Abstract
Major losses of fresh horticultural produce transpire during postharvest storage due to prompt senescence and diseases. The traditional postharvest preservation techniques used after harvest are based on cooling and the application of chemical preservation techniques. As a residue-free physical sterilization and preservation method, light-emitting diode (LED) treatment, has recently been applied for postharvest storage of fruits and vegetables by numerous researchers. This paper reviews the recent applications of LEDs in postharvest storage of fresh produce, including its effect on physiological characteristics, secondary metabolism, nutritional attributes, ripening process, senescence, shelf-life improvement, and pathogenic microbial spoilage of fruits and vegetables. LED treatment has promoted the accumulation of different phytochemicals, such as phenolic compounds, vitamins, glucosinolates, chlorophyll, total soluble solids, and carotenoids. Changes in the nutritional content, anthocyanin content, antioxidant capacity, and ripening were also observed after the treatment. Reduction in microbial spoilage and delay senescence were evident after the LED exposure. The influence of LED light depended on the fruit and vegetable variety. Therefore, LED treatment is an efficient and promising strategy for extending the storage life of fruits and vegetables with enhanced nutritional values.
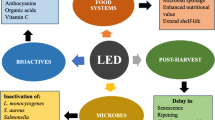
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruits and vegetables are well known for their health-promoting properties due to their contents of vitamins, minerals, and antioxidants, which reduce the risk of chronic diseases and increasing their demand in the consumer diet (Aghdam et al. 2018; Pinela and Ferreira 2017; Slavin and Lloyd Beate 2012). The Food and Agriculture Organization (FAO) has reported that fruits and vegetables are the most wasted commodities (Dou et al. 2017; Miguel et al. 2008). The primary causes for this wastage are inefficient postharvest infrastructure and poor harvesting and storage techniques ( Dou et al. 2017; Dueck et al. 2016; Kozai 2013; Kozai and Niu 2015; Regnier and Combrinck 2009). The postharvest problem is a major global issue, in both developed and developing countries. Many studies have shown that there are minimal storage facilities for various horticultural crops, especially in developing countries, which has influenced postharvest losses, including over-ripening, softening, decay, weight loss, senescence, firmness loss, and specific physiological disorders (Bantis et al. 2018; Dou et al. 2017). The shortening of fruits and vegetable loss is a leading issue for providing long-term storage across the global population (Capone et al. 2014). However, due to the speedy senescence and susceptible diseases of postharvest fruits and vegetables, the preservation technology has become the research focus (Mari et al. 2016; Usall et al. 2016).
Currently, the techniques used for preserving fruits and vegetables are mainly chemical additives, such as the use of 1-methyl cyclopropane (Amornputti et al. 2014; Chiabrando and Giacalone 2011), ozone treatment (Song et al. 2016), application of a high oxygen atmosphere (Zheng et al. 2008), chitosan coating (Kerch 2015), and physical techniques, including controlled atmosphere storage (Skrovankova et al. 2015), forced air cooling air packaging (Castro et al. 2004), and UV-C (Amornputti et al. 2014; Wang et al. 2009), and biological techniques such as biocontrol agent (Amornputti et al. 2014) to reduce losses. As a result of limitations on chemical additives technologies, thus, the food processing industries have shifted toward nonthermal approaches, including irradiation, ultraviolet light (UV), pulsed light (PL), high-pressure processing (HPP), ultrasound (US), and cold plasma (Naveenkumar et al. 2017; Castillo et al. 2010; Osae et al. 2020; Pinela and Ferreira 2017).
Recently, greater emphasis has been paid to the influence of LEDs (narrow-bandwidth illumination) on the postharvest storage of fresh horticultural produce. Recent research has shown that LEDs enhance the traits of numerous postharvest fresh produce, including lettuce, cabbage, kale plants, white mustard, sweet pepper, cucumber and blueberries (Hung et al. 2016), peaches (Gong et al. 2015), strawberries (Xu et al. 2014a, 2014b), tomatoes (Dhakal and Baek 2014), citrus fruits (Ma et al. 2012), Chinese bayberries (Shi et al. 2014), Spinach and kale (Erwin and Gesick 2017), and grapes (Rodyoung et al. 2016).
In addition, there have been many researches on the application of LEDs in the field of postharvest fruits and vegetables and several reviews on plant physiology (D’Souza et al. 2015). However, there is a lack of usable information on the advantages of LED techniques when applied at the time of postharvest storage. There is also no synopsis of the influence of LEDs on quality attributes. This review provides information on the application of LEDs for the postharvest preservation of fresh horticultural crops. An overview of the effect of LEDs as a nonhazardous technique for improving the ripening process, nutritional parameters, and secondary metabolites, delaying senescence, and leading to quality improvement of fruit is discussed.
Overview of LED (NBI) Technology in the Postharvest Period of Fruits and Vegetables
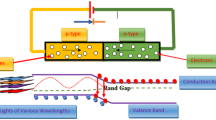
LEDs are a solid-state (i.e., semiconductor-based) system that converts electrical energy to electromagnetic radiation (D’Souza et al. 2015; Schultz et al. 2008), typically in the infrared or visible regimes (Rodyoung et al. 2016), and occasionally in ultraviolet regimes (Bantis et al. 2018; Dou et al. 2017; Krames et al. 2007). A depiction of a LED system is shown in (Fig. 1). The LEDs comprised of a semiconductor with a p-n (positive-negative) junction, consisting of p-type (positive-doped) and n-type (negative-doped) semiconductor on the right and left, respectively. A depletion region, defined as the barrier between p-type and n-type regions that prevent the movement of electrons from n-type and holes from p-type semiconductors, is created due to diffusion currents. The depletion region left with positive and negative charged ions. Electrons and holes (being the absence of particles) recombine spontaneously when a forward bias (i.e., applied electric field) forces them to interact within the depletion region. This recombination process produces light (as photons) in a spontaneous emission process, defining the mechanism of the LEDs (Bantis et al. 2018; Folta et al. 2005; Hasan et al. 2017a, 2017b). This emitted light has a wavelength dictated by the bandgap energy of the semiconductor between the conduction and valence energy bands (D’Souza et al. 2015). The emission of light from LED can vary from a narrow band of single colors like red, green, or blue to the wider band for white light (Mitchell et al. 2012). A white LED is typically a blue LED that has been infused with phosphorous to broaden the emission spectrum. A phenomenon involved in the operation of LEDs is electroluminescence, which is a non-thermal process of emitting light when the electric current passes through the semiconductor (Lai et al. 2016). The electroluminescence phenomenon was first detected when H. J. Round was working with silicon carbide in 1907. However, the first patent based on the emission of infrared radiation from a semiconductor due to the passage of electricity was obtained by James Biard and Gary Pittman in 1962 (Taulavuori et al. 2017).
LEDs have many advantageous properties that mark their use in postharvest storage of fruits and vegetables (Bantis et al. 2018; Branas et al. 2013; Lee et al. 2014a, 2014b ). LEDs have the potential to regulate senescence, lead to ripening, and improve fruit quality and nutritional attributes in fruits and vegetables (Dutta Gupta 2017; Bantis et al. 2018). The potential of LEDs to extend the storage life of horticultural crops is advancing. Other advantageous properties of LEDs include their monochromatic nature, long life, prevention of thermal degradation, and high photon efficiency. All these favorable characteristics make LED application beneficial for extended the storage life of fruits and vegetables (Krames et al. 2007; Yu et al. 2017). LEDs do not involve the use of heavy metals that are toxic and operate at a low temperature and low direct current (DC) voltages (Dutta Gupta et al. 2019; D’Souza et al. 2015; Yang et al. 2018). Furthermore, the monochromatic nature of LEDs allows for the selection of the wavelength-specific emission light spectra desired for the production, preservation, and storage of fresh horticultural produce (Krames et al. 2007; Yu et al. 2017). The application of LEDs in postharvest has expanded due to its numerous advantages over conventional light sources. LEDs have more efficient energy utilization and a longer lifespan (approximately 5 × 104 versus 7–15 × 103 h) compared with traditional light sources (Krames et al. 2007; Yu et al. 2017). Additionally, LED lights have some properties such as the capability to restrain spectral composition, adjustable sizes, durability, a longer operating time, and relatively cold emitting surfaces (Folta et al. 2005; Yu et al. 2020). LEDs facilitate nonthermal operations and therefore minimize thermal degradation that might have any detrimental influence on the quality of fresh produce. It is also mechanically robust, which prevents damage due to vibrations. LEDs do not present any mechanical damage as a result of transportation, thus enabling their use in cold storage (D’Souza et al. 2015).
Characteristics of LEDs (NBI)
LEDs emit low radiant heat and are highly effective at a low temperature. LEDs have long-term effects and can last for 50,000–100,000 h working against traditional lighting, which can last for 15000 h (Jatothu 2013; Zhmakin 2011). LEDs have a close-packed size that improves flexibility while designing lighting systems. LEDs can control ultraviolet (UV) and infrared (IR) LEDs, while both UV and IR LEDs cannot control high-pressure lamps (HPL) and fluorescent lights (Denbaars et al. 2013; Munthikote 2018). LED components can be assembled into a compact system of various shapes, but this cannot be achieved with HPL and fluorescent lights due to their bulkiness (Ibrahim et al. 2014; Munthikote 2018). LEDs have a color mixture of white of 100–150 lm/W, whereas high-pressure and fluorescent lamps have values of 45–80 lm/W, and 65–150 lm/W, respectively. Many properties of LEDs have facilitated their use over traditional light, such as sodium and xenon lamps (D’Souza et al. 2015), high-intensity discharge lamps (Dutta Gupta 2017). LEDs are energy efficient with high luminosity and high photon flux (Dutta Gupta 2017). They do not involve the use of heavy metals that are toxic in cases of leakage (D’Souza et al. 2015). Additionally, LEDs are safe to operate and have adjustable on/off properties.
Effects of LEDs (NBI) on Postharvest Physiochemical Characteristics of Fruits and Vegetables
During postharvest storage, the majority of fruits and vegetables undergo rapid age and deterioration in quality, such as a rise in respiration process, softening, tissue destruction, and lipid peroxidation, increases in water loss, and postharvest quality characteristics such as the visual scale, flavor, texture, aroma, and nutritional quality; therefore, the primary purpose of postharvest storage of horticultural crops is to postpone senescence and to maintain the trait of fruits and vegetables during storage (Hodges and Toivonen 2008; Martínez-Romero et al. 2007; Pinela and Ferreira 2017; Prasad and Chakravorty 2018; Sharma et al. 2017). LEDs have been proven to be an effective inhibitory treatment for postharvest senescence of fresh produce in recent years, by extending the shelf life and maintain the quality of fresh produce (Table 1) (Colquhoun et al. 2013; D’Souza et al. 2015; Dhakal and Baek 2014; Dutta Gupta 2017; Gong et al. 2015; Hodges and Toivonen 2008; Kader 2005; Lichtfouse 2015; Prasad and Chakravorty 2018; Xu et al. 2014a, 2014b). The most recent study mainly focused on the application of LEDs in horticulture, and some studies have explored the application of LEDs in the postharvest stage (Dutta Gupta 2017). The study of the effect of LEDs on postharvest quality is relatively recent but has resulted in important findings.
Color Development
The color of a fruit is an important attribute that defines fruit acceptability and quality. In most fruit such as blueberries, the presence of anthocyanins is responsible for their color. The color of the fruit is affected by multiple factors, such as temperature, pH, and fruit physiology (Casati et al. 2012). Similarly, light has a significant impact on the color indices of fruits and vegetables, which regulate their shelf life. The development of color in fruits after harvest is related to the accumulation of various pigments such as lycopene, chlorophyll, or anthocyanin (Büchert et al. 2011; Xu et al. 2014a, 2014b). The accumulation and production of pigments are affected mainly by light (Xu et al. 2014a, 2014b).
In brussels sprouts (Brassica oleracea gemmifera) treated with white and blue LEDs for 10 days of storage, LEDs affect the color indices of the vegetables (Hasperué et al. 2016a, 2016b). Similarly, lower L (where L* lightness-darkness color) values also reported in broccoli florets exposed to red LEDs (630 nm) at a fluence rate of 50 W m−2 for 5 days. The red light was able to inhibit the increase in L values, leading to the higher chlorophyll content of 59.1% compared with the control untreated samples; likewise, the a/b (where the positive a* represents the redness and the negative a* represents the greenness whereas the positive b* represents the yellowness and the negative b* represents the blueness) value of broccoli generally decreased during storage, regardless of treatment; however, it was higher in LED-treated broccoli after 3 days until the end of the experiment (Jin et al. 2015). It has been reported that blue LED treatment (85.72 W m−2) in green tomatoes fruit was able to prevent red color development as compared with red light (102.70 W m−2) and untreated samples after 7 days of storage. The L values were significantly lower for tomatoes treated with blue light with less reddish tomatoes visually. However, tomato treated with the red light turned red at room temperature for given intensity (Dhakal and Baek 2014). Also, when the treatment of strawberries was carried out with blue light at a fluence rate of 40 W m−2 for 12 days at 5 °C and evaluated for color changes, no significant differences were observed. Also, there was an increase in CRIG values during postharvest storage as compared with control samples (Xu et al. 2014a, 2014b). In another study conducted by Büchert et al. (2011), there was an increase in L value for broccoli when treated with white light using fluorescent tubes at a fluence rate of 12, 25, and 50 W m−2 at 22 °C. This increase explained that with an increase in the treatment period, fruit or vegetable heads towards the senescence stage. However, broccoli heads remained green under treatment with light than those kept in the dark, implying that treated samples showed a lower increase in L value as compared with untreated ones (Büchert et al. 2011).
The influence of light at different wavelengths (463, 520, and 630 nm) and fluence rates (20, 40, and 60 W m−2) at two different temperatures of 21 °C and 2 °C on blueberries showed variable responses (Zhou et al. 2014). At 21 °C, the lowest L value was observed for 20 and 40 W m−2, respectively, under blue light treatment for 15 days. In contrast, at 2 °C, red light resulted in the lowest L value under the same conditions. However, with an increased influence rate to (60 Wm−2), green light had the lowest L value for 15 days at 21 °C and 2 °C, followed by blue and red light. At higher fluence rates, blue and red (40 and 60 W m−2) light exhibited an increased L value compared with the control samples at 21 °C, which was inconsistent with the 2 °C (control samples) with the highest L values. Overall, enhanced L values were reported for red light treatment for all three fluence rates at 21 °C. However, a decrease in the L value was observed under blue light treatment followed by green light. At 2 °C, green light showed a decrease in L value with an increased influence rate (Zhou et al. 2014).
Total Soluble Solids
The content of total soluble solids in both climacteric and non-climacteric fruit determines the maturity index of fruits and vegetables. Increased levels of total soluble solids (TSS) in fruit indicate an acceleration of the ripening process, while a decrease in TSS content indicates slow ripening and enhanced shelf life of fruit (Gong et al. 2015). Similarly, the application of LED treatment in brussels sprouts (Brassica oleracea gemmifera) inhibited the increase in TSS within the first 5 days of storage; however, after 10 days of storage, the TSS content significantly decreased (Hasperué et al. 2016a, 2016b; Shi et al. 2014). Likewise, the application of blue, green, and red LEDs in blueberry fruit inhibited the maximum increase in TSS content during postharvest storage (Taulavuori et al. 2017). At 21 °C, red light showed a maximum increase in TSS for 5, 10, and 15 days, followed by blue and green light at a fluence rate of 20 Wm−2. Equivalently, for 40 and 60 W m−2, red light showed a maximum increase in TSS for 5 and 10 days. However, over 15 days, blue light had maximum TSS values. At 2 °C, blue light resulted in enhanced levels of TSS at 20, 40, and 60 W m−2 during the 15-day treatment period. However, the TSS content of blueberries should remain constant as an increase in TSS results in ripening and reduces the shelf life of fruit. Green LED light showed lower values for TSS compared with red and blue light and control samples over 15 days of treatment at 20, 40, and 60 Wm−2 at both 21 °C and 2 °C (Taulavuori et al. 2017). Also, after 4 days of LED treatment at 5 °C in strawberry fruit, the contents of vitamin C and sugar significantly increased during postharvest storage. However, the significant increase in TSS in strawberries could be associated with the solubilization of cell wall components of crops, such as polyuronides and hemicelluloses, rather than the conversion of starch to sugars, as there is very little accumulation of glycogen during fruit development (Kim et al. 2011). There was an increase in TSS levels in Chinese bayberries (Myrica rubra Sieb) under the treatment of blue (470 nm) LEDs at 40 Wm−2 for 8 days at 10 °C. There were enhanced levels of glucose, fructose, and sucrose as compared with untreated samples (Shi et al. 2016). In addition, the application of white and red:far-red LED treatment at a fluence rate of 476 W m−2 significantly increased the firmness parameters compared with other white LED light conditions. Furthermore, the highest R: FR ratio increased the TSS and TA of the tomato fruit compared with white LED light and control sample after 6 days of storage at 23 °C (Nájera et al. 2018)
Conversely, the TSS content significantly increased under blue light treatment. A similar trend of enhanced ethylene production was observed in postharvest peaches treated with blue (470 nm) LEDs for 15 days at 10 °C (Gong et al. 2015). However, maximum ethylene production was delayed from 6 to 9 days of storage compared with the untreated control samples. The TSS content significantly increased, and the acidity decreased after treatment, in addition to color development in peaches; blue LEDs helped to regulate the synthesis of pigments and internal ethylene in fruit, accelerating the ripening during a postharvest period (Gong et al. 2015).
Weight Loss
Weight loss is an important problem of quality deterioration in fruits and vegetables during postharvest storage (Zhan et al. 2012a, 2012b). The weight loss of vegetables was inhibited when treated with LED light, including brussels sprouts (Brassica oleracea gemmifera) and broccoli (Brassica oleracea) (Hasperué et al. 2016a, 2016b), also brussels sprouts treated with a white and blue light at a fluence rate of 20 W m−2 for 5 and 10 days at 22 °C. There was higher weight loss under light treatment as compared with control untreated samples. Also, the weight losses increased with an increase in the treatment period. These clearly explain the interaction of blue light with stomatal cells. Blue light affects the opening of stomata leading to higher respiration rates, which in return affects the overall metabolism of fruits and vegetables (Hasperué et al. 2016a, 2016b). In addition to respiration, the other process affected by light is transpiration that is also associated with stomatal opening regulated by light (Zhan et al. 2012a, 2012b). In another study conducted by Xu et al. (2014a, 2014b) found that levels of respiration rates were higher in strawberries treated with blue light after 4 days of storage at 5 °C. Treatment was carried out at a fluence rate of 40 W m−2 for 12 days.
Similarly, a higher increase in weight loss was observed in broccoli during storage in both treated and untreated samples. The level of increase in weight loss was significantly lower in the control group relative to treated broccoli on 4 and 5 days (Jin et al. 2015). Red and blue LED (456 nm) and (655 nm) treatment at a fluence rate of 500 W m−2 at 23 °C significantly increased the weight of lettuce first and then decreased after 10 days of storage (Shao et al. 2020).
Antioxidant Compounds
It is well known that plant senescence is mostly as a result of the production of reactive oxygen species which can provoke oxidative damage to lipids and protein of plant cells, thus controlling the production of ROS is a significant way to retard the senescence of fresh horticultural produce during postharvest storage (Skrovankova et al. 2015). The removal of ROS is classified into two categories: antioxidant substances (AS) and antioxidant enzymes (AE). AS comprising (carotenoids, polyphenols, vitamin E, and reduced glutathione), while AE mainly comprises (glutathione oxidase, catalase, ascorbate oxidase, superoxide dismutase, and glutathione oxidase) (Xu et al. 2014a, 2014b).
The antioxidant system of fruits and vegetables was significantly increased by LED treatment (Table 1). On the other hand, LED treatment increases the content of other antioxidants such as phenolic compounds (i.e., flavonols, anthocyanins, and ascorbic acid), carotenoids, and GSH, as shown in (Table 1). The increased content of antioxidant and DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical scavenging activity was observed in immature strawberries treated with blue light (470 nm) at 40 W m−2 for 12 days at 5 °C compared with untreated samples (Xu et al. 2014a, 2014b). In addition, red (660 nm) and blue (470 nm) light treatment at a fluence rate of 50 W m−2 at 20 °C decreased the contents of carotenoid in citrus fruit compared with the control, demonstrating a gradual increase towards the end of treatment (Ma et al. 2017). However, blue (470 nm) LEDs did not have any significant effect (Ma et al. 2014). Similar results have been reported for fresh-cut broccoli using green light treatment (Jin et al. 2015) and strawberries with a blue light at (40 W m−2) (Xu et al. 2014a, 2014b). Research showed that LED treatment increased the antioxidant activity of blueberries at 40 W m−2 at 21 °C and 2 °C (Dinardo et al. 2018). Similarly, the application of blue (470 nm) and red (660 nm) LED light in citrus fruits at 20 °C for 6 days at a fluence rateof 50 W m−2 both the light significantly increased the antioxidant present in citrus fruit such as ẞ-cryptoxanthin (Ma et al. 2012). Also, blue and UV-C LED light treatment at a fluence rate and dosage (0, 1.5 W m−2 and 0, 0.5 kJ m−2) increased the content of all bioactive compounds and antioxidant capacity in habanero pepper vegetables for 30 days at 4–5 °C during storage. Also, in some bioactive compounds such as chlorophylls and total carotenoids, the effect was appreciable only in the 1 day of storage (Pérez-Ambrocio et al. 2018). Additionally, LED treatment increased antioxidant characteristics of many leafy vegetables such as tomato (Solanum Lycopersicum), Chinese kale (Brassica sp.), Chinese cabbage (Brassica napus L.), and pea (Pisum sativum L.), during storage as a result of ROS-scavenging enzymes, DPPH free radicals, β-carotene, and glucosinolates (Hee-Sun Kook 2013; Johkan et al. 2010; Ki Lee et al. 2016; Wu et al. 2007). The increased activity of this antioxidant helps in curing many diseases such as cancer and ultimately promotes the functional properties of citrus fruit (Ma et al. 2012).
Effects of LEDs (NBI) on Secondary Metabolites in Postharvest Fruits and Vegetables
Lights of different wavelengths regulate key photochemical processes include photosynthesis and the production/assimilation of secondary metabolites in horticultural crops (Xu et al. 2014a, 2014b). The increase in gene expressions such as MdMYB10 and MdUFGT under the effect of light aids in the accumulation of compounds such as vitamin C, soluble sugars, anthocyanin, or organic acids. Additionally, enzymes such as phenylalanine ammonia-lyase are responsible for the production of secondary metabolites (Hasan et al. 2017a, 2017b). The effect of light on various photoreceptors or the production of secondary metabolites is essential for its application of crop productivity and quality (Dutta Gupta et al. 2017). Crops respond to a natural light source, the sun, to carry out the process of photosynthesis. Also, plants react to other variables such as the light intensity, duration, wavelength, surrounding temperature, humidity, and carbon dioxide, among others, which leads to the initiation of signals that affect photoreceptors. For example, the phytochrome photoreceptor is responsible for the absorption of far-red and red light (Briggs and Olney 2001; Bentsink and Koornneef 2008; Brazaityte et al. 2006; Casal 2013; Cookson and Granier 2006; Dutta Gupta 2017; Ouzounis et al. 2014; Samuoliene et al. 2013; Reinbothe and Reinbothe 1996; Park and Runkle 2017), whereas cryptochrome and phototropin are responsible for the absorption of blue light (Kong and Okajima 2016; Wang and Folta 2013). The responses affect processes such as ripening, senescence, nutritional quality, and overall fruits and vegetable quality (Solovchenko and Merzlyak 2008; Seigler 2000; Wang and Folta 2013). Also, light exposure affects the production of secondary metabolites and the accumulation of phytochemicals (Ki Lee et al. 2016). Light sensors such as phytochrome, cryptochrome, and phototropin perceive light at variable wavelengths and affect the metabolic pathways (Braidot et al. 2014; Jones 2018; Kong and Okajima 2016).
In fresh horticultural produce, secondary metabolites produced are different from primary metabolites such as carbohydrates and amino acids (Nhut et al. 2011). The role of LEDs in secondary metabolite induction in crops seems to be associated with the phenylalanine ammonia-lyase enzyme. The upregulation of PAL by blue light and red LEDs increases the production of secondary metabolites (Bian et al. 2014; Heo et al. 2012; Hasan et al. 2017a, 2017b). The major secondary metabolite is ginsenoside, which is generated via the isoprenoid pathway in ginseng. The effects of LEDs on secondary metabolites has not been well researched. However, studies have shown that blue LED treatment (450–470 nm) increases the concentration of flavonoids in Chinese foxglove (Rehmannia sp.) from 2–74% compared with the sample that is stored under dark conditions (Manivannan et al. 2015; Nhut et al. 2011).
Phenolic Compounds
Phenolic compounds are the main nutrient present in fruits and vegetables, which not only gives fruits and vegetables the function of promoting human health but also plays a vital role in plants themselves as secondary metabolites produced in response to biological and abiotic stresses. The significant phenolics found in fruits and vegetables are flavonoids (cyanidin, petunidin, delphinidin), flavonols (myricetin, quercetin, kaempferol, gallic acid), flavanols (catechins, epicatechin), and tannins (ellagitannins and phenolic acid) (Dutta Gupta et al. 2019; Skrovankova et al. 2015). Light stress promotes the biosynthesis of these compounds. As abiotic stress, LEDs induce the change of phenolic metabolism of postharvest fruits and vegetables (Table 2). For fruits and vegetables, this stimulatory influence was likely to increase the total phenolic content over a long time. LEDs as a source of light with high photosynthetically active radiation efficiency induces responses through photoreceptors (blue light) (Wang et al. 2009), as well as phytochrome (red light) (Zhan et al. 2012a, 2012b), which are involved in the accumulation of compounds such as flavonoids, quercetin glycosides, or kaempferol. So, blue light plays a significant role in activating a biosynthetic pathway to produce phenolic compounds in the fruit. It regulates and stimulates the enzymes such as phenylalanine ammonia-lyase (PAL) associated with the synthesis and accumulation of secondary metabolites (Wang et al. 2009). Red light helps in enhancing the photosynthetic ability and induces photophosphorylation for stomatal opening. It converts the phytochrome from an inactive state to active form and regulates the accumulation of phytochemicals such as tocopherols and terpenes (Zhan et al. 2012a, 2012b). Additionally, light affects the expression of genes encoding enzymes, including phenyl ammonia-lyase (PAL), chalcone synthase, and flavanone-3-hydroxylase, among others, involved in various processes (Dutta Gupta 2017).
In blueberries fruit, treatment with LEDs blue light (470 nm) significantly increases the total phenolic content at a fluence rate of 40 W m−2 after treatment (Xu et al. 2014a, 2014b). In addition, After 3 days of red (630 nm), blue (463 nm), and green treatment (520 nm) at a fluence rate of 20 W m−2 in the blueberry (Vaccinium) fruit, the content of non-flavonoids including ascorbic, chlorogenic, caffeic acid, and rutin in blueberries showed a maximum increasing trend overall. However, the content of phenolic compounds was significantly increased (Kim et al. 2011). Similar results have been reported for immature broccoli treated with blue (660 nm) LEDs, showing a positive effect on the phenolic compounds within 7 days of storage (Zhan et al. 2012a, 2012b). Some recent studies have shown that LED treatment increases the phenolic content in blueberries at 12 and 24 h (Patel 2014). Also, several studies have reported that the application of blue (450 nm, 440 nm, 450 nm, 470 nm, 456 nm, 405 nm, 465 nm, and 460 nm) LED light enhanced the phenolic content during storage of Tartary buckwheat sprouts (Fagopyrum sp.) (Thwe et al. 2014), grape (Vitis sp.) (González et al. 2015), citrus fruit (Citrus sp.) (Ballester and Lafuente 2017a), Chinese foxglove (Rehmannia sp.) (Ahn et al. 2015), cherry tomato (Solanum sp.) (Kim et al. 2014), kalanchoe pinata (Nascimento et al. 2013), strawberry fruit (Fragaria sp.) (Choi et al. 2013), and lettuce (Lactuca sp.) (Son and Oh 2013). The increased contents of phenolic, ascorbic acid, and acidity were observed in immature strawberry fruits (Fragaria sp.) treated with blue (λ = 470 nm) LED light at a fluence rate 40 W m−2 for 12 days at 5 °C, as compared with control samples; also, the sugar content was significantly decreased during the initial 6 days of treatment in both the control and treated samples, followed by an increase in the later stage of storage (Kim et al. 2011). After 2 days of LED treatment in strawberries, the content of total phenol showed an increasing trend overall compared with the untreated samples (Kim et al. 2011; Wang et al. 2009). Furthermore, the significant increase in the phenolic compound could be attributed to the substantial decrease in acidity and organic acids over time, which aid in the provision of carbon skeletons for the formation of phenolic compounds (Wang et al. 2009). Similarly, the application of blue (436 nm), green (524 nm), and red (665 nm) LEDs on cabbage Brassica oleracea L.) vegetable inhibited the increase of phenolic content, total chlorophyll, and vitamin C during postharvest storage (Lee et al. 2014a, 2014b). On the other hand, UV-LED treatment increases the production of flavonoids content (Yang et al. 2019).
Anthocyanin Content
Different fluence rates and wavelengths affect the anthocyanin content of fruits and vegetables during the postharvest period (Table 2). However, at the postharvest stage, minimal studies have examined the effect of light on anthocyanin content (Kim et al. 2011). LEDs modulate anthocyanin biosynthesis by controlling the expression of anthocyanin synthesis genes in some fruit such as Chinese bayberry fruit (Myrica rubra Sieb) and immature strawberry (Fragaria sp.) (Shi et al. 2014). In addition, the application of blue (470 nm), red (525 nm), and green (630 nm) LED treatment at a fluence rate of 20 and 60 W m−2 and 40 W m−2)increased the content of anthocyanin in blueberries fruits for 10 days at 2 °C and 21 °C (Shi et al. 2014). Similarly, the application of UV-B (310 nm), blue (450 nm), a combination of white, blue and green LED light (450, 473, and 532 respectively) at a fluence rate of 23, 0.046, 3.6, and 0.02 W m−2 increased the content of anthocyanin in sweet cherries fruit for 10 days at 1 ± 0.5 °C (cyaniding 3 Oglucoside, cyanidin 3-O-rutinoside) and significantly influenced the CIE color parameters. Furthermore, a combination of white-blue-green light provoked similar but less pronounced effects, while UV-B light was similar to the control untreated sample (Kokalj et al. 2019). Also, some studies have reported for red-leaf cabbage (Brassica oleracea L.), buckwheat sprouts (Fagopyrum sp.), red leaf lettuce (Lactuca sp.), and Chinese kale sprouts (Brassica sp.) with red, blue light, and far-red light LEDs maintaining the green color during the postharvest period (Seo et al. 2015; Shi et al. 2014). In the strawberry fruit, anthocyanin content increased during LED treatment compared with the control samples (Xu et al. 2014a, 2014b). Similar results were also reported for Chinese bayberries (Myrica rubra Sieb) at 40 W m−2 for 8 days, where blue light treatment significantly increased the anthocyanin content (Shi et al. 2014). Conversely, blue LED treatment enhanced the anthocyanin content of strawberry fruit both at the ripening stage and during postharvest storage (Bantis et al. 2016; Kadomura-Ishikawa et al. 2013; Xu et al. 2014a, 2014b). In the previous study, treatment with blue light LEDs shown to induce anthocyanin accumulation and total flavonoid content in Chinese herbal medicine during storage (Ren et al. 2014) and Chinese kale fruit (Brassica sp.) (Qian et al. 2016). Nevertheless, it has been reported that LED treatment has a positive influence on the anthocyanin accumulation in grapes (Vitis sp.) and leaf baby lettuce (Lactuca sativa) (Kondo et al. 2014; Samuoliene et al. 2012).
The effect of different LED treatments blue, red, and green (385 nm, 470 nm, 525 nm, and 630 nm) in immature strawberries (Fragaria ananassa) at 5 °C for 4 days was reported compared with control samples (Kim et al. 2011). The anthocyanin content showed a significant increase by 21% in the treated strawberries during storage compared with the untreated controls, as a result of variable light wavelengths, which resulted in an increased of anthocyanin via the expression and enzymatic activities of genes such as chalcone isomerase (FaCHI), flavanone 3-hydroxylase (FaF3H), and anthocyanidin synthase (FaANS) (Kim et al. 2011). Additionally, when photoreceptors respond to light signals, they coordinate anthocyanin biosynthesis. compared with green and red LEDs, phototropin such as FaPHOT2 respond to blue LEDs mostly involved in inducing flavonoid pathway gene expression for the accumulation of anthocyanin and other flavonoids (Kadomura-Ishikawa et al. 2013). Similarly, the application of blue LED light at a fluence rate (40 W m−2) for 8 days on Chinese bayberries fruit (Myrica rubra) inhibited the increased of anthocyanin content by 1.8% compared with the untreated control samples (Gupta et al. 2017; Shi et al. 2014). Blue light induces the expression of genes involved in the synthesis of pigments such as anthocyanin, including MrMYB1, MrANS, MrF3H, and MrDFR1 (Gupta et al. 2017; Shi et al. 2014).
Influence of LEDs (NBI) on Nutritional Parameters in Postharvest Fruits and Vegetables
Light stimulates the making of several secondary metabolites, nutrients content as well as an antioxidant which supply (ROS) in the course of photosynthesis (Darko et al. 2014). LEDs intensify the redolent characteristics of fresh horticultural products (Colquhoun et al. 2013), also improve the nutritive value of horticultural crops at the postharvest level, and increase the accumulation of vitamin C, anthocyanin, and total phenolic compounds (Table 3) (Dutta Gupta et al. 2017a; D’Souza et al. 2015). Thus, the treatment of fruit with LEDs of different light (wavelength) helps to maintain the nutritional quality in the postharvest stage (Samuoliene et al. 2013). The effect of the different types of LEDs diverse for a variety of fruits and vegetables, affecting their nutritional value and thereby affecting their functional components and quality (Muneer et al. 2014). The use of LEDs has made the application of monochromatic light convenient with well-defined wavelengths and emission intensities. Thus, blue (400-470 nm) and red (600-650 nm) LEDs reported having a significant effect on nutritional value (Colquhoun et al. 2013; Holopainen et al. 2018; Taulavuori et al. 2017). For instance, blue light is associated with its effect on various metabolic pathways and accumulation of phenolic compounds, carotenoid, anthocyanin, ascorbic acid, and polyphenols (Taulavuori et al. 2017). In contrast, red light regulates the concentration of phytochemicals like terpenes, sesquiterpenes, and tocopherols in fruits and vegetables (Holopainen et al. 2018). The influence of different types of LEDs (wavelength) on fresh horticultural produce varies because of the impact generated by the lights on the specific receptors in fruits and vegetables (Samuoliene et al. 2013). Kitayama et al. (2019), studied the effect of LED light superiority, fluence rate, and photoperiod on the nutritional increase in many fruits and vegetables in controlled conditions. In general, studies have shown that LED treatment of different colors red (665 nm), blue (436 nm), green (524 nm), and white (375 nm) improve the nutritional quality and other bioactive compounds of some harvested fruits and vegetables during the postharvest period such as Chinese bayberry fruit (Myrica rubra Sieb), cabbage (Brassica oleracea L.), broccoli (Brassica oleracea), romaine lettuce (Lactuca sp.), spinach Spinacia oleracea), and pea seedling (Kanazawa et al. 2012; Lee et al. 2014a, 2014b; Lester et al. 2010; Zhan et al. 2012a). It also reported that during postharvest storage, chlorophyll, vitamin C, and total phenolic content were significantly increased by LED light (Zhan et al. 2012b). Consequently, the use of a particular wavelength supports the initiation of responses in fruits and vegetables, which may lead to the enrichment of functional compounds (Table 3). These findings demonstrated that the nutritional value of fruits and vegetables is enhanced by the application of LEDs of different colors/wavelengths during postharvest storage. However, ultraviolet (UV) LEDs are not part of the visible spectrum; they are suited for postharvest storage, improving nutritive value and retarding microbial growth in fruits and vegetables (Yang et al. 2019). The higher increase in quercetin-glycoside was observed in garden pea sprouts and watercress by UV-A LED (375 nm) at a fluence rate of 33 W m−2 for 5 days (Kanazawa et al. 2012). Also, proper UV LED exposure improved phytochemical traits of fruits and vegetables (Luksiene and Zukauskas 2009).
The precise biological processes of how nutrients improved through light are not fully understood. But current studies seem to move towards tracing the modifications in terms of biochemical response and comparing in nutrition with an expression of the gene via techniques like real-time- quantitative reverse transcription-polymerase chain reaction (qRT-PCR) investigation (Park et al. 2020). Consequently, the tractability in modification LEDs (optimum spectral composition) would be convenient in discovery the (OSP) of light for fresh horticultural crops, and well understanding of biological responses to various spectral composition (SP) of light. This will permit growers and vendors to exploit the light schemes that increase the nutritive trait of their fresh produce in the future.
Influence of LEDs (NBI) on the Ripening Process in Postharvest Fruits and Vegetables
Ripening is the most evident phenomenon defining the storage life of fresh produce during the postharvest period. Providing palatability in fruits and vegetables, a proper ripening stage is a critical step in the growth phase (Aked 2002). However, it causes changes in acidity, firmness, sweetness, color, aroma, and nutritional composition of fruits and vegetables, which can be both desirable (consumer acceptability) and undesirable (towards senescence). Factors such as the respiration rate, chlorophyll loss, degradation of complex molecules (organic acids, pectin, cellulose, etc.), and changes in biochemical and physiological properties of tissue cells affect this process (Lichtfouse 2015). Similarly, light is also an important parameter affecting the ripening of fruits and vegetables. Light accelerate or postpone the maturation of fruits and vegetables by controlling the amount of ethylene production. LEDs influence the rapid ripening of crops species as a result of respiration and ethylene output amplification (Xu et al. 2014a, 2014b). Throughout this process, ethylene production is triggered by various parameters, including LED light, which can affect the yang cycle of ethylene synthesis (Gong et al. 2015). In this metabolic pathway, receptors cause an increase or decrease in the expression of auxin-related or photosynthetic genes that interfere with the ripening process (Tadiello et al. 2016). In addition to ethylene production, pigment accumulation is another relevant regulatory event associated with fruit ripening. An increase in gene expression and corresponding mutations in fruit resulted in an intensification of carotenoid and flavonoid concentrations. LED light interacts with these gene modifications through light signal transduction and photo-oxidation processes, targeting the overall ripening phase of horticultural crops (Adams-Phillips et al. 2004). The influence of LED light depends on the type of fruits and vegetables, light intensity, wavelength, and treatment parameters. Many studies suggested the use of LED sources increased the ripening process of fruits and vegetable during storage (Table 4), which aid in enhancing the storage life and quality of fresh produce for consumers.
The effects of blue and red LED treatments (440–450 nm and 650–660 nm) on green tomatoes (Solanum Lycopersicum) at 85.72 and 102.70 μE m−2 s−1 for 7, 14, and 21 days, respectively, have been reported (Dhakal and Baek 2014). The blue light treatment was able to retard the red color in tomatoes compared with the control and the red light. After 14 days of treatment, the maturation of red color in green tomatoes treated with blue LEDs was reduced compared with the red light (Dhakal and Baek 2014). However, after 21 days of storage, almost the same observations were obtained irrespective of the type of treatment. Similar results have been obtained for lycopene, where blue LEDs was effective in delaying the development of pigment in tomatoes compared with those kept in the dark and those treated with red LEDs, demonstrating higher levels of lycopene (Dhakal and Baek 2014). This sequence of trials performed by (Dhakal and Baek 2014) confirmed that yellow color was more prominent in tomatoes treated with blue light.
Additionally, with fruit ripening, firmness tends to decrease, making fruit more palatable but reducing the storage life. In this research, firmness has been reduced significantly with the increase in storage time for all treatments. These findings showed that blue light was able to delay firmness compared with red light and control samples, helping to increase the shelf life of fruit during storage (Dhakal and Baek 2014). In another study conducted by Xu et al. (2014a, 2014b), blue LED light (470 nm) treatment was applied to immature strawberries (Fragaria ananassa) at 40 W m−2 for 12 days, which resulted in an increase in color development after 4 days of storage, implying that the ripening process accelerated in strawberries. The respiration rate was significantly decreased during the initial 2 days of storage, followed by a gradual increase in the control and blue light treatment groups when compared with the control untreated samples. Also, ethylene production and higher respiration process were significantly enhanced in the samples treated with blue light LEDs (Xu et al. 2014a, 2014b).
The effect of blue light (470 nm) on the ripening process of peaches (Prunus persica cv.) was evaluated by Gong et al. (2015), who observed that 15 days of treatment at 10 °C at a fluence rate of (40 W m−2) accelerated the ripening process during storage. Ethylene synthesis was significantly increased in fruit after illumination with blue light due to an increase in the activity of lipoxygenase, 1-aminocyclopropane-1-carboxylic acid, ethylene response factors, ethylene sensors and ethylene receptors (Gong et al. 2015). Similar results also reported for sweet oranges, in which blue (450 nm) LED treatment at a fluence rate between 60 and 630 (W m−2) at 20 °C led to an enhancement of ethylene production during postharvest storage (Ballester and Lafuente 2017). This result implies that blue light treatment accelerated the ripening process in citrus fruits (Citrus sp.) by increasing the synthesis of ethylene at higher intensities, as in the case of peaches (Gong et al. 2015) and strawberries (Xu et al. 2014a, 2014b). Blue LED treatment enhances the ripening of fruit during storage in conjunction with the initiation of synthesis of gama-tocopherol, alper-tocopherol, lutein, and beta-carotene compared with the control untreated samples (Xu et al. 2014a, 2014b). Also, blue LED treatment stimulates the expression of genes, like PpACS3 and PpACO1, which indicates the molecular mechanism of LED-mediation fruit ripening (Gong et al. 2015). Overall, the studies suggest that the application of LEDs at specific wavelengths and fluence rates is an effective approach for regulating the processes of ripening and is also used to accelerate or retard the ripening process during the postharvest stage. In conclusion, the influence of other LEDs of different wavelengths and fluence rates should be studied on other climacteric fruits and equated the impact of secondary ripening in fruit presented in the literature.
Influence of LEDs (NBI) on Delaying Senescence in Postharvest Fruits and Vegetables
Senescence is an undesirable phenomenon in some horticultural crops, instigating deterioration of fresh produces, thus results in a loss of nutritional and commercial value (Pogson and Morris 2003). The mechanism of the senescence process involves the allocation of nutrients from one part of the tissue to another and modification in both the physical characteristics and biochemical attributes of the cell structure (Aked 2002; Buchanan-Wollaston 2007). Moreover, oxidative metabolism results in the production of (ROS) in fruits and vegetables. Overproduction and accumulation of ROS facilitate the degradation of membrane lipids, proteins, macromolecules, and enzymatic changes, causing cell death (Drobot et al. 2013). Additionally, structural changes, chlorophyll degradation, protein and macromolecule breakdown, and gene expression in horticultural crops cells are the factors responsible for the trait, texture, and shelf life deterioration of fruit. Simultaneously, these lead to the death of cells (Aked 2002; Buchanan-Wollaston 2007). Cytokinins, gibberellins, and some auxins slow down senescence while other phytohormones such as ethylene, abscisic acid, and jasmonates increase senescence during postharvest storage of fruits and vegetables (Pogson and Morris 2003).
LEDs have significant utility in delay senescence as the optimum amount of light of a specific wavelength and frequency affects stress conditions in fruits and vegetables (Noodén and Schneider 2003). The point at which the light required for the pace of photosynthesis and respiration becomes adequate is called the light compensation point, which defines this optimum level of light intensity. Senescence occurs below or above this illumination level of brightness (D’Souza et al. 2015). Mostly, blue LEDs (460 nm) and white LED (420–700) lights are known to control the senescence by lowering the pigment degradation and affecting the transpiration and respiration processes (Hasperué et al. 2016a, 2016b). Blue LED light reduces the moisture content of fresh produce during postharvest storage of crops, while the red LED light supports the moisture of tissues of horticultural crops (Dutta Gupta 2017; Muneer et al. 2014; Massa et al. 2010). Both blue and red LEDs delay the senescence of vegetables and fruit by decreasing the production of ethylene and ascorbates by regulating other hormones (Ma et al. 2014; Xu et al. 2014a, 2014b). Light intensities below the light compensation point resulted in a net loss of sugar in fruits and vegetables, but not under all circumstances (Eldeen Nour Eldaime Elgimabi 2017). The study showed that irradiated horticultural crops with a light intensity below light compensation resulted in better storage conditions than those in the dark. In another study, a light intensity below the light compensation point below (30 W m−2 ) was irradiated on Chinese kale, basal leave, and fresh-packaged spinach leaves recorded higher nutrients and bioactive compounds in the light than in the dark (Costa et al. 2013; Lester et al. 2010).
Light quality increases the production of nutrients and delay senescence during the storage of fruits and vegetables (Braidot et al. 2014). Low intensity has been reported to improve the postharvest attribute of fruits and prevent senescence during storage than those stored in the darkness (Braidot et al. 2014). Senescence may vary in criteria with different vegetables, and loss of chlorophyll and wilting usually indicates senescence in leafy vegetables. Also, in broccoli and Mature kale, treatment with blue LEDs (21.8 W m−2) within 1 to 2 days at 20 °C reduces the senescence as compared with those in the dark (control) (Zhan et al. 2012a). Furthermore, several studies have reported that the application of LEDs postpone senescence and prevent yellowing in some fruits and vegetables including cabbage (Brassica oleracea L.), mandarin fruit (Citrus reticulate), spinach leaves (Spinacia oleracea), Chinese kale (Brassica sp.) and kiwifruit (Actinidia deliciosa) (Liu et al. 2015; Lester et al. 2010; Zhan et al. 2012a, 2012b). Many studies have been performed in this area to examine the effect of light (variable wavelengths) from LED systems on the rate of senescence and, ultimately, control the quality attributes of fresh horticultural produce (Table 5).
In Brussels sprouts, (Brassica oleracea gemmifera), treatment with white and blue LEDs (420–700 nm and 460 nm) significantly increase the contents of chlorophyll and total soluble sugars and weight loss compared with untreated samples of outer leaves after 10 days of treatment (Hasperué et al. 2016a, 2016b). Moreover, Newman and Sherman (1978) reported that illumination with blue light causes maximum absorption of chlorophyll b as light signals are gathered by phototropin, namely phot-1 and phot-2, creating the stimulus. Likewise, the total soluble sugar content was significantly higher in treated internal and external foliage compared with untreated samples. Also, a decrease in sugar content can be due to starvation in leafy vegetables, implying that high sugar content enhances green vegetables (Hasperué et al. 2016a, 2016b). The effect of white and blue LEDs on broccoli (Brassica oleracea L.) was studied under the same treatment conditions mentioned above at (20 W m−2) LED treatment showed the smallest quantity of yellowing. At the same time, chlorophyll a/b and carotenoid contents significantly increased in the treated sample and maintained until the end of the storage period, facilitating the preservation of the green color of broccoli (Hasperué et al. 2016a, 2016b). Also, withholding of sucrose, fructose, and glucose was experimental during storage treatment. This phenomenon can be attributed to the ability of blue light to maximize the absorption of lutein and beta-carotene.
Additionally, white and blue LEDs (420–700 nm and 460 nm) also enhanced the expression of genes such as phytoene synthase (PSY), lycopene beta-cyclase (LCYb), and zeaxanthin epoxidase in broccoli (Dean Kopsell and Carl Sams 2013). Moreover, the sucrose content was significantly increased after 35 days in both 5 °C and 22 °C storage, which means that white-blue LEDs were able to regulate the metabolic process. The higher sucrose was detected in the 5 °C storage treatment compared with the elevated temperature of 22 °C because of the lower temperature and lower rate of metabolic activities. All eminence pointers of senescence were better for broccoli irradiated by LEDs compared with the one stored under darkness even up to 42 days when stored at 5 °C. In general, low fluence rates can prevent senescence from continuing, and also can keep the sample as fresh as possible in good commercial value. The study showed that white-blue light treatment of broccoli was effective in delaying yellowing and regulating senescence during postharvest storage (Hasperué et al. 2016a, 2016b).
The effect of red (λ = 660 nm) and blue LED (λ = 470 nm) lights on broccoli (Brassica oleracea) have been studied over 4 days of treatment at 20 °C. The samples were compared with those treated with white light (control) to study the effect of different colors. After treatment, the red light was effective in controlling the yellowing of leaves, but the blue light showed no impact on preserving the green color (Ma et al. 2012). The effect of light varies with the absorption effect on chlorophyll, as red and blue light promotes the absorption of chlorophyll a and b, respectively contributing to the accumulation of chlorophyll levels and optimal photosynthetic efficiency (Dean Kopsell and Carl Sams 2013). Also, the effect of red light was more prominent in decreasing ethylene production and increasing in ascorbic acid content compared with the blue LEDs and control samples, demonstrating that as ethylene reduced, the respiration rate also decreased (Ma et al. 2014). Similarly, a rise in ethylene production and respiration rate was reported in strawberries by Xu et al. (2014a, 2014b). The fruit was illuminated with blue (470 nm) LED light at 5 °C for 12 days and compared with strawberries kept in the dark. A decrease in ethylene production was observed upon an initial treatment, followed by a significant increase from 2 to 6 days of storage.
In lettuce (Lactuca sativa), treated with blue (λ = 450 nm), green (λ = 516 nm), white (λ = 400-700 nm), and red (λ = 630 nm) LEDs, the red and white light was more effective than the other two colors in preserving the greenness of lettuce leaves (Kasim and Kasim 2017). This finding showed that red light significantly affected the associated receptors. Since red light is part of white light, the effect of preservation for red and white color was observed during the postharvest period. All the treated samples showed reduced yellowing during postharvest storage. After treatment, the content of chlorophyll a and b showed an increasing trend overall beyond the initial ratio and slowly decreased in pheophytin, hence suggesting a postponed in senescence. However, the degradation of pigment was less when treated with white and red LEDs, lights compared with green, blue LEDs, and control samples. After the experiment, the content of glucose was significantly enhanced during storage with a maximum effect of white LEDs, followed by blue and green LEDs, light (Kasim and Kasim 2017). These studies showed that LEDs, light colors were effective in enhancing the shelf life of lettuce leaves and delaying the senescence compared with control samples during the storage period. Similar results were reported in lettuce at 1.4 W m−2 for 8 days, where white LED significantly increased chl a and b ratio above the initial rate and pulsed lighting using 16 cycles of 30 min pulses where reduced the degradation of chl a and b resulted in a slower decline of carotenoids content in lettuce, thereby delaying the loss of postharvest nutritional quality and postponed senescence (Braidot et al. 2014). Also, the application of red LEDs, at a wavelength of 660 nm on lettuce, delayed yellowing during postharvest storage (Holopainen et al. 2018), while the yellow LEDs, suppressed the growth of lettuce, suggesting that the yellow LEDs, decreased photosynthesis (Dean Kopsell and Carl Sams 2013; Ma et al. 2012).
Influence of LEDs (NBI) on Pathogenic Microorganisms in Postharvest Fruits and Vegetables
The diseases caused by fungi during the storage period of fresh horticultural produce will cause a lot of economic losses, which has been the focus of attention among researchers. Currently, chemical synthesizes fungicides are still the best ones, but the application is limited to its safety (Assefa 2019; Xu et al. 2019). LEDs are among the several techniques developed and introduced to reduce pathogenic microorganisms in fruits and vegetables. Upon illumination with LEDs, photosensitizers or photoreceptors absorbs light to form reactive oxygen species that react with the biomolecules to trigger the effect. These reactive oxygen species interfere with the microbial cell structure, causing breakdown and preventing spoilage (Luksiene and Zukauskas 2009). Moreover, the application of LEDs triggers defense-related gene expression or the accumulation of defense hormones in fruits and vegetables, providing resistance to microbial growth and damage. However, different LED wavelengths initiated different molecular responses in microbial cells (Hasan et al. 2017a, 2017b).
As a residue-free physical sterilization technique, LEDs have been related to the postharvest anti-fungal effects of fruits and vegetables (Table 6) (D’Souza et al. 2015). All along, LEDs have always been exhibited an improved impact on postharvest fungal infections of fruits and vegetables during postharvest storage (Liao et al. 2013). Liu et al. (2011) reported that LED treatment inhibits fungal spoilage conidial germination and sporulation of Colletotrichum on mango fruit. Research showed that blue LEDs at a fluence rate of 40 W m−2 decrease rotten areas, mycelial growth, and the formation of various fungi including Penicillium digitatum, Penicillium italicum, and Phomopsis citri, when compared with white LED and dark control (Alferez et al. 2012; Liao et al. 2013). LED treatment with blue (450 nm), red (630 nm), white (400–700 nm), and green (516 nm) light at a fluence rate of 10 W m−2 significantly inhibit the decay rate of lettuce during storage (Kasim and Kasim 2017). Likewise, LEDs of blue (410–540 nm), green (47–620 nm), and red (580–670 nm) light at a fluence rate of 40 W m−2 for 1 to 2 days inhibit fungal pathogenic microorganisms, including Penicillium citri, Penicillium italicum, and Penicillium digitatum in Fallglo, sweet orange (Citrus sp.), tomato (Solanum Lycopersicum), and tangerine fruits (Citrus reticulate L.) during postharvest storage (Alferez et al. 2012; Ghate et al. 2013; Imada et al. 2014; Liao et al. 2013). Alferez et al. (2012) reported that the application of blue LEDs during postharvest storage of fruits and vegetables minimize postharvest losses by reducing fungal infections and fruit decay. Also, another study showed that blue LEDs at a fluence rate (40 W m−2) in tangerine fruit (Citrus reticulate L.) reduced fungal infection against Penicillium digitatum (Alferez et al. 2012). Blue LED treatment of citrus fruits (Citrus sp.) for 18 h at a fluence rate of 120 W m−2 and 700 W m−2 controlled fungal infections by Penicillium digitatum and Penicillium italicum, thereby inhibiting the germination of spores during storage (Lafuente and Alférez 2015). The study has shown that LED treatment at 461 nm (blue), 521 nm (green), and 642 nm (red) of fruits and vegetables enhanced the antibacterial activity against Staphylococcus aureus, Salmonella typhimurium, and Escherichia coli during storage (Ghate et al. 2013; Koutchma and Popović 2019). Additionally, blue LED treatment (405 nm) at a fluence rate of 50 W m−2 for 15 days inhibited bacteria (Botrytis cinerea) growth on the leaves of tomato fruit during postharvest storage (Imada et al. 2014).
Trends and Future Perspectives of LED Use During Postharvest Storage
A growing interest in research related to postharvest fruits and vegetables exposed to LEDs has occurred from 2000 to 2019. A review of Science direct databases showed increased use of LEDs in the postharvest preservation of fresh produce (Fig. 2), implying that LEDs are serviceable in the postharvest storage of horticultural crops. The use of nonthermal techniques has been studied to avoid the use of chemicals. The application of LEDs in the postharvest stage has dramatically increased due to its advantages over other types of conventional light (Taulavuori et al. 2017).
The application of LED technology is widely gaining interest due to its enhanced properties over conventional light (Gatamaneni et al. 2020). Moreover, the effects of LED technology on processes such as senescence, ripening, nutritional aspects, improved shelf life, and pathogenic microbial prevention have enormous potential during the postharvest period. Consequently, the approach of the LED system for postharvest activities is in a phase of continuous growth (Abewoy 2018). However, most works in this field are lab-based and need to be applied in the actual food supply chain. Such studies are important to harness the potential of the technology for extended shelf life, provision of quality and safe, fresh produce over long-term storage, as well as long-distance transportation (Taulavuori et al. 2017).
Additionally, with advancements and further studies, the application of LED technology could be a promising approach in developing countries where critical issues are involved in the delivery of quality and safe, fresh produce to consumers. In developed countries, this technology can aid in ensuring wholesomeness and reducing fruit and vegetable losses. This technology can be implemented in retail locations and linked with other parameters, such as temperature or packaging, to increase efficiency (Dueck et al. 2016). Ultimately, further development and scaling up of the LED system to the food industry level swill be of great benefit and importance for postharvest management. Another primary concern for the application of LEDs for postharvest management is the risk assessment of the technology. Studies have revealed that LEDs stimulate the accumulation of phytochemicals in vegetables and fruits, which can be both harmful and beneficial. The production of nitrate, a toxic substance, is primarily regulated by light quality, especially during artificial lighting in greenhouses (Bian et al. 2014). The effect of light on consumer health and eyes while installing in-store shelves remains to be explored. However, some studies have revealed an influence of blue light (450–475 nm) on the retina and toxicity associated with its use (Bian et al. 2014). Similarly, the effect of light at other wavelengths also needs to be studied to examine the influence on consumer health. Further studies are required in this field to address the emerging concept of applying LEDs in postharvest handling of horticultural crops.
Conclusion
Growers and vendors currently face challenges in maintaining the quality of fresh produce after harvest. Possible ways to circumvent these challenges are to increase the production rate of fruits and vegetables and prevent waste to ensure the provision of quality and safe, fresh produce to consumers. LEDs play an essential role in agriculture and also crucial in the postharvest stage to fulfill the above requirements. The distinctive properties of LEDs make them an outstanding choice. Lights of different wavelengths regulate key photochemical processes in fruits and vegetables, such as photosynthesis and secondary metabolite yields. Similarly, the use of LEDs regulates senescence, controls ripening rates, affect the nutritional quality, and reduce pathogenic microbial spoilage in fresh produce during the postharvest period. The application of LEDs holds the potential for life-enhancing storage and handling during postharvest activities, also can assist growers and vendors in reducing waste as well as aid in the provision of long-term storage and transportation.
References
Abewoy, D. (2018). Review on Impacts of climate change on vegetable production and its management practices. Advances in Crop Science and Technology, 06(01), 1–7. https://doi.org/10.4172/2329-8863.1000330.
Adams-Phillips, L., Barry, C., & Giovannoni, J. (2004). Signal transduction systems regulating fruit ripening. Trends in Plant Science, 9(7), 331–338. https://doi.org/10.1016/j.tplants.2004.05.004.
Aghdam, M. S., Jannatizadeh, A., Luo, Z., & Paliyath, G. (2018). Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends in Food Science and Technology, 76(September 2017), 67–81. https://doi.org/10.1016/j.tifs.2018.04.003.
Ahn, S. Y., Kim, S. A., Choi, S. J., & Yun, H. K. (2015). Comparison of accumulation of stilbene compounds and stilbene related gene expression in two grape berries irradiated with different light sources. Horticulture, Environment and Biotechnology, 56(1), 36–43. https://doi.org/10.1007/s13580-015-0045-x.
Aked, J. (2002). Maintaining the post-harvest quality of fruits and vegetables. In Fruit and Vegetable Processing. https://doi.org/10.1533/9781855736641.2.119
Alferez, F., Liao, H., & Burns, J. K. (2012). Postharvest Biology and Technology Blue light alters infection by Penicillium digitatum in tangerines. Postharvest Biology and Technology, 63(1), 11–15. https://doi.org/10.1016/j.postharvbio.2011.08.001.
Amornputti, S., Ketsa, S., & van Doorn, W. G. (2014). Effect of 1-methylcyclopropene (1-MCP) on storage life of durian fruit. Postharvest Biology and Technology, 97, 111–114. https://doi.org/10.1016/j.postharvbio.2014.06.011.
Assefa, A. (2019). Review on effect of light on disease development and management of horticultural crops under protected cultivations. International Journal of Forestry and Horticulture, 5(3), 5–18. https://doi.org/10.20431/2454-9487.0503002.
Ballester, A. R., & Lafuente, M. T. (2017). LED blue light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chemistry, 218, 575–583. https://doi.org/10.1016/j.foodchem.2016.09.089.
Bantis, F., Ouzounis, T., & Radoglou, K. (2016). Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Scientia Horticulturae, 198, 277–283. https://doi.org/10.1016/j.scienta.2015.11.014.
Bantis, F., Smirnakou, S., Ouzounis, T., Koukounaras, A., Ntagkas, N., & Radoglou, K. (2018). Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Scientia Horticulturae, 235(January), 437–451. https://doi.org/10.1016/j.scienta.2018.02.058.
Bentsink, L., & Koornneef, M. (2008). Seed Dormancy and Germination. The Arabidopsis Book, 6, e0119. https://doi.org/10.1199/tab.0119.
Bárcena, A., Gustavo Martínez, G., & Costa, L. (2019). Low intensity light treatment improves purple kale (Brassica oleracea var. sabellica) postharvest preservation at room temperature. Heliyon, 5, 9. https://doi.org/10.1016/j.heliyon.2019.e02467.
Bian, Z. H., Yang, C., & Ke, W. (2014). Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments : a review. Journal of the Scinence of Food and Agriculture, (June). https://doi.org/10.1002/jsfa.6789
Braidot, E., Petrussa, E., Peresson, C., Patui, S., Bertolini, A., Tubaro, F., Wählby, U., Coan, M., Vianello, A., & Zancani, M. (2014). Low-intensity light cycles improve the quality of lamb’s lettuce (Valerianella olitoria [L.] Pollich) during storage at low temperature. Postharvest Biology and Technology, 90, 15–23. https://doi.org/10.1016/j.postharvbio.2013.12.003.
Branas, C., Azcondo, F. J., & Alonso, J. M. (2013). Solid-state lighting: A system review. IEEE Industrial Electronics Magazine, 7(4), 6–14. https://doi.org/10.1109/MIE.2013.2280038.
Brazaityte, A., Ulinskaite, R., Duchovskis, P., Samuoliene, G., Šikšnianiene, J. B., Jankauskiene, J., et al. (2006). Optimization of lighting spectrum for photosynthetic system and productivity of lettuce by using light-emitting diodes. Acta Horticulturae, 711, 183–188. https://doi.org/10.17660/ActaHortic.2006.711.22.
Briggs, W. R., & Olney, M. A. (2001). Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiology, 125(1), 85–88. https://doi.org/10.1104/pp.125.1.85.
Buchanan-Wollaston, V. (2007). Senescence processes in plants. Annual Plant Reviews, Volume 26. Annals of Botany, 101(1), 197–197. https://doi.org/10.1093/aob/mcm286.
Büchert, A. M., Gómez Lobato, M. E., Villarreal, N. M., Civello, P. M., & Martínez, G. A. (2011). Effect of visible light treatments on postharvest senescence of broccoli (Brassica oleracea L.). Journal of the Science of Food and Agriculture, 91(2), 355–361. https://doi.org/10.1002/jsfa.4193.
Capone, R., El Bilali, H., Debs, P., Cardone, G., & Driouech, N. (2014). Food System Sustainability and Food Security: Connecting the Dots. Journal of Food Security, 2(1), 13–22. https://doi.org/10.12691/jfs-2-1-2
Casal, J. J. (2013). Photoreceptor Signaling Networks in Plant Responses to Shade. Annual Review of Plant Biology, 64(1), 403–427. https://doi.org/10.1146/annurev-arplant-050312-120221.
Casati, C. B., Sánchez, V., Baeza, R., Magnani, N., Evelson, P., & Zamora, M. C. (2012). Relationships between colour parameters, phenolic content and sensory changes of processed blueberry, elderberry and blackcurrant commercial juices. International Journal of Food Science and Technology, 47(8), 1728–1736. https://doi.org/10.1111/j.1365-2621.2012.03027.x.
Castillo, S., Navarro, D., Zapata, P. J., Guillén, F., & Valero, D. (2010). Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biology and Technology, 57(3), 183–188. https://doi.org/10.1016/j.postharvbio.2010.04.006.
Castro, L. R., Vigneault, C., & Cortez, L. a. B. (2004). Container opening design for horticultural produce cooling efficiency. Food, Agriculture & Environment, 2(1), 135–140.
Charles, F., Nilprapruck, P., Roux, D., & Sallanon, H. (2018). Visible light as a new tool to maintain fresh-cut lettuce post-harvest quality. Postharvest Biology and Technology, 135, 51–56. https://doi.org/10.1016/j.postharvbio.2017.08.024.
Chiabrando, V., & Giacalone, G. (2011). Shelf-life extension of highbush blueberry using 1-methylcyclopropene stored under air and controlled atmosphere. Food Chemistry, 126(4), 1812–1816. https://doi.org/10.1016/j.foodchem.2010.12.032.
Choi, H. G., Kwon, J. K., Moon, B. Y., Kang, N. J., Park, K. S., Cho, M. W., & Kim, Y. C. (2013). Effect of different Light Emitting Diode (LED) lights on the growth characteristics and the phytochemical production of strawberry fruits during cultivation. Korean Journal of Horticultural Science and Technology, 31(1), 56–64. https://doi.org/10.7235/hort.2013.12100.
Colquhoun, T. A., Schwieterman, M. L., Gilbert, J. L., Jaworski, E. A., Langer, K. M., Jones, C. R., Rushing, G. V., Hunter, T. M., Olmstead, J., Clark, D. G., & Folta, K. M. (2013). Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biology and Technology, 86, 37–44. https://doi.org/10.1016/j.postharvbio.2013.06.013.
Cookson, S. J., & Granier, C. (2006). A dynamic analysis of the shade-induced plasticity in Arabidopsis thaliana rosette leaf development reveals new components of the shade-adaptative response. Annals of Botany, 97(3), 443–452. https://doi.org/10.1093/aob/mcj047.
Costa, L., Millan Montano, Y., Carrión, C., Rolny, N., & Guiamet, J. J. (2013). Application of low intensity light pulses to delay postharvest senescence of Ocimum basilicum leaves. Postharvest Biology and Technology, 86, 181–191. https://doi.org/10.1016/j.postharvbio.2013.06.017.
D’Souza, C., Yuk, H. G., Khoo, G. H., & Zhou, W. (2015). Application of light-emitting diodes in food production, postharvest preservation, and microbiological food safety. Comprehensive Reviews in Food Science and Food Safety, 14(6), 719–740. https://doi.org/10.1111/1541-4337.12155.
Darko, E., Heydarizadeh, P., Schoefs, B., & Sabzalian, M. R. (2014). Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philosophical Transactions of the Royal Society, B: Biological Sciences, 369(1640), 20130243. https://doi.org/10.1098/rstb.2013.0243.
Dean Kopsell, A., & Carl Sams, E. (2013). Increases in Shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. American Society of Horticultural Science, 1(138), 31–37.
Denbaars, S. P., Feezell, D., Kelchner, K., Pimputkar, S., Pan, C. C., Yen, C. C., et al. (2013). Development of gallium-nitride-based light-emitting diodes (LEDs) and laser diodes for energy-efficient lighting and displays. Acta Materialia, 61(3), 945–951. https://doi.org/10.1016/j.actamat.2012.10.042.
Dhakal, R., & Baek, K. H. (2014). Metabolic alternation in the accumulation of free amino acids and γ-aminobutyric acid in postharvest mature green tomatoes following irradiation with blue light. Horticulture, Environment and Biotechnology, 55(1), 36–41. https://doi.org/10.1007/s13580-014-0125-3.
DiNardo, A., Subramanian, J., & Singh, A. (2018). Investigation of antioxidant content and capacity in yellow European plums. International Journal of Fruit Science, 18(1), 99–116. https://doi.org/10.1080/15538362.2017.1381873.
Dou, H., Niu, G., Gu, M., & Masabni, J. G. (2017). Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae, 3(2), 1–11. https://doi.org/10.3390/horticulturae3020036.
Drobot, L. B., Samoylenko, A. A., Vorotnikov, A. V., Tyurin-Kuzmin, P. A., Bazalii, A. V., Kietzmann, T., et al. (2013). Reactive oxygen species in signal transduction. Ukrain’skyi Biokhimichnyi Zhurnal, 85(6), 209–217. https://doi.org/10.15407/ubj85.06.209.
Dueck, T., van Ieperen, W., & Taulavuori, K. (2016). Light perception, signalling and plant responses to spectral quality and photoperiod in natural and horticultural environments. Environmental and Experimental Botany, 121, 1–3. https://doi.org/10.1016/j.envexpbot.2015.06.012.
Dutta Gupta, S. (2017). Light emitting diodes for agriculture: Smart lighting. Light Emitting Diodes for Agriculture: Smart Lighting, 1–334. https://doi.org/10.1007/978-981-10-5807-3
Dutta Gupta, S., Kumar, A., & Agarwal, A. (2019). Impact of light-emitting diodes (LEDs) on the growth and morphogenesis of encapsulated shoot buds of Curculigo orchioides Gaertn., an endangered medicinal herb. Acta Physiologiae Plantarum, 41(4). https://doi.org/10.1007/s11738-019-2840-y.
Eldeen Nour Eldaime Elgimabi, M. (2017). Effect of light irradiance on regulation of leaf senescence. Journal of Botanical Sciences, 6(4).
Erwin, J., & Gesick, E. (2017). Photosynthetic responses of swiss chard, kale, and spinach cultivars to irradiance and carbon dioxide concentration. HortScience, 52(5), 706–712. https://doi.org/10.21273/HORTSCI11799-17.
Favre, N., Barcena, A., Bahima, J. V., Martinez, G., & Costa, L. (2018). Pulses of low intensity light as promising technology to delay postharvest senescence of broccoli. Postharvest Biology and Technology, 142, 107–114. https://doi.org/10.1016/j.postharvbio.2017.11.006.
Folta, K. M., Koss, L. L., McMorrow, R., Kim, H. H., Kenitz, J. D., Wheeler, R., & Sager, J. C. (2005). Design and fabrication of adjustable red-green-blue LED light arrays for plant research. BMC Plant Biology, 5(1), 1–11. https://doi.org/10.1186/1471-2229-5-17.
Gatamaneni, B., Valerie, L., Mark, O., Bo, L., & Wu, S. (2020). A comprehensive study on the effect of light quality imparted by light - emitting diodes ( LEDs) on the physiological and biochemical properties of the microalgal consortia of Chlorella variabilis and Scenedesmus obliquus cultivated in dairy wastewater. Bioprocess and Biosystems Engineering, 1(123), 56–78. https://doi.org/10.1007/s00449-020-02338-0.
Ghate, V. S., Ng, K. S., Zhou, W., Yang, H., Khoo, G. H., Yoon, W. B., & Yuk, H. G. (2013). Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. International Journal of Food Microbiology, 166(3), 399–406. https://doi.org/10.1016/j.ijfoodmicro.2013.07.018.
Gong, D., Cao, S., Sheng, T., Shao, J., Song, C., & Wo, F. (2015). Effect of blue light on ethylene biosynthesis, signalling and fruit ripening in postharvest peaches. Scientia Horticulturae, 197, 657–664.
González, C. V., Fanzone, M. L., Cortés, L. E., Bottini, R., Lijavetzky, D. C., Ballaré, C. L., & Boccalandro, H. E. (2015). Fruit-localized photoreceptors increase phenolic compounds in berry skins of field-grown Vitis vinifera L. cv. Malbec. Phytochemistry, 110, 46–57. https://doi.org/10.1016/j.phytochem.2014.11.018.
Gupta, D. S., Agarwal, A., Ibaraki, Y., Pocock, T., van Iersel, M. W., Bugbee, B., … Wojciechowska, R. (2017). Light emitting diodes for health. https://doi.org/10.1007/978-981-10-5807-3
Hasan, M. M., Bashir, T., & Bae, H. (2017a). Use of utrasonication tchnology for the icreased poduction of pant scondary mtabolites. Molecules, 22(7). https://doi.org/10.3390/molecules22071046.
Hasan, M. M., Bashir, T., Ghosh, R., Lee, S. K., & Bae, H. (2017b). An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules, 22(9), 1–12. https://doi.org/10.3390/molecules22091420.
Hasperué, J. H., Guardianelli, L., Rodoni, L. M., Chaves, A. R., & Martínez, G. A. (2016a). Continuous white-blue LED light exposition delays postharvest senescence of broccoli. LWT - Food Science and Technology, 65, 495–502. https://doi.org/10.1016/j.lwt.2015.08.041.
Hasperué, J. H., Rodoni, L. M., Guardianelli, L. M., Chaves, A. R., & Martínez, G. A. (2016b). Use of LED light for Brussels sprouts postharvest conservation. Scientia Horticulturae, 213, 281–286. https://doi.org/10.1016/j.scienta.2016.11.004.
Hee-Sun Kook, K. K. (2013). The Effect of blue-light-emitting diodes on antioxidant properties and resistance to Botrytis cinerea in tomato. Journal of Plant Pathology & Microbiology, 04(09). https://doi.org/10.4172/2157-7471.1000203.
Heo, J. W., Kang, D. H., Bang, H. S., & Hong, S. G. (2012). Early Growth , pigmentation , protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean Journal of Horticultural Science & Technology, 30(1), 6–12.
Hodges, D. M., & Toivonen, P. M. A. (2008). Quality of fresh-cut fruits and vegetables as affected by exposure to abiotic stress. Postharvest Biology and Technology, 48(2), 155–162. https://doi.org/10.1016/j.postharvbio.2007.10.016.
Holopainen, J. K., Kivimäenpää, M., & Julkunen-Tiitto, R. (2018). New Light for Phytochemicals. Trends in Biotechnology, 36(1), 7–10. https://doi.org/10.1016/j.tibtech.2017.08.009.
Hung, C. D., Hong, C. H., Kim, S. K., Lee, K. H., Park, J. Y., Nam, M. W., et al. (2016). LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiologiae Plantarum, 38(6). https://doi.org/10.1007/s11738-016-2164-0.
Ibrahim, M. A. S., Macadam, J., Autin, O., & Jefferson, B. (2014). Evaluating the impact of LED bulb development on the economic viability of ultraviolet technology for disinfection. Environmental Technology (United Kingdom), 35(4), 400–406. https://doi.org/10.1080/09593330.2013.829858.
Imada, K., Tanaka, S., Ibaraki, Y., Yoshimura, K., & Ito, S. (2014). Antifungal effect of 405-nm light on Botrytis cinerea. Letters in Applied Microbiology, 59(6), 670–676. https://doi.org/10.1111/lam.12330.
Jatothu, S. D. G. B. (2013). Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. 211–220. https://doi.org/10.1007/s11816-013-0277-0
Jiang, A. L., Zuo, J. H., Zheng, Q. L., Guo, L., Gao, L. P., Zhao, S. G., Wang, Q., Hu, & W, Z. (2019). Red LED irradiation maintains the postharvest quality of broccoli by elevating antioxidant enzyme activity and reducing the expression of senescence-related genes. Scientia Horticulturae, 251, 73–79. https://doi.org/10.1016/j.scienta.2019.03.016.
Jin, P., Yao, D., Xu, F., Wang, H., & Zheng, Y. (2015). Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chemistry, 172, 705–709. https://doi.org/10.1016/j.foodchem.2014.09.134.
Johkan, M., Shoji, K., Goto, F., Hashida, S., & Yoshihara, T. (2010). Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortucultural Science, 45(12), 1809–1814.
Jones, M. A. (2018). Using light to improve commercial value. Horticulture Research, 5(1). https://doi.org/10.1038/s41438-018-0049-7.
Kader, A. A. (2005). Increasing Food availability by reducing postharvest losses of fresh produce (pp. 2169–2176). Davis: Department of Pomology, University of California Davis.
Kadomura-Ishikawa, Y., Miyawaki, K., Noji, S., & Takahashi, A. (2013). Phototropin 2 is involved in blue light-induced anthocyanin accumulation in Fragaria x ananassa fruits. Journal of Plant Research, 126(6), 847–857. https://doi.org/10.1007/s10265-013-0582-2.
Kanazawa, K., Hashimoto, T., Yoshida, S., Sungwon, P., & Fukuda, S. (2012). Short photoirradiation induces flavonoid synthesis and increases its production in postharvest vegetables. Journal of Agricultural and Food Chemistry, 60(17), 4359–4368. https://doi.org/10.1021/jf300107s.
Kasim, M. U., & Kasim, R. (2017). While continuous white LED lighting increases chlorophyll content (SPAD), green LED light reduces the infection rate of lettuce during storage and shelf-life conditions. Journal of Food Processing and Preservation, 41(6), 1–7. https://doi.org/10.1111/jfpp.13266.
Kerch, G. (2015). Chitosan films and coatings prevent losses of fresh fruit nutritional quality: a review. Trends in Food Science and Technology, 46(2), 159–166. https://doi.org/10.1016/j.tifs.2015.10.010.
Ki Lee, M., HaeByeon, D., Chung, S.-O., & UnPark, S. (2016). LED Lights enhance metabolites and antioxidants in Chinese cabbage and kale. Brazilian Archives of Biology and Technology. An I n t e r n a t i o n a l j o u r n a L, 59(December), 1–9. https://doi.org/10.1590/1678-4324-2016150546.
Kim, B. S., Lee, H. O., Kim, J. Y., Kwon, K. H., Cha, H. S., & Kim, J. H. (2011). An effect of light emitting diode (LED) irradiation treatment on the amplification of functional components of immature strawberry. Horticulture, Environment and Biotechnology, 52(1), 35–39. https://doi.org/10.1007/s13580-011-0189-2.
Kim, E. Y., Park, S. A., Park, B. J., Lee, Y., & Oh, M. M. (2014). Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Horticulture, Environment and Biotechnology, 55(6), 506–513. https://doi.org/10.1007/s13580-014-0121-7.
Kitayama, M., Nguyen, D. T. P., Lu, N., & Takagaki, M. (2019). Effect of light quality on physiological disorder, growth, and secondary metabolite content of water spinach (Ipomoea aquatica forsk) cultivated in a closed-type plant production system. Horticultural Science and Technology, 37(2), 206–218. https://doi.org/10.12972/kjhst.20190020.
Kokalj, D., Zlatić, E., Cigić, B., & Vidrih, R. (2019). Postharvest light-emitting diode irradiation of sweet cherries (Prunus avium L.) promotes accumulation of anthocyanins. Postharvest Biology and Technology, 148, 192–199. https://doi.org/10.1016/j.postharvbio.2018.11.011.
Kondo, S., Tomiyama, H., Rodyoung, A., Okawa, K., Ohara, H., Sugaya, S., Terahara, N., & Hirai, N. (2014). Abscisic acid metabolism and anthocyanin synthesis in grape skin are affected by light emitting diode (LED) irradiation at night. Journal of Plant Physiology, 171(10), 823–829. https://doi.org/10.1016/j.jplph.2014.01.001.
Kong, S. G., & Okajima, K. (2016). Diverse photoreceptors and light responses in plants. Journal of Plant Research, 129(2), 111–114. https://doi.org/10.1007/s10265-016-0792-5.
Koutchma, T., & Popović, V. (2019). UV light-emitting diodes (LEDs) and food safety. Ultraviolet LED Technology for Food Applications, 91–117. https://doi.org/10.1016/b978-0-12-817794-5.00005-4
Kozai, T. (2013). Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 89(10), 447–461. https://doi.org/10.2183/pjab.89.447.
Kozai, T., & Niu, G. (2015). Introduction. In Plant Factory: an indoor vertical farming system for efficient quality food production. https://doi.org/10.1016/B978-0-12-801775-3.00001-9
Krames, M. R., Shchekin, O. B., Mueller-Mach, R., Mueller, G. O., Zhou, L., Harbers, G., & Craford, M. G. (2007). Status and future of high-power light-emitting diodes for solid-state lighting. IEEE/OSA Journal of Display Technology, 3(2), 160–175. https://doi.org/10.1109/JDT.2007.895339.
Lafuente, M. T., & Alférez, F. (2015). Effect of LED blue light on Penicillium digitatum and Penicillium italicum strains. Photochemistry and Photobiology, 91(6), 1412–1421. https://doi.org/10.1111/php.12519.
Lai, M. L., Tay, T. Y., Sadhanala, A., Dutton, S. E., Li, G., Friend, R. H., et al. (2016). Tunable near-infrared luminescence in tin halide perovskite devices. Journal of Physical Chemistry Letters, 7(14), 2653–2658. https://doi.org/10.1021/acs.jpclett.6b01047.
Lee, S. W., Seo, J. M., Lee, M. K., Chun, J. H., Antonisamy, P., Arasu, M. V., Suzuki, T., al-Dhabi, N. A., & Kim, S. J. (2014a). Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Industrial Crops and Products, 54, 320–326. https://doi.org/10.1016/j.indcrop.2014.01.024.
Lee, Y. J., Ha, J. Y., Oh, J. E., & Cho, M. S. (2014b). The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Science and Biotechnology, 23(4), 1087–1093. https://doi.org/10.1007/s10068-014-0149-6.
Lester, G. E., Makus, D. J., & Mark Hodges, D. (2010). Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: Effects of cultivar, leaf size, and storage duration. Journal of Agricultural and Food Chemistry, 58(5), 2980–2987. https://doi.org/10.1021/jf903596v.
Liao, H., Alferez, F., & Burns, J. K. (2013). Postharvest biology and technology assessment of blue light treatments on citrus postharvest diseases. Postharvest Biology and Technology, 81, 81–88. https://doi.org/10.1016/j.postharvbio.2013.02.019.
Lichtfouse, E. (2015). Sustainable agriculture reviews (Vol. 15). Environmental Chemistry. Green Chemistry and Pollutants in Ecosystems Springer 2005.
Liu, Y., Tao, J., Yan, Y., Li, B., Li, H., & Li, C. (2011). Biocontrol efficiency of Bacillus subtilis SL-13 and characterization of an antifungal chitinase. Chinese Journal of Chemical Engineering, 19(1), 128–134. https://doi.org/10.1016/S1004-9541(09)60188-9.
Liu, J. D., Goodspeed, D., Sheng, Z., Li, B., Yang, Y., Kliebenstein, D. J., & Braam, J. (2015). Keeping the rhythm: Light/dark cycles during postharvest storage preserve the tissue integrity and nutritional content of leafy plants. BMC Plant Biology, 15(1), 1–9. https://doi.org/10.1186/s12870-015-0474-9.
Luksiene, Z., & Zukauskas, A. (2009). Prospects of photosensitization in control of pathogenic and harmful micro-organisms. Journal of Applied Microbiology, 107(5), 1415–1424. https://doi.org/10.1111/j.1365-2672.2009.04341.x.
Ma, G., Zhang, L., Kato, M., Yamawaki, K., Kiriiwa, Y., Yahata, M., Ikoma, Y., & Matsumoto, H. (2012). Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Journal of Agricultural and Food Chemistry, 60, 197–201. https://doi.org/10.1016/j.postharvbio.2014.08.002.
Ma, G., Zhang, L., Setiawan, C. K., Yamawaki, K., Asai, T., Nishikawa, F., Maezawa, S., Sato, H., Kanemitsu, N., & Kato, M. (2014). Effect of red and blue LED light irradiation on ascorbate content and expression of genes related to ascorbate metabolism in postharvest broccoli. Postharvest Biology and Technology, 94, 97–103. https://doi.org/10.1016/j.postharvbio.2014.03.010.
Ma, L., Zhang, M., Bhandari, B., & Gao, Z. (2017). Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends in Food Science and Technology, 64, 23–38. https://doi.org/10.1016/j.tifs.2017.03.005.
Manivannan, A., Soundararajan, P., Halimah, N., Ko, C. H., & Jeong, B. R. (2015). Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Horticulture, Environment and Biotechnology, 56(1), 105–113. https://doi.org/10.1007/s13580-015-0114-1.
Mari, M., Bautista-Baños, S., & Sivakumar, D. (2016). Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biology and Technology, 122, 70–81. https://doi.org/10.1016/j.postharvbio.2016.04.014.
Martínez-Romero, D., Bailén, G., Serrano, M., Guillén, F., Valverde, J. M., Zapata, P., Castillo, S., & Valero, D. (2007). Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: a review. Critical Reviews in Food Science and Nutrition, 47(6), 543–560. https://doi.org/10.1080/10408390600846390.
Massa, G. D., Drive, A. M., Lafayette, W., Kim, H., Wheeler, R. M., & Mitchell, C. A. (2010). Plant Productivity in Response to LED Lighting., 43(7), 1951–1956.
Miguel, J., Javier, P., Castillo, S., & Valero, D. (2008). The addition of essential oils to MAP as a tool to maintain the overall quality of fruits. Trends in Food Science & Technology, 19(9), 464–471. https://doi.org/10.1016/j.tifs.2008.01.013.
Mitchell, N., Eljach, C., Lodge, B., Sharp, J. L., Desjardins, J. D., & Kennedy, M. S. (2012) Single and reciprocal friction testing of micropatterned surfaces for orthopedic device design. Journal of the Mechanical Behavior of Biomedical Materials, 7, 106–115. https://doi.org/10.1016/j.jmbbm.2011.08.022.
Muneer, S., Kim, E. J., Park, J. S., & Lee, J. H. (2014). Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves ( Lactuca sativa L.). International Journal of Molecular Sciences, 15, 4657–4670. https://doi.org/10.3390/ijms15034657.
Munthikote, S. (2018). Review article role of light emitting diode in food industry. 1, 1–9.
Nájera, C., Guil-Guerrero, J. L., Enríquez, L. J., Álvaro, J. E., & Urrestarazu, M. (2018). LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biology and Technology, 145, 151–156. https://doi.org/10.1016/j.postharvbio.2018.07.008.
Nascimento, L. B. S., Leal-Costa, M. V., Coutinho, M. A. S., Moreira, N. D. S., Lage, C. L. S., Barbi, N. D. S., Costa, S. S., & Tavares, E. S. (2013). Increased antioxidant activity and changes in phenolic profile of kalanchoe pinnata (Lamarck) persoon (crassulaceae) specimens grown under supplemental blue light. Photochemistry and Photobiology, 89(2), 391–399. https://doi.org/10.1111/php.12006.
Naveenkumar, R., Muthukumar, A., & Mohanapriya, R. (2017). Occurrence , Virulence and Evaluation of Essential Oils against Fusarium oxysporum. Vegetos- An International Journal of Plant Research, 30(4), 6. https://doi.org/10.4172/2229-4473.1000303.
Newman, P. J., & Sherman, L. A. (1978). Isolation and characterization of Photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 503(2), 343–361. https://doi.org/10.1016/0005-2728(78)90193-7.
Nhut, D. T., Don, P. L. H. N. N. T., Hien, N. T. T., Huy, N. P., Nam, N. B., Vinh, B. T., & Luan, T. C. (2011). Effects of light quality on the growth and essential oil content in sweet basil. Acta Biologica Cracoviensia Series Botanica, 56(2), 91–94.
Noodén, L. D., & Schneider, M. J. (2003). Light Control of Senescence. Plant Cell Death Processes, 1, 375–383. https://doi.org/10.1016/B978-012520915-1/50029-1.
Ouzounis, T., Fretté, X., Ottosen, C., & Rosenqvist, E. (2014). Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘ Vivien ’ and ‘ Purple Star’. International Journal of Plnat Biology, 1. https://doi.org/10.1111/ppl.12300.
Panjai, L., Noga, G., Fiebig, A., & Hunsche, M. (2017). Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Scientia Horticulturae, 226, 97–103. https://doi.org/10.1016/j.scienta.2017.08.035.
Papoutsis, K., Mathioudakis, M. M., Hasperué, J. H., & Ziogas, V. (2019). Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends in Food Science & Technology, 86, 479–491. https://doi.org/10.1016/j.tifs.2019.02.053.
Park, Y., & Runkle, E. S. (2017). Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. In Environmental and Experimental Botany, 136, 41–49. https://doi.org/10.1016/j.envexpbot.2016.12.013.
Park, W. T., Yeo, S. K., Sathasivam, R., Park, J. S., Kim, J. K., & Park, S. U. (2020). Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Applied Biological Chemistry, 63(1). https://doi.org/10.1186/s13765-020-00510-4.
Patel, S. (2014). Blueberry as functional food and dietary supplement: the natural way to ensure holistic health. Mediterranean Journal of Nutrition and Metabolism, 7(2), 133–143. https://doi.org/10.3233/MNM-140013.
Pérez-Ambrocio, A., Guerrero-Beltrán, J. A., Aparicio-Fernández, X., Ávila-Sosa, R., Hernández-Carranza, P., Cid-Pérez, S., & Ochoa-Velasco, C. E. (2018). Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biology and Technology, 135, 19–26. https://doi.org/10.1016/j.postharvbio.2017.08.023.
Pinela, J., & Ferreira, I. C. F. R. (2017). Nonthermal physical technologies to decontaminate and extend the shelf-life of fruits and vegetables: trends aiming at quality and safety. Critical Reviews in Food Science and Nutrition, 57(10), 2095–2111. https://doi.org/10.1080/10408398.2015.1046547.
Pogson, B. J., & Morris, S. C. (2003). Postharvest senescence of vegetables and its regulation. Plant Cell Death Processes, 319–329. https://doi.org/10.1016/B978-012520915-1/50025-4.
Prasad, B. V. G., & Chakravorty, S. (2018). Effects of climate change on vegetable cultivation - a review. Nature Environment and Pollution Technology An International Quarterly Scientific Journal, 14(4), 923–929.
Qian, H., Liu, T., Deng, M., Miao, H., Cai, C., Shen, W., & Wang, Q. (2016). Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chemistry, 196, 1232–1238. https://doi.org/10.1016/j.foodchem.2015.10.055.
Regnier, T., & Combrinck, S. (2009). Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biology and Technology, 53(3), 117–122. https://doi.org/10.1016/j.postharvbio.2009.04.005.
Reinbothe, S., & Reinbothe, C. (1996). Regulation of chlorophyll biosynthesis in angiosperms. Plant Physiology, 111(1), 1–7. https://doi.org/10.1104/pp.111.1.1.
Ren, J., Guo, S., Xu, C., Yang, C., Ai, W., Tang, Y., & Qin, L. (2014). Effects of different carbon dioxide and LED lighting levels on the anti-oxidative capabilities of Gynura bicolor DC. Advances in Space Research, 53(2), 353–361. https://doi.org/10.1016/j.asr.2013.11.019.
Rodyoung, A., Masuda, Y., Tomiyama, H., Saito, T., Okawa, K., Ohara, H., & Kondo, S. (2016). Effects of light emitting diode irradiation at night on abscisic acid metabolism and anthocyanin synthesis in grapes in different growing seasons. Plant Growth Regulation, 79(1), 39–46. https://doi.org/10.1007/s10725-015-0107-1.
Samuoliene, G., Sirtautas, R., Brazaityte, A., & Duchovskis, P. (2012). LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chemistry, 134(3), 1494–1499. https://doi.org/10.1016/j.foodchem.2012.03.061.
Samuoliene, G., Brazaityte, A., Sirtautas, R. n., Viršile, A., Sakalauskaite, J., Sakalauskiene, S., & Duchovskis, P. (2013). LED illumination affects bioactive compounds in romaine baby leaf lettuce. Journal of the Science of Food and Agriculture, 93(13), 3286–3291. https://doi.org/10.1002/jsfa.6173.
Schultz, C., Platonova, I., Doluweera, G., & Irvine-Halliday, D. (2008). Why the developing world is the perfect market place for solid state lighting. Eighth International Conference on Solid State Lighting, 7058, 705802. https://doi.org/10.1117/12.800718
Seigler, B. D. S. (2000). Books Plant secondary metabolism Biochemistry of plant secondary metabolism (pp. 483–485). Dordrecht: Kluwer Academic Publishers.
Seo, J. M., Arasu, M. V., Kim, Y. B., Park, S. U., & Kim, S. J. (2015). Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chemistry, 177, 204–213. https://doi.org/10.1016/j.foodchem.2014.12.094.
Shao, M., Liu, W., Zha, L., Zhou, C., Zhang, Y., & Li, B. (2020). Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Scientia Horticulturae, 268, 109–366. https://doi.org/10.1016/j.scienta.2020.109366.
Sharma, S., Pareek, S., Sagar, N. A., Valero, D., & Serrano, M. (2017). Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: a review. International Journal of Molecular Sciences, 18(8). https://doi.org/10.3390/ijms18081789.
Shezi, S., Magwaza, L. S., Mditshwa, A., & Tesfay, S. Z. (2020) Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Scientia Horticulturae, 269, 109397. https://doi.org/10.1016/j.scienta.2020.109397
Shi, L., Cao, S., Chen, W., & Yang, Z. (2014). Blue light induced anthocyanin accumulation and expression of associated genes in Chinese bayberry fruit. Scientia Horticulturae, 179, 98–102. https://doi.org/10.1016/j.scienta.2014.09.022.
Shi, L., Cao, S., Shao, J., Chen, W., Yang, Z., & Zheng, Y. (2016). Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biology and Technology, 111, 197–204. https://doi.org/10.1016/j.postharvbio.2015.08.013.
Shi Y., Wang B. L., Shui D. J., Cao L. L., Wang C., Yang T., et al. (2014). Effect of 1-methylcyclopropene on shelf life, visual quality and nutritional quality of netted melon. Food Science and Technology International, 21(3), 175–87. https://doi.org/10.1177/1082013214520786.
Skrovankova, S., Sumczynski, D., Mlcek, J., Jurikova, T., & Sochor, J. (2015). Bioactive compounds and antioxidant activity in different types of berries. International Journal of Molecular Sciences, 16(10), 24673–24706. https://doi.org/10.3390/ijms161024673.
Slavin, J. U. o. M., & Lloyd Beate, P. N. G. R. (2012). Health Benefits of cassava-karrapendalam. American Society for Nutrition, 3(4), 506–516. https://doi.org/10.3945/an.112.002154.506.
Solovchenko, A. E., & Merzlyak, M. N. (2008). Screening of visible and UV radiation as a photoprotective mechanism in plants. Russian Journal of Plant Physiology, 55(6), 719–737. https://doi.org/10.1134/S1021443708060010.
Song, K., Mohseni, M., & Taghipour, F. (2016). Application of ultraviolet light-emitting diodes ( UV-LEDs) for water disinfection : a review. Water Research, 94, 341–349. https://doi.org/10.1016/j.watres.2016.03.003.
Tadiello, A., Longhi, S., Moretto, M., Ferrarini, A., Tononi, P., Farneti, B., Busatto, N., Vrhovsek, U., Molin, A. ., Avanzato, C., Biasioli, F., Cappellin, L., Scholz, M., Velasco, R., Trainotti, L., Delledonne, M., & Costa, F. (2016). Interference with ethylene perception at receptor level sheds light on auxin and transcriptional circuits associated with the climacteric ripening of apple fruit (Malus x domestica Borkh.). Plant Journal, 88(6), 963–975. https://doi.org/10.1111/tpj.13306.
Taulavuori, E., Taulavuori, K., Holopainen, J. K., Julkunen-Tiitto, R., Acar, C., & Dincer, I. (2017). Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment. Journal of the Science of Food and Agriculture, 97(15), 5059–5064. https://doi.org/10.1002/jsfa.8492.
Thwe, A. A., Kim, Y. B., Li, X., Seo, J. M., Kim, S. J., Suzuki, T., Chung, S. O., & Park, S. U. (2014). Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in fagopyrum tataricum sprouts. Journal of Agricultural and Food Chemistry, 62(21), 4839–4845. https://doi.org/10.1021/jf501335q.
Usall, J., Ippolito, A., Sisquella, M., & Neri, F. (2016). Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biology and Technology, 122, 30–40. https://doi.org/10.1016/j.postharvbio.2016.05.002.
Wang, Y., & Folta, K. M. (2013). Contributions of green light to plant growth and development. American Journal of Botany, 100(1), 70–78. https://doi.org/10.3732/ajb.1200354.
Wang, S. Y., Chen, C. T., & Wang, C. Y. (2009). The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chemistry, 112(3), 676–684. https://doi.org/10.1016/j.foodchem.2008.06.032.
Wu, M. C., Hou, C. Y., Jiang, C. M., Wang, Y. T., Wang, C. Y., Chen, H. H., & Chang, H. M. (2007). A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chemistry, 101(4), 1753–1758. https://doi.org/10.1016/j.foodchem.2006.02.010.
Xu, F., Cao, S., Shi, L., Chen, W., Su, X., & Yang, Z. (2014a). Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. Journal of Agricultural and Food Chemistry, 62(20), 4778–4783. https://doi.org/10.1021/jf501120u.
Xu, F., Shi, L., Chen, W., Cao, S., Su, X., & Yang, Z. (2014b). Scientia Horticulturae Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae, 175, 181–186. https://doi.org/10.1016/j.scienta.2014.06.012.
Xu, Y., Charles, M. T., Luo, Z., Mimee, B., Tong, Z., Véronneau, P. Y., & Rolland, D. (2019). Ultraviolet-C priming of strawberry leaves against subsequent Mycosphaerella fragariae infection involves the action of reactive oxygen species, plant hormones, and terpenes. Plant, Cell & Environment, 42(3), 815–831. https://doi.org/10.1111/pce.13491.
Yang, X., Xu, H., Shao, L., Li, T., Wang, Y., & Wang, R. (2018). Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environmental and Experimental Botany, 150(March), 161–171. https://doi.org/10.1016/j.envexpbot.2018.03.013.
Yang, Q., Pan, J., Shen, G., & Guo, B. (2019). Yellow light promotes the growth and accumulation of bioactive flavonoids in Epimedium pseudowushanense. Journal of Photochemistry and Photobiology B: Biology, 197(151), 111550. https://doi.org/10.1016/j.jphotobiol.2019.111550.
Yu, W., Liu, Y., Song, L., Jacobs, D. F., Du, X., Ying, Y., et al. (2017). Effect of Differential Light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. Journal of Plant Growth Regulation, 36(1), 148–160. https://doi.org/10.1007/s00344-016-9625-y.
Yu, S., Wang, Z., Fan, J., Qian, C., Deng, Z., & Gui, D. (2020). High temperature performance evaluation and life prediction for titanium modified silicone used in light-emitting diodes chip scale packages. Journal of Electronic Packaging, 142(2), 1–8. https://doi.org/10.1115/1.4045568.
Zhan, L., Hu, J., Li, Y., & Pang, L. (2012a). Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.). Postharvest Biology and Technology, 72, 76–81. https://doi.org/10.1016/j.postharvbio.2012.05.001.
Zhan, L., Li, Y., Hu, J., Pang, L., & Fan, H. (2012b). Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innovative Food Science and Emerging Technologies, 14, 70–76. https://doi.org/10.1016/j.ifset.2012.02.004.
Zheng, Y., Yang, Z., & Chen, X. (2008). Effect of high oxygen atmospheres on fruit decay and quality in Chinese bayberries, strawberries and blueberries. Food Control, 19(5), 470–474. https://doi.org/10.1016/j.foodcont.2007.05.011.
Zhmakin, A. I. (2011). Enhancement of light extraction from light emitting diodes. Physics Reports, 498(4–5), 189–241. https://doi.org/10.1016/j.physrep.2010.11.001.
Zhou, F., Zuo, J., Gao, L., Sui, Y., Wang, Q., Jiang, A., & Shi, J. (2019). An untargeted metabolomic approach reveals significant postharvest alterations in vitamin metabolism in response to LED irradiation in pak-choi (Brassica campestris L. ssp. chinensis (L.) Makino var. communis Tsen et Lee). Metabolomics, 15(12), 155. https://doi.org/10.1007/s11306-019-1617-z.
Zhou, Q., Ma, C., Cheng, S., Wei, B., Liu, X., & Ji, S. (2014). Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biology and Technology, 88, 88–95. https://doi.org/10.1016/j.postharvbio.2013.10.003.
Funding
This work was supported by the National Key Research and Development Program of China (2019YFC1604504, 2019YFD1002300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nassarawa, S.S., Abdelshafy, A.M., Xu, Y. et al. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: a Review. Food Bioprocess Technol 14, 388–414 (2021). https://doi.org/10.1007/s11947-020-02534-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02534-6