA-T activity, which led to GABA accumulation. In summary, our findings suggest that irradiation with red or blue light treatment is likely a useful technique for maintaining sugar metabolism and the γ-aminobutyric acid shunt pathway, improving grape berries’ quality and extended postharvest storage life.
Data Availability Statement
On reasonable request, the datasets generated and/or analyzed during the current study are available from the corresponding author.
References
Avalos-Llano, K. R., Molina, R. S., & Sgroppo, S. C. (2020). UV-C Treatment applied alone or combined with orange juice to improve the bioactive properties, microbiological, and sensory quality of fresh-cut strawberries. Food and Bioprocess Technology, 13(9), 1528–1543. https://doi.org/10.1007/s11947-020-02491-0
Azuma, A., Ito, A., Moriguchi, T., Yakushiji, H., & Kobayashi, S. (2012). Light emitting diode irradiation at night accelerates anthocyanin accumulation in grape skin. Acta Horticulturae, 956, 341–347. https://doi.org/10.17660/ActaHortic.2012.956.39
Azuma, A., Yakushiji, H., & Sato, A. (2019). Postharvest light irradiation and appropriate temperature treatment increase anthocyanin accumulation in grape berry skin. Postharvest Biology and Technology, 147, 89–99. https://doi.org/10.1016/j.postharvbio.2018.09.008
Balic, I., Moreno, A., Sanhueza, D., Huerta, C., Orellana, A., Defilippi, B. G., & Campos-Vargas, R. (2012). Molecular and physiological study of postharvest rachis browning of table grape cv Red Globe. Postharvest Biology and Technology, 72, 47–56. https://doi.org/10.1016/j.postharvbio.2012.05.005
Barut Gök, S. (2021). UV-C Treatment of apple and grape juices by modified UV-C reactor based on dean vortex technology: Microbial, physicochemical and sensorial parameters evaluation. Food and Bioprocess Technology, 14(6), 1055–1066. https://doi.org/10.1007/s11947-021-02624-z
Bhavya, M. L., Shewale, S. R., Rajoriya, D., & Hebbar, H. U. (2021). Impact of blue LED illumination and natural photosensitizer on bacterial pathogens, enzyme activity and quality attributes of fresh-cut pineapple slices. Food and Bioprocess Technology, 14(2), 362–372. https://doi.org/10.1007/s11947-021-02581-7
Castagna, A., Dall’Asta, C., Chiavaro, E., Galaverna, G., & Ranieri, A. (2014). Effect of post-harvest UV-B irradiation on polyphenol profile and antioxidant activity in flesh and peel of tomato fruits. Food and Bioprocess Technology, 7(8), 2241–2250. https://doi.org/10.1007/s11947-013-1214-5
Choi, H. G., Moon, B. Y., & Kang, N. J. (2015). Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Scientia Horticulturae, 189, 22–31. https://doi.org/10.1016/j.scienta.2015.03.022
Crupi, P., Pichierri, A., Basile, T., & Antonacci, D. (2013). Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chemistry, 141(2), 802–808. https://doi.org/10.1016/j.foodchem.2013.03.055
Dhakal, R., & Baek, K. H. (2014a). Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biology and Technology., 90, 73–77. https://doi.org/10.1016/j.postharvbio.2013.12.007
Dhakal, R., & Baek, K. H. (2014b). Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Horticulture, Environment, and Biotechnology, 55(1), 36–41. https://doi.org/10.1007/s13580-014-0125-3
Falguera, V., Garvín, A., Garza, S., Pagán, J., & Ibarz, A. (2014). Effect of UV-vis photochemical processing on pear juices from six different varieties. Food and Bioprocess Technology, 7(1), 84–92. https://doi.org/10.1007/s11947-013-1069-9
Falguera, V., Garza, S., Pagán, J., Garvín, A., & Ibarz, A. (2013). Effect of UV-Vis irradiation on enzymatic activities and physicochemical properties of four grape musts from different varieties. Food and Bioprocess Technology, 6(8), 2223–2229. https://doi.org/10.1007/s11947-012-0781-1
Farhadi, K., Esmaeilzadeh, F., Hatami, M., Forough, M., & Molaie, R. (2016). Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chemistry, 199, 847–855. https://doi.org/10.1016/j.foodchem.2015.12.083
Gómez, P. L., Schenk, M. L., Salvatori, D. M., & Alzamora, S. M. (2015). Potential of UV-C light for preservation of cut apples fortified with calcium: Assessment of optical and rheological properties and native flora dynamics. Food and Bioprocess Technology, 8(9), 1890–1903. https://doi.org/10.1007/s11947-015-1545-5
Gong, D., Cao, S., Sheng, T., Shao, J., Song, C., & Wo, F. (2015). Effect of blue light on ethylene biosynthesis, signalling and fruit ripening in postharvest peaches. Scientia Horticulturae, 197, 657–664.
Hägele, F., Nübling, S., Schweiggert, R. M., Baur, S., Weiss, A., Schmidt, H., et al. (2016). Quality improvement of fresh-cut endive (Cichorium endivia L.) and recycling of washing water by low-dose UV-C irradiation. Food and Bioprocess Technology, 9(12), 1979–1990. https://doi.org/10.1007/s11947-016-1782-2
Harindra Champa, W. A., Gill, M. I. S., Mahajan, B. V. C., & Bedi, S. (2015). Exogenous treatment of spermine to maintain quality and extend postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless under low temperature storage. LWT, 60(1), 412–419. https://doi.org/10.1016/j.lwt.2014.08.044
Jagadeesh, S. L., Charles, M. T., Gariepy, Y., Goyette, B., Raghavan, G. S. V., & Vigneault, C. (2011). Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food and Bioprocess Technology, 4(8), 1463–1472. https://doi.org/10.1007/s11947-009-0259-y
Jiang, T., Jahangir, M. M., Jiang, Z., Lu, X., & Ying, T. (2010). Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biology and Technology, 56(3), 209–215. https://doi.org/10.1016/j.postharvbio.2010.01.011
Kaewsuksaeng, S., Urano, Y., Aiamla-or, S., Shigyo, M., & Yamauchi, N. (2011). Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biology and Technology, 61(2–3), 124–130. https://doi.org/10.1016/j.postharvbio.2011.02.014
Khan, M. I. R., Jalil, S. U., Chopra, P., Chhillar, H., Ferrante, A., Khan, N. A., & Ansari, M. I. (2021). Role of GABA in plant growth, development and senescence. Plant Gene, 26, 100283. https://doi.org/10.1016/j.plgene.2021.100283
Khan, S., Hussain, A., Rafeeya, D., Mohsin, S., Aga, B., & Wasim, M. (2022). Applications of ultraviolet light-emitting diodestechnology in horticultural produce: A systematic review and meta-analysis. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-021-02742-8
Li, D., Luo, Z., Mou, W., Wang, Y., Ying, T., & Mao, L. (2014). ABA and UV-C effects on quality, antioxidant capacity and anthocyanin contents of strawberry fruit (Fragaria ananassa Duch.). Postharvest Biology and Technology, 90, 56–62. https://doi.org/10.1016/j.postharvbio.2013.12.006
Li, D., Zhang, X., Li, L., Soleimani, M., Wei, X., & Liu, J. (2019). Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chemistry, 285, 163–170. https://doi.org/10.1016/j.foodchem.2019.01.150
Li, Y., Xin, G., Wei, M., Shi, Q., Yang, F., & Wang, X. (2017). Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Scientia Horticulturae, 225, 490–497. https://doi.org/10.1016/j.scienta.2017.07.053
Lo’ay, A. A., & Dawood, H. D. (2017). Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Scientia Horticulturae, 224, 286–292. https://doi.org/10.1016/j.scienta.2017.06.043
Lu, H., Wu, W., Limwachiranon, J., Yang, D., Xiao, G., Luo, Z., & Li, L. (2018). Effect of micro-perforated film packing on fatty acid-derived volatile metabolism of “Red Globe” table grapes. Food and Bioprocess Technology, 11(10), 1807–1817. https://doi.org/10.1007/s11947-018-2142-1
Nassarawa, S. S., Abdelshafy, A. M., Xu, Y., Li, L., & Luo, Z. (2020). Effect of light-emitting diodes (LEDs) on the quality of fruits and vegetables during postharvest period: A review. Food and Bioprocess Technology, 20(6), 11947–25340. https://doi.org/10.1007/s11947-020-02534-6
Nassarawa, S. S., Belwal, T., Javed, M., & Luo, Z. (2022a). Influence of the red LEDs light irradiation on the quality and chemical attributes of postharvest table grape (Vitis vinifera L.) during storage. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02824-1
Nassarawa, S. S., Luo, Z., & Lu, Y. (2022b). Conventional and emerging techniques for detection of foodborne pathogens in horticulture crops: A leap to food safety. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-021-02730-y
Nia, A. E., Taghipour, S., & Siahmansour, S. (2022). Effects of salicylic acid preharvest and aloe vera gel postharvest treatments on quality maintenance of table grapes during storage. South African Journal of Botany. https://doi.org/10.1016/j.sajb.2022.05.010
Ortega-Hernández, E., Welti-Chanes, J., & Jacobo-Velázquez, D. A. (2018). Effects of UVB light, wounding stress, and storage time on the accumulation of betalains, phenolic compounds, and ascorbic acid in red prickly pear (Opuntia ficus-indica cv. Rojo Vigor). Food Bioprocess and Technology, 11(12), 2265–2274. https://doi.org/10.1007/s11947-018-2183-5
Pendyala, B., Patras, A., Ravi, R., Gopisetty, V. V. S., & Sasges, M. (2020). Evaluation of UV-C irradiation treatments on microbial safety, ascorbic acid, and volatile aromatics content of watermelon beverage. Food and Bioprocess Technology, 13(1), 101–111. https://doi.org/10.1007/s11947-019-02363-2
Pinto, E. P., Perin, E. C., Schott, I. B., da Silva Rodrigues, R., Lucchetta, L., Manfroi, V., & Rombaldi, C. V. (2016). The effect of postharvest application of UV-C radiation on the phenolic compounds of conventional and organic grapes (Vitis labrusca cv. ‘Concord’). Postharvest Biology and Technology, 120, 84–91. https://doi.org/10.1016/j.postharvbio.2016.05.015
Pombo, M. A., Rosli, H. G., Martínez, G. A., & Civello, P. M. (2011). UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biology and Technology, 59(1), 94–102. https://doi.org/10.1016/j.postharvbio.2010.08.003
Rodyoung, A., Masuda, Y., Tomiyama, H., Saito, T., Okawa, K., Ohara, H., & Kondo, S. (2016). Effects of light emitting diode irradiation at night on abscisic acid metabolism and anthocyanin synthesis in grapes in different growing seasons. Plant Growth Regulation, 79(1), 39–46. https://doi.org/10.1007/s10725-015-0107-1
Shao, M., Liu, W., Zha, L., Zhou, C., Zhang, Y., & Li, B. (2020). Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Scientia Horticulturae, 268, 109–366. https://doi.org/10.1016/j.scienta.2020.109366
Shen, Y., Sun, Y., Qiao, L., Chen, J., Liu, D., & Ye, X. (2013). Effect of UV-C treatments on phenolic compounds and antioxidant capacity of minimally processed Satsuma mandarin during refrigerated storage. Postharvest Biology and Technology, 76, 50–57. https://doi.org/10.1016/j.postharvbio.2012.09.006
Sheng, K., Zheng, H., Shui, S. S., Yan, L., Liu, C., & Zheng, L. (2018). Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biology and Technology, 138, 74–81. https://doi.org/10.1016/j.postharvbio.2018.01.002
Shi, L., Cao, S., Shao, J., Chen, W., Yang, Z., & Zheng, Y. (2016). Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biology and Technology, 111, 197–204. https://doi.org/10.1016/j.postharvbio.2015.08.013
Shing, L., Lasekan, O., Mohd, N., & Hashim, N. (2016). The effect of combinations of UV-C exposure with ascorbate and calcium chloride dips on the enzymatic activities and total phenolic content of minimally processed yam slices. Postharvest Biology and Technology, 120, 138–144. https://doi.org/10.1016/j.postharvbio.2016.06.008
Song, Z., Pang, Q., Lu, S., Yu, L., Pervaiz, T., Fu, W., et al. (2022). Transcriptomic and metabolmic approaches to counter the effect of Botrytis cinerea in grape berry with the application of nitric oxide. Scientia Horticulturae, 296, 110–901. https://doi.org/10.1016/j.scienta.2022.110901
Sun, Y., Qian, M., Wu, R., Niu, Q., Teng, Y., & Zhang, D. (2014). Postharvest pigmentation in red chinese sand pears (Pyrus pyrifolia Nakai) in response to optimum light and temperature. Postharvest Biology and Technology, 91, 64–71. https://doi.org/10.1016/j.postharvbio.2013.12.015
Wang, C. Y., Chen, C., & Wang, S. Y. (2009). Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chemistry, 117(3), 426–431. https://doi.org/10.1016/j.foodchem.2009.04.037
Wilawan, N., Ngamwonglumlert, L., Devahastin, S., & Chiewchan, N. (2019). Changes in enzyme activities and amino acids and their relations with phenolic compounds contents in okra treated by LED lights of different colors. Food and Bioprocess Technology, 12(11), 1945–1954. https://doi.org/10.1007/s11947-019-02359-y
Wu, J., Liu, W., Yuan, L., Guan, W. Q., Brennan, C. S., Zhang, Y. Y., et al. (2017). The influence of postharvest UV-C treatment on anthocyanin biosynthesis in fresh-cut red cabbage. Science and Reports, 7(1), 1–11. https://doi.org/10.1038/s41598-017-04778-3
Xijia, Z., Min, Z., Bimal, C., & Sakamon, Z. G. (2022). Novel combined use of red‑white LED illumination and modified atmosphere packaging for maintaining storage quality of postharvest pakchoi. Food Bioprocess and Technology, 591–605.
Xu, F., Cao, S., Shi, L., Chen, W., Su, X., & Yang, Z. (2014a). Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. Journal of Agriculture and Food Chemistry, 62(20), 4778–4783. https://doi.org/10.1021/jf501120u
Xu, F., & Liu, S. (2017). Control of postharvest quality in blueberry fruit by combined 1-methylcyclopropene (1-MCP) and UV-C irradiation. Food and Bioprocess Technology, 10(9), 1695–1703. https://doi.org/10.1007/s11947-017-1935-y
Xu, F., Shi, L., Chen, W., Cao, S., Su, X., & Yang, Z. (2014b). Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae, 175, 181–186. https://doi.org/10.1016/j.scienta.2014.06.012
Xu, F., Wang, S., Xu, J., Liu, S., & Li, G. (2016). Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biology and Technology, 117, 125–131. https://doi.org/10.1016/j.postharvbio.2016.01.012
Xue, M., & Yi, H. (2017). Induction of disease resistance providing new insight into sulfur dioxide preservation in Vitis vinifera L. Scientia Horticulturae, 225, 567–573. https://doi.org/10.1016/j.scienta.2017.07.055
Yan, L., Zheng, H., Liu, W., Liu, C., Jin, T., Liu, S., & Zheng, L. (2021). UV-C treatment enhances organic acids and GABA accumulation in tomato fruits during storage. Food Chemistry. https://doi.org/10.1016/j.foodchem.2020.128126
Yuan, Z., Deng, L., Yin, B., Yao, S., & Zeng, K. (2017). Effects of blue LED light irradiation on pigment metabolism of ethephon-degreened mandarin fruit. Postharvest Biology and Technology, 134, 45–54. https://doi.org/10.1016/j.postharvbio.2017.08.005
Zeng, F., Jiang, T., Wang, Y., & Luo, Z. (2015). Effect of UV-C treatment on modulating antioxidative system and proline metabolism of bamboo shoots subjected to chilling stress. Acta Physiologiae Plantarum, 37(11), 1–10. https://doi.org/10.1007/s11738-015-1995-4
Funding
The authors received financial support from the National Natural Science Foundation of China (U2003213).
Author information
Authors and Affiliations
Contributions
SSN: writing—first draft, revision and editing, conceptualization, data curation, formal analysis. ZL: conceptualization, funding, resources, review, and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nassarawa, S.S., Luo, Z. Effect of Light Irradiation on Sugar, Phenolics, and GABA Metabolism on Postharvest Grape (Vitis vinifera L.) During Storage. Food Bioprocess Technol 15, 2789–2802 (2022). https://doi.org/10.1007/s11947-022-02919-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02919-9