Abstract
Prickly pear (Opuntia ficus-indica cv. Rojo Vigor) fruit is an excellent source of secondary metabolites with health-promoting properties (i.e., betalains, flavonoids, and ascorbic acid), and thus, it is relevant to find postharvest treatments that increase their concentration. Postharvest abiotic stresses such as wounding and ultraviolet radiation can induce the accumulation of secondary metabolites in different horticultural crops. In the present study, the effect of ultraviolet B (UVB) radiation applied alone or combined with wounding stress on the accumulation of betalains, phenolics, and ascorbic acid in red prickly pear was evaluated. Whole and wounded fruit samples were treated with UVB radiation (6.4 W m−2) for 0, 15, 90, and 180 min and stored for 24 h at 16 °C. The content of bioactive compounds was evaluated before and after storage. The application of UVB radiation for 15 min was the most adequate treatment to induce the accumulation of bioactive compounds. In this context, UVB radiation (15 min) of the wounded tissue resulted on an immediate accumulation of betalains (33–40%) and ascorbic acid (54–58%) in the pulp and peel of the fruit. Likewise, after storage, the pulp of irradiated whole fruits showed the highest accumulation of phenolics (125.8%) and betalains (49.8%) as compared with the control, whereas the stored wounded tissue treated with UVB presented accumulation of ascorbic acid in the pulp (67.2%) and peel (84.6%). The stressed tissue with enhanced concentration of nutraceuticals could be transformed into functional processed foods or used as raw material for the extraction of compounds with applications in health-related markets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Opuntia ficus-indica (L.) Mill., commonly known as prickly pear, is a tropical or subtropical plant belonging to the Cactaceae family (De Cotazar and Nobel 1992). In Mexico, the stem of Opuntia ficus-indica has been used for the treatment of different health-disorders including ulcers, cancer, high cholesterol levels, and hypoglycemia (Chauhan et al. 2010). Furthermore, recent reports indicate that compounds present in the pulp and peel of prickly pear fruit (i.e., betalains, flavonoids, and ascorbic acid) also exert biological activity (Hameed-Abdel et al. 2014; Bisson et al. 2010).
The most apparent characteristic of cactus pear fruits and flowers is the yellow and red color. This feature is exerted by betalains, which are nitrogen-containing vacuolar pigments that replace anthocyanins in most plant families of the Caryophyllales (Albano et al. 2015). In addition to color, betalains have shown antioxidant, anti-inflammatory, and vascular-protective effects (Stintzing et al. 2005; Tesoriere et al. 2005). Phenolic compounds are secondary metabolites produced via the shikimate and phenylpropanoid pathways (Cartea et al. 2010). Their presence has been detected in the peel and pulp of cactus fruit (Yeddes et al. 2013). It has been found that the antioxidant activity of prickly pear is mainly attributed to quercetin, kaempferol, and isorhamnetin, which are the main flavonoids present in the fruit (Kuti 1992). Ascorbic acid is a water-soluble vitamin required to form and preserve bones, blood vessels, and skin. Cactus pear fruits contain ascorbic acid in concentrations around 20 to 40 mg/100 g of fresh weight (Russel and Felker 1987), content that is higher than the values reported for apples, pears, grapes, and bananas (Kristbergsson and Ötleş 2016).
The application of postharvest abiotic stresses [i.e., wounding and ultraviolet (UV) radiation] in fruits and vegetables has received significant attention because they allow the accumulation of health-promoting compounds (Cisneros-Zevallos 2003). The production of secondary metabolites in plant tissue can be promoted by ultraviolet B (UVB radiation) without concern about conflicting interactions in the plant tissue matrix used itself as a food (Schreiner et al. 2009). UVB radiation (280–315 nm) induces the biosynthesis of flavonoids and phenolic acids, capable of attenuating UV spectrum without interfering with the absorption of active photosynthetic radiation (Lavola 1998). Likewise, wounding stress induces the accumulation of bioactive compounds. The wound-induced biosynthesis of plant secondary metabolites is mediated by molecules such as reactive oxygen species (ROS), ethylene (ET), and jasmonic acid (JA) that act as signals to activate the phenylpropanoid pathway, producing the synthesis of phenolic compounds implicated in the lignification process during wounding-healing (Jacobo-Velázquez et al. 2015). In this context, the application of abiotic stresses on prickly pear may be an attractive strategy to increase its nutraceutical content.
Little is known on the effect of UVB radiation applied alone or in combination with wounding stress on the accumulation of phytochemicals in red prickly pear. Therefore, the objective of the present study was to evaluate the effect of UVB radiation and wounding stress applied alone or combined on the accumulation of total phenolics, betalains and ascorbic acid before and after storage (24 h in at 16 °C and 70% relative humidity) of red prickly pear (Opuntia ficus-indica cv. Rojo vigor).
Materials and Methods
Chemicals
Sodium hydroxide (NaOH), ferric chloride (FeCl3), and phosphoric acid (H3PO4) were obtained from Desarrollo de Especialidades Químicas (San Nicolás de los Garza, N.L., México). The other chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Plant Material
Red prickly pears (Opuntia ficus-indica cv. Rojo Vigor) were obtained from the agroproducer La Flor de Villanueva, Tuna y Nopal (Puebla, Mexico) during the last week of June 2017. After harvesting, fruits were classified by ripening stage considering the following indicators: size of fruit, external changes of color from green to red, total soluble solids (TSS), total titratable acids (TTA), firmness, and pH of the fruit. With these parameters, minimum ripening stage was selected based on the UC Davis Postharvest Technology Center database (Kader 1999). The selected fruits to carry out the experiments presented the following characteristics: external color was red in the middle and green at the ends, fresh weight of 142.40 ± 5.30, polar length of 15.16 ± 0.28, equatorial length of 11.58 ± 0.58, TSS of 13.1 ± 1.09 °Brix, TTA of 0.03%, pH of 6.10 ± 0.60, and firmness was acceptable in 22.41 ± 2.04 N.
UVB Treatments and Storage
UVB light chambers consisted of a steel framework of 60 cm (width) × 30 cm (depth) × 60 cm (height) covered with aluminum and equipped with four 40 W UVB lamps: Philips TL 40 W/12RS (Philips, USA). The irradiation dose was determined before the experiment as 6.4 W m−2 using a PMA 2106 UVB sensor (Solar Light, Glenside, PA, USA) measuring in the spectral range from 280 to 320 nm. Fruits were placed 45 cm below the light source. In order to ensure uniform UVB dose, fruits were aligned in rows parallel to the lamp tubes and inverted at the middle of the radiation process to achieve optimum exposure on all sides of the tissue.
Four UV treatments consisting of a single UVB exposure for 0, 15, 90, and 180 min were carried out. These conditions were selected based on previous reports from our research group where UV treatments were applied in carrots and broccoli sprouts to induce the accumulation of different phytochemicals (Surjadinata et al. 2017; Moreira et al. 2017a, b). After washing and disinfecting the fruits with chlorinated water (200 ppm, pH 6.5), ten whole and four wounded fruits (cut in four pieces, with a cross and a longitudinal section, using a commercial straight-edged knife) were selected for each UVB treatment. In this experiment, total betalains, phenolic compounds, and ascorbic acid content were evaluated before and after 24 h of storage in the whole and wounded prickly pears treated with UVB light.
Samples were stored for 24 h in an incubator (VWR, Radnor, PA, USA) at 16 °C and 70% relative humidity in darkness. Due to the quantities of tissue required for analysis of tissue metabolites and the small size of our samples, it was necessary to pool samples. The samples were obtained from five whole fruits and two pieces from each wounded fruit at 0 and 24 h of storage. The peel (∼ 3 mm thickness) and pulp tissues were separated, cut in small pieces, immediately frozen in liquid nitrogen, and stored at − 80 °C until further analysis.
Color, Firmness, TSS, and TTA Analyses
Prickly pear peel color was evaluated using a Konica Minolta™ CM-5 (Osaka, Japan). The color was recorded using the L*, a*, b* scale CIELAB system. Results of L*, a*, and b* were obtained from five different red prickly pears, in three different sites of the peel and used to calculate Chroma (C = [(a*2 + b*2)1/2]) and Hue angle (h°) (h° = tg−1(b/a)). Firmness was directly determined in five whole red prickly pear fruits with a texture analyzer (TA. XT Plus, StableMicroSystem, Godalming, UK), using a load cell of 10 kg and a speed penetration of 1 mm s−1. The puncture test was performed using a stainless-steel probe with 5 mm of diameter. The texture was measured as the maximum force (expressed in Newtons, 1 N = 0.1 kg F) required to achieve probe penetration of 10 mm at three different locations in each sample.
pH and acidity determinations were performed in homogenized pulp tissue without seeds of five different red prickly pears. pH was measured by direct immersion on the samples with a potentiometer (Thermo Scientific Orion, Singapore). Titratable acidity was determined at room temperature by titration of 10 mL prickly pear juice (obtained from five different fruits) with 0.1 N NaOH solution to an endpoint of pH 8.3 and expressed as a percentage of citric acid (1 mL of 0.1 N NaOH = 0.006404 g of citric acid). Total soluble solids determination was performed in the pulp of five prickly pears by direct measure with a digital refractometer (ATAGO-PR-32α, Brix 0.0 to 32.0%) and expressed in °Brix. Moisture content was determined with five replications by the method AACC 44-15A, using aluminum plates with sand (10 g) and a rod each, previously placed at a constant weight. Prickly pear samples (3 g) were weighed before and after 8 h of drying at 110 °C.
Total Phenolics, Total Betalains, and Ascorbic Acid Determinations
Extraction and Quantification of Total Phenolic Compounds
For the extraction of total phenolics, prickly pear sample (250 mg fresh weight) was suspended in methanol (1 mL). The mixture was immersed in an ultrasound bath (Branson 2510, Branson Ultrasonic Corporation, CT, USA) at room temperature for 5 min. Centrifugation was performed at 13,000×g for 5 min at 4 °C. After that, the supernatant (200 μL) was diluted with methanol (800 μL) and shacked during 10 s on a vortex mixer (VWR Digital Vortex Mixer, USA). Total phenolics were determined by the Folin-Ciocalteu colorimetric method described by Swain and Hillis (1959) adapted to 96-well microplate format (Sánchez-Rangel et al. 2013). Gallic acid was used as standard, and the total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per 100 g of prickly pear dry weight (DW).
Extraction and Quantification of Total Betalains
Quantification of betalains was performed with a colorimetric method stated by Stintzing et al. (2005). Briefly, the prickly pear sample (250 mg fresh weight) was mixed with distilled water (1 mL) and immersed into the ultrasound bath for 5 min. The extracts were then clarified by centrifugation at 13,000×g for 5 min at 4 °C (Eppendorf AG Centrifuge 5417R), and the supernatant was diluted (1:1 v/v) with water.
Betacyanins and betaxanthins content were reported as milligrams of equivalent of betanin and indicaxanthin per 100 g of prickly pear DW, respectively. Betacyanins were detected at 535 nm and betaxanthins at 483 nm, according to the following equation proposed by Sumaya-Martínez et al. (2011) with some modifications:
where A = absorbance at 535 or 483 nm, DF = dilution factor, MW = molecular weight, E = extinction coefficient, and B = width of the spectrophotometer well. The method was optimized for a 96-well plate with a truncated cone formula, and the calculated width was 5.335 mm. For betacyanins the extinction coefficient was 60,000 L/(mol cm) and MW = 550 g/mol, whereas for betaxanthins, the extinction coefficient was 48,000 L/(mol cm) and MW = 308 g/mol.
Determination of Ascorbic Acid
Ascorbic acid analyses were determined using the method reported by Gillespie and Ainsworth (2007). Extractions were performed at room temperature and under dark conditions. Prickly pear sample (1 g fresh weight) was homogenized with trichloroacetic acid (TCA, 6%, 5 mL) and centrifuged at 13,000×g for 20 min at 4 °C. For total ascorbic acid, the extract (100 μL) was mixed with DDT solution (32 mM, 50 μL) and incubated for 10 min at room temperature in the dark. Then, N-ethylmaleimide (NEM) solution (12 mM, 50 μL) was added to the mixture and incubated for 30 s. On the other hand, reduced ascorbic acid was determined by adding distilled water (100 μL). Subsequently, a mixture of TCA (10%, 250 μL), H3PO4 (43%, 200 μL), α,α′-bipyridyl (4%, 200 μL), and FeCl3 (3%, 100 μL) solutions was added to both reactions (total and reduced). After incubation at 37 °C for 60 min, the reactions (200 μL) were placed in a 96-well microplate, and absorbance readings were recorded at 525 nm. Absorbance values were compared against an ascorbic acid standard curve (0.10–3 mM) prepared in TCA (6%). Results were expressed as milligrams of ascorbic acid per 100 g of prickly pear DW.
Statistical Analysis
Statistical analyses of phytochemical analyses were performed using five treatment repetitions. Data represent the mean values of samples and their standard error. A full-factorial design was applied to study the main effects and interactions of the variables evaluated. Analyses of variance (ANOVA) were conducted using JMP software version 13.0 (SAS Institute Inc., Cary, NC, USA) and mean separations performed using the LSD test (p < 0.05).
Results and Discussion
Effects of UVB Treatments (0, 15, 90, and 180 min), Wounding Stress, and Storage Time (0 and 24 h) on Total Phenolics
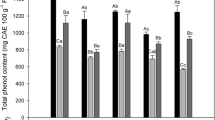
Total phenolic content before and after 24 h of storage of red prickly pear subjected to different UVB light doses (6.4 W m−2 for 0, 15, 90, and 180 min) is shown in Fig. 1. As observed, the peel showed 285.3% higher content of total phenolics than the pulp. These results are in agreement with Jiménez-Aguilar et al. (2014) and Yeddes et al. (2013), who reported that the concentration of total phenolics in the peel is 220–300% higher than the pulp in different varieties of prickly pear. Likewise, Fernández-López et al. (2010) reported values from 1266 to 1683 mg GAE per 100 g in extracts from whole fruits, including pulp and peels, in different species of red-skinned prickly pears, which are in accordance with the results presented herein.
Concentration of total phenolics before and after storage (24 h at 16 °C) of whole and wounded red prickly pear (a) pulp and (b) peel treated with UVB light (6.4 W m−2) for 0, 15, 90, and 180 min. (c) Full factorial analyses of variance showing the main effects and interactions of the variables evaluated. Bars are means of five replicates ± standard error. Different letters among bars indicate statistical difference between treatments using the LSD test (p < 0.05). Asterisks indicate that main effects and interactions are significantly different by analyses of variance (ANOVA). NS non-significant, *p < 0.05, **p < 0.01, ***p < 0.001. Results are expressed as milligrams of equivalents of gallic acid per 100 g of sample in dry weight basis
The application of different UVB treatments did not induce an immediate significant change in the phenolic content of red prickly pear fruit pulp and peel when the whole fruit was subjected to different UVB light exposure times. On the other hand, for the wounded fruit pulp, the content of total phenolic increased by 52.5%, 101.8%, and 38.8%, in samples treated with UVB for 15, 90, and 180 min, respectively, as compared with the control, whereas the phenolic content in the wounded peel was not affected by UVB radiation. The immediate increase in the concentration of phenolic compounds in the wounded pulp can be attributed in part to changes in the permeability of the cell membrane induced by the wave of free radical produced by wounding stress and UV light that derives from peroxidation within the lipid bilayer of the tissues (Surjadinata et al. 2017). The free radical reaction may lead to ruptured cell membranes, which may allow a higher release of phenolic compounds mainly at 90 min of UVB light exposure. When the fruit was exposed to UVB light for a more extended period, a degradation effect of the phenolic compounds was observed as compared with 90 min UVB-treated wounded fruit pulp. Results suggest that the whole fruit was protected with UVB-filtering compounds present in the peel, which impeded the production of free radicals in the pulp, inhibiting the release of phenolic compounds from the plant cell of pulps (Surjadinata et al. 2017).
After storage, the effect of UVB and wounding stress on the content of phenolic compounds was tissue-dependent (Fig. 1). No significant increment in the phenolic content of untreated whole red prickly pear fruit pulp and peel was detected after 24 h of storage time. On the other hand, wounding stress alone induced a significant increment (27.7%) in the content of phenolic compounds in the pulp after storage, whereas the phenolic content in the wounded peel was not affected. Previous reports have shown that wounding is an effective stress that allows the accumulation of phenolics in different horticultural crops such as potatoes (Torres-Contreras et al. 2014a, b), broccoli (Villarreal-García et al. 2016; Torres-Contreras et al. 2017), and carrots (Jacobo-Velázquez et al. 2011, 2015). The wound-induced accumulation of phenolics in plants is triggered by extracellular ATP (eATP) produced at the site of injury, which diffuses and binds to ATP receptors of adjacent cells. Upon eATP binding to receptors, the production of reactive oxygen species (ROS) is triggered, which are the central signaling molecules involved on the wound-induced biosynthesis of phenolic compounds in plants (Jacobo-Velázquez et al. 2011, 2015).
The application of UVB for 15 and 90 min in the whole fruit induced increases in phenolic content after storage, which showed 125.8–101.5% and 33.8–28.2% higher levels in the pulp and peel, respectively, as compared with the control stored for 24 h. However, the highest effect was observed in samples treated with UVB light for 15 min, whereas no accumulation of phenolic compounds was detected in samples treated with 180 min of UVB. The UVB-induced production of phenolic compounds in horticultural crops has been previously reported in different tissues such as carrot (Surjadinata et al. 2017), broccoli sprouts (Moreira-Rodríguez et al. 2017a, b) and lemons (Interdonato et al. 2011). Phenolic compounds selectively absorb light in the UVB region of the spectrum, while not decreasing penetration of photosynthetic radiation (Jansen et al. 2008). Thus, it is likely that red prickly pear is producing phenolics as a mechanism to filter the high-intensity radiation of UVB light to which it was subjected. The accumulation of phenolic compounds was repressed in red prickly pear treated with 180 min of UVB light. This result suggests that prolonged radiation induced a failure in the plant’s repair mechanism, inhibiting the synthesis of phenolic compounds.
The combination of both stresses only induced accumulation of phenolics when the wounded tissue was treated with 15 min of UVB light. In these samples, the application of UVB light increased the concentration of phenolics in the pulp and peel, by 17.7 and 19.2%, respectively, as compared with wounded control samples after storage. As indicated by the results, the accumulation of phenolics was favored in the whole fruit treated with UV-light for 15 min as compared with the wounded fruit. Besides, the wounded pulp treated with UVB for 90 and 180 min showed a decrease of − 12.6 and − 32.6%, respectively, in the content of total phenolics. These results indicate that wounding stress exerts an antagonistic effect on the UVB induced accumulation of phenolics in the pulp. This antagonistic effect can be attributed to the presence of oxidative enzymes such as polyphenol oxidase which is decompartmentalized after cutting the tissue and uses as substrate phenolic compounds (Tomás-Barberán et al. 1997). Likewise, the wounded tissue is highly exposed to oxygen, which is an additional substrate required for oxidative enzymatic reactions.
Effects of UVB Treatments (0, 15, 90, and 180 min), Wounding Stress, and Storage Time (0 and 24 h) on Total Betalains
The content of betalains before and after 24 h of storage of red prickly pear exposed to different UVB light doses (6.4 W m−2 for 0, 15, 90, and 180 min) is shown in Fig. 2. As observed, the pulp showed a 10.9% higher content of total betalains than the peel. The betalain content determined in this study was similar to values previously reported for Sicilian and San Martín cultivars (Butera et al. 2002; Aparicio-Fernández et al. 2017).
Concentration of total betalains before and after storage (24 h at 16 °C) of whole and wounded red prickly pear (a) pulp and (b) peel treated with UVB light (6.4 W m−2) for 0, 15, 90, and 180 min. (c) Full factorial analyses of variance showing the main effects and interactions of the variables evaluated. Bars are means of five replicates ± standard error. Different letters among bars indicate statistical difference between treatments using the LSD test (p < 0.05). Asterisks indicate that main effects and interactions are significantly different by analyses of variance (ANOVA). NS non-significant, *p < 0.05, **p < 0.01, ***p < 0.001. Results are expressed as milligrams of equivalents of betanin per 100 g of sample and milligrams of equivalents of indicaxanthin per 100 g of sample in dry weight basis for betacyanins and betaxanthins, respectively
The application of UVB light for 15 min did not induce an immediate effect on the concentration of total betalains in the whole red prickly pear. However, UVB treatments of 90 and 180 min induced an immediate decrement in the betalain content of the pulp (12–17%) and peel (19–24%). The wounded fruit presented the same behavior, showing decreases in the betalain content of the pulp (13–21%) and peel (17–14%) exposed to 90 and 180 min, whereas UVB light exposure for 15 min did not induce changes in the betalain concentration. The degradation of betalains observed in samples treated with 90 and 180 min of UVB light can be attributed to cellular alkalization due to degradation of the membrane by the presence of reactive oxygen species and the influx of ions produced. Alkalization may induce the hydrolysis of aldimine bond in the betalain structure, resulting in the production of a phenolic acid (Herbach et al. 2006).
After storage, the betalain content of untreated whole red prickly pear fruit pulp and peel did not change. On the other hand, at 24 h of storage, the wounded pulp and peel showed 13.6 and 13.2%, respectively, higher levels of total betalains as compared with the stored whole fruit. As discussed for phenolics, these results suggest that betalains may play a role in the defense mechanism of prickly pear against wounding stress. Previous reports suggest that ROS produced by wounding could also serve as signaling molecules to induce the biosynthesis of betalains in plants, mainly as a defense response because betalains may act as ROS scavengers, limiting the damage caused by wounding stress (Sepúlveda-Jiménez et al. 2004). Although little is known about the wound-induced biosynthesis of betalains, these results agree with a previous report in beets (Sepúlveda-Jimenez et al. 2005). The authors reported that ROS act as a signal to induce BvGT expression, which encodes for a glucosyltransferase related to betacyanin synthesis.
The application of UVB alone induced a significant increment in betalains after storage of whole fruit pulp (49, 36, and 29%) irradiated for 15, 90, and 180 min with UVB. Likewise, betalain content in the whole peel increased by 17.9% with UVB for 15 min, as compared with the control. Vogt et al. (1999) have previously reported similar results in the synthesis of betacyanin, induced as a consequence of UVA and UVB exposure in Mesembryanthemum crystallinum. Accumulation of betalains in UVB-treated plant tissues could be due to their high molar absorption coefficients, which allow them to function as protectors against UV radiation (Clement and Mabry 1996). Also, betalains accumulate in the vacuoles (Tanaka et al. 2008), acting as a potential sink for excess H2O2 produced in the chloroplast due to UVB and wounding, alleviating the photo-oxidative damage in the plant (Kytridis and Manetas 2006). Besides, the greater accumulation was observed at 15 min of exposure, indicating that longer exposures begin to saturate the absorption capacity of the betalain molecules and a photo-bleaching process was activated.
The combination of both stresses (UVB + wounding) induced a significant increase of 39.6%, 25.6%, and 16.2% in the concentration of betalains after storage of the pulp treated with 15, 90, and 180 min of UVB radiation, respectively, as compared with the wounded control, whereas the peel showed increments of 33%, 19%, and 11% in samples treated with 15, 90, and 180 min of UVB radiation, respectively. UVB and wounding stress showed a synergistic effect in the accumulation of betalains. Besides, this effect was dose-dependent, where higher UVB light doses resulted in a lower accumulation of betalains in the wounded tissue. As earlier explained for the whole tissue treated with UVB, prolonged UVB exposures times may result in a photo-bleaching process of betalains in wounded samples treated with 90 and 180 min of radiation.
Effects of UVB Treatments (0, 15, 90, and 180 min), Wounding Stress, and Storage Time (0 and 24 h) on Ascorbic Acid Content
The ascorbic acid content at 0 and 24 h of storage of red prickly pear exposed to different UVB light is shown in Fig. 3. As observed, the content of ascorbic acid is similar in the pulp and the peel. Similar values of ascorbic acid content have been previously reported in different species of prickly pear (Butera et al. 2002).
Concentration of ascorbic acid before and after storage (24 h at 16 °C) of whole and wounded red prickly pear (a) pulp and (b) peel treated with UVB light (6.4 W m−2) for 0, 15, 90, and 180 min. (c) Full factorial analyses of variance showing the main effects and interactions of the variables evaluated. Bars are means of five replicates ± standard error. Different letters among bars indicate statistical difference between treatments using the LSD test (p < 0.05). Asterisks indicate that main effects and interactions are significantly different by analyses of variance (ANOVA). NS nonsignificant, *p < 0.05, **p < 0.01, ***p < 0.001. Results are expressed as milligrams of equivalents of ascorbic acid per 100 g of sample in dry weight basis
The application of UVB treatment induced an immediate significant increment in the ascorbic acid content of red prickly pear fruit pulp when the whole fruit was subjected to 90 min (37.4%) of radiation, whereas the whole pulp treated for 15 and 180 min did not show significant changes. For the whole fruit peel, the content of ascorbic acid did not change in samples treated with 15 min of UVB light; however, it showed a decrement in samples treated for 90 (− 15.4%) and 180 min (− 15.1%) of radiation as compared with the control. Ascorbic acid (vitamin C) is the most abundant antioxidant in plants (Smirnoff et al. 2001) and has a role in redox signaling. Ascorbate peroxidase (APX) mediates the scavenging of H2O2 by ascorbate. Therefore, the ascorbic acid should be accumulated in the presence of UVB as a defense mechanism against ROS production and for the regeneration of α-tocopherol (Niki 1987; Akram et al. 2017).
On the other hand, the wounded pulp treated with UVB for 15 and 90 min showed an increment in the content of ascorbic acid of 54.1% and 37.5% as compared with the control, respectively, whereas the wounded peel presented significant increments of 58.4%, 38.9%, and 29.1% for the 15, 90, and 180 min treatments, respectively. In accordance with these results, it has been reported that UVB exposure induces the accumulation of ascorbic acid in Landsberg erecta, as well as its redox state compared to control plants (Kovácsa and Keresztesb 2002). This immediate increase in ascorbic acid levels could be related with a defense mechanism of the plant in the detoxification of ROS production after UV radiation. However, 15 min of radiation is the adequate time, since prolonged radiation resulted in a lower accumulation of ascorbic acid. Interestingly, the wounded tissue showed higher accumulation of ascorbic acid when exposed to UVB as compared with the whole tissue. It is likely that the skin partially filtered UVB radiation, and thus, lower oxidative stress was generated in the whole tissue as previously reported for carrot (Surjadinata et al. 2017), resulting in lower accumulation of ascorbic acid.
After storage, the application of UVB alone induced a significant increment in ascorbic acid content in the whole fruit pulp irradiated with UVB for 15 (41.4%) and 90 min (46.2%), whereas the pulp of whole tissue treated with 180 min of UVB did not show significant difference in ascorbic acid concentration after storage as compared with time 0 h control samples. Furthermore, after storage, the ascorbic acid content in the peel of whole fruits treated with UVB increased by 23.4% and 13.3% in samples irradiated for 15 and 90 min, respectively, as compared with the untreated stored sample, whereas peel of fruits treated with 180 min of UVB showed a significant decrement (− 23.1%) in ascorbic acid content as compared with the stored whole control. Castagna et al. (2013) obtained similar results; the authors reported an increase in the total ascorbic acid concentration of 34–41% for the flesh of tomato after 10 d of storage of fruits treated for 1 h of UVB (1.69 W/m2) as compared with the control. As earlier mentioned, UV radiation produces ROS in the plant tissue, and thus, ascorbic acid biosynthesis during storage would be acting as an antioxidant to neutralize free radicals. The decreases in the concentration of ascorbic acid detected in the pulp of whole fruit, treated with 180 min of UVB, can be related with a higher rate of degradation (Kd) to neutralize free radicals than the rate of UVB-induced biosynthesis (Kb).
When the two stresses were combined (wounding + UVB light), a significant increase was detected in the concentration of ascorbic acid after storage of samples treated with 15 (67.5%) and 90 min (40.4%) of UVB as compared with wounded control samples. Likewise, the wounded peel treated with 15, 90, and 180 min of UVB light showed a significant increase of ascorbic acid of 84.5%, 54.5%, and 19.3%, respectively, as compared with the wounded control stored for 24 h. In previous reports, non-significant changes in ascorbic acid content due to wounding stress was found in different plants including sweet potato, radish and red cabbage (Reyes et al. 2007). However, to the best of our knowledge, the combined effect of wounding stress and UVB on ascorbic acid in horticultural crops had not been previously studied.
UVB and wounding stress presented a synergistic effect on the elicitation of ascorbic acid biosynthesis. This synergistic effect could be attributed to the combination of signaling pathways that activates the biosynthesis of vitamin C. UVB induces the monomerization and accumulation of UVR8 photoreceptor, which initiates a sequence of metabolic events leading with the expression of genes related with the synthesis of secondary metabolites (Tilbrook et al. 2013); likewise, UVB induces the generation of ROS, enhancing the wound-induced production of ROS, which also are important signaling molecules involved in the production of antioxidants in plants (Jacobo-Velázquez et al. 2011, 2015; Surjadinata et al. 2017). Likewise, it has been reported that wounding stress leads to the generation of methyl jasmonate (Jacobo-Velázquez et al. 2015), a signaling molecule that has been strongly linked with the activation of ascorbic acid biosynthesis in different plants (Sasaki-Sekimoto et al. 2005; Wolucka et al. 2005).
Conclusions
In the present study, results showed that the application of UVB light and wounding stress can induce the accumulation of secondary metabolites in red prickly pear. From the different conditions tested, UVB radiation (6.4 W m−2) for 15 min before 24 h of storage (at 16 °C and 70% relative humidity) was the most adequate treatment to accumulate phenolics (125.8%), betalains (49.8%), and ascorbic acid (84.6%). Depending on the target compound to accumulate, the combination of UVB radiation and wounding stress showed either a synergistic or antagonistic effect on its biosynthesis. Data presented would help prickly pear producers to add value to the crop, by selecting appropriate postharvest treatments to increase the content of specific compounds. The stressed tissue with enhanced concentration of bioactive compound could be used as raw material to produce processed foods or for the extraction of nutraceuticals with applications in health-related markets. Further experiments should be focused into the optimization of UVB conditions that achieve the highest accumulation of phytochemicals in prickly pear. Likewise, it will be relevant to design experiments that could increase our understanding on the physiological and molecular mechanisms involved on the accumulation of secondary metabolites in prickly pear treated with UVB alone or combined with wounding stress.
References
Akram, N. A., Shafiq, F., & Ashraf, M. (2017). Ascorbic acid—a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science, 8(613), 1–17.
Albano, C., Negro, C., Tommasi, N., Gerardi, C., Mita, G., Miceli, A., et al. (2015). Betalains, phenols and antioxidant capacity in cactus pear [Opuntia ficus-indica (L.) Mill.] fruits from Apulia (South Italy) genotypes. Antioxidants, 4(2), 269–280.
Aparicio-Fernández, X., Loza-Cornejo, S., Torres-Bernal, M. G., Velázquez-Placencia, N. J., & Arreola-Nava, H. J. (2017). Physicochemical characteristics of fruits from wild Opuntia species from two semiarid regions of Jalisco, Mexico. Polibotanica, 43, 219–244.
Bisson, J.-F., Daubié, S., Hidalgo, S., Guillemet, D., & Linarés, E. (2010). Diuretic and antioxidant effects of Cacti-Nea®, a dehydrated water extract from prickly pear fruit, in rats. Phytotherapy Research, 24(4), 587–594.
Butera, D., Tesoriere, L., Di Gaudio, F., Bongiorno, A., Allegra, M., Pintaudi, A. M., et al. (2002). Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. Journal of Agricultural and Food Chemistry, 50(23), 6895–6901.
Cartea, M. E., Francisco, M., Soengas, P., & Velasco, P. (2010). Phenolic compounds in Brassica vegetables. Molecules, 16(1), 251–280.
Castagna, A., Chiavaro, E., Dall’asta, C., Rinaldi, M., Galaverna, G., & Ranieri, A. (2013). Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chemistry, 137(1–4), 151–158.
Chauhan, S. P., Sheth, N. R., Jivani, N. P., Rathod, I. S., & Shah, P. I. (2010). Biological actions of Opuntia species. Systematic Reviews in Pharmacy, 1(2), 146–151.
Cisneros-Zevallos, L. (2003). The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. Journal of Food Science, 68(5), 1560–1565.
Clement, J. S., & Mabry, T. J. (1996). Pigment evolution in the Caryophyllales: a systematic overview. Botanica Acta, 109(5), 360–367.
De Cotazar, V. G., & Nobel, P. S. (1992). Biomass and fruit production for the prickly pear cactus Opuntia ficus-indica. Journal of the American Society for Horticultural Science, 117(4), 558–562.
Fernández-López, J. A., Almela, L., Obón, J. M., & Castellar, R. (2010). Determination of antioxidant constituents in cactus pear fruits. Plant Foods for Human Nutrition, 65(3), 253–259.
Gillespie, K., & Ainsworth, E. (2007). Measurement of reduced, oxidized and total ascorbate content in plants. Nature Protocols, 2(4), 871–874.
Hameed-Abdel, E.-S., Nagaty, M. A., Salman, M. S., & Bazaid, S. A. (2014). Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chemistry, 160, 31–38.
Herbach, K. M., Stintzing, F. C., & Carle, R. (2006). Betalain stability and degradation—structural and chromatic aspects. Journal of Food Science, 71(4), R41–R50.
Interdonato, I., Rosa, M., Nieva, C. B., González, J. A., Hilal, M., & Prado, F. E. (2011). Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environmental and Experimental Botany, 70(2–3), 204–211.
Jacobo-Velázquez, D., Martínez-Hernández, G., Rodríguez, S. C., Cao, C. M., & Cisneros-Zevallos, L. (2011). Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. Journal of Agricultural and Food Chemistry, 59(11), 6583–6593.
Jacobo-Velázquez, D. A., González-Agüero, M., & Cisneros-Zevallos, L. (2015). Cross-talk between signaling pathways: the link between plant secondary metabolite production and wounding. Scientific Reports, 5(1), 8608.
Jansen, M. A. K., Hectors, K., O’Brien, N. M., Guisez, Y., & Potters, G. (2008). Plant stress and human health: Do human consumer benefit from UV-B acclimated crops? Plant Science, 175(4), 449–458.
Jiménez-Aguilar, D., Mújica-Paz, H., & Welti-Chanes, J. (2014). Phytochemical characterization of prickly pear (Opuntia spp.) and of its nutritional and functional properties: a review. Current Nutrition & Food Science, 10(1), 57–69.
Kader, A. (1999). Cactus (prickly) pear. Recommendations for maintaining postharvest quality. http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Fruit_English/?uid=13&ds=798. Accessed 19 May 2017.
Kovácsa, E., & Keresztesb, Á. (2002). Effect of gamma and UV-B/C radiation on plant cells. Micron, 33(2), 199–210.
Kristbergsson, K., & Ötleş, S. (2016). Functional properties of traditional foods. 1st ed. (p. 258). US: Springer.
Kuti, J. O. (1992). Growth and compositional changes during the development of prickly pear fruit. Journal of Horticultural Science, 67(6), 861–868.
Kytridis, V. P., & Manetas, Y. (2006). Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. Journal of Experimental Botany, 57(10), 2203–2210.
Lavola, A. (1998). Accumulation of flavonoids and related compounds in birch induced by UV-B irradiance. Tree Physiology, 18(1), 53–58.
Moreira-Rodríguez, M., Nair, V., Benavides, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017a). UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. International Journal of Molecular Sciences, 18(11), 2330.
Moreira-Rodríguez, M., Nair, V., Benavides, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017b). UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules, 22(7), E1065.
Niki, E. (1987). Interaction of ascorbate and alpha-tocopherol. Annals of the New York Academy of Sciences, 498, 186–199.
Reyes, L. F., Villarreal, J. E., & Cisneros-Zevallos, L. (2007). The increase in antioxidant capacity after wounding depends on the type of fruit and vegetable tissue. Food Chemistry, 101(3), 1254–1262.
Russel, C., & Felker, P. (1987). The prickly pear (Opuntia spp., Cactaceae): a source of human and animal food in semi-arid regions. Economic Botany, 41(3), 433–445.
Sánchez-Rangel, J. C., Benavides, J., Heredia, J. B., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2013). The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Analytical Methods, 5(21), 5990–5999.
Sasaki-Sekimoto, Y., Taki, N., Obayashi, T., Aono, M., Matsumoto, F., Sakurai, N., et al. (2005). Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. The Plant Journal, 44(4), 653–668.
Schreiner, M., Krumbein, A., Mewis, I., Ulrichs, C., & Huyskens-Keil, S. (2009). Short-term UV-B radiation effects on secondary metabolism in different organs of Tropaeolum majus L. Innovative Food Science and Emerging Technologies, 10(1), 93–96.
Sepúlveda-Jiménez, G., Rueda-Benítez, P., Porta, H., & Rocha-Sosa, M. (2004). Betacyanin synthesis in red beet (Beta vulgaris) leaves induced by wounding and bacterial infiltration is preceded by an oxidative burst. Physiological and Molecular Plant Pathology, 64(3), 125–133.
Sepúlveda-Jimenez, G., Rueda-Benítez, P., & Rocha-Sosa, M. (2005). A red beet (Beta vulgaris) UDP-glucosyltransferase gene induced by wounding, bacterial infiltration and oxidative stress. Journal of Experimental Botany, 56(412), 605–611.
Smirnoff, N., Conklin, P. L., & Loewus, F. A. (2001). Biosynthesis of ascorbic acid in plants: a renaissance. Annual Review of Plant Physiology, 52(1), 437–467.
Stintzing, F., Herbach, K., Mosshammer, M., Carle, R., Yi, W., Sellappan, S., et al. (2005). Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. Journal of Agricultural and Food Chemistry, 53(2), 442–451.
Sumaya-Martínez, M., Cruz-Jaime, S., Madrigal-Santillán, E., García-Paredes, J., Cariño-Cortés, R., Cruz-Cansino, N., et al. (2011). Betalain, acid ascorbic, phenolic contents and antioxidant properties of purple, red, yellow and white cactus pears. International Journal of Molecular Sciences, 12(10), 6452–6468.
Surjadinata, B. B., Jacobo-Velázquez, D. A., & Cisneros-Zevallos, L. (2017). UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules, 22(4), 668.
Swain, T., & Hillis, W. (1959). The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10(1), 63–68.
Tanaka, Y., Sasaki, N., & Ohmiya, A. (2008). Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. The Plant Journal, 54(4), 733–749.
Tesoriere, L., Butera, D., Allegra, M., Fazzari, M., & Livrea, M. (2005). Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. Journal of Agricultural and Food Chemistry, 53(4), 1266–1270.
Tilbrook, K., Arongaus, A. B., Binkert, M., Heijde, M., Yin, R., & Ulm, R. (2013). The UVR8 UV-B photoreceptor: perception, signaling and response. The Arabidopsis Book / American Society of Plant Biologists, 11, e0164.
Tomás-Barberán, F. A., Gil, M. I., Castañer, M., Artés, F., & Saltveit, M. E. (1997). Effect of selected browning inhibitors on phenolic metabolism in stem tissue of harvested lettuce. Journal of Agricultural and Food Chemistry, 45(3), 583–589.
Torres-Contreras, A. M., Nair, V., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2014a). Plants as biofactories: stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Industrial Crops and Products, 62, 61–66.
Torres-Contreras, A. M., Nair, V., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2014b). Effect of exogenous amylolytic enzymes on the accumulation of chlorogenic acid isomers in wounded potato tubers. Journal of Agriculture and Food Chemistry, 62(31), 7671–7675.
Torres-Contreras, A. M., Nair, V., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017). Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules, 22(4), 636.
Villarreal-García, D., Nair, V., Cisneros-Zevallos, L., & Cisneros-Zevallos, D. A. (2016). Plants as biofactories: postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Frontiers in Plant Science, 7, 45.
Vogt, T., Ibdah, M., Schmidt, J., Wray, V., Nimtz, M., & Strack, D. (1999). Light-induced betacyanin and flavonol accumulation in bladder cells of Mesembryanthemum crystallinum. Phytochemistry, 52(4), 583–592.
Wolucka, B. A., Goossens, A., & Inzé, D. (2005). Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. Journal of Experimental Botany, 56(419), 2527–2538.
Yeddes, N., Chérif, J., Guyot, S., Sotin, H., & Ayadi, M. (2013). Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants, 2(4), 37–51.
Acknowledgments
The authors would like to thank Agroproductores La Flor de Villanueva, Tuna, and Nopal (Puebla, Mexico) for the donation of the prickly pear fruit (Rojo vigor) necessary to carry out this project.
Funding
This study was supported by funds from Tecnologico de Monterrey (Bioprocess, Bioengineering and Synthetic Biology Research Group; Emerging Technologies and Molecular Nutrition Research Group). Author E.O.-H. also acknowledges the scholarship (573045) from CONACYT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortega-Hernández, E., Welti-Chanes, J. & Jacobo-Velázquez, D.A. Effects of UVB Light, Wounding Stress, and Storage Time on the Accumulation of Betalains, Phenolic Compounds, and Ascorbic Acid in Red Prickly Pear (Opuntia ficus-indica cv. Rojo Vigor). Food Bioprocess Technol 11, 2265–2274 (2018). https://doi.org/10.1007/s11947-018-2183-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2183-5