Abstract
Existence of cuticle wax layer not only reduces the drying rate of blueberries but also causes the fruit to burst during drying. Such a phenomenon results in undesirable appearance as well as in losses of bioactive compounds responsible for health benefits and in sugars adhering to the surface and hence increased moisture adsorption capability of the dried fruit. In this study, uses of CO2 laser perforation, ultrasound and freezing–thawing as skin pretreatments prior to infrared freeze drying and their effects on drying characteristics as well as selected properties, i.e., shrinkage, color, rehydration capacity, as well as total anthocyanins and phenolics contents, of blueberries were investigated. Fourier transform infrared (FTIR) spectra of the dried fruit were also determined. Pretreatments increased drying rate and rehydration capacity of blueberries; shrinkage reduced from around 57 to 25%. Laser perforation and freezing–thawing but not ultrasonic pretreatments exhibited no significant effects on color of blueberries. Laser perforation and ultrasonic pretreatments exerted positive effects on the total phenolic and anthocyanin contents of the dried samples, respectively. FTIR spectra illustrated that all pretreatments did not alter the chemical fingerprints of the dried fruit powder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blueberry (Vaccinium corymbosum L.) is a popularly consumed fruit that belongs to the genus Vaccinium in the family Ericaceae. The fruit is sometimes regarded as king of berries due to its wide array of health benefits, which include antioxidant, antibacterial, and antiviral activities (Aranaz et al., 2017). However, blueberry has very short shelf life of only 2–4 days at a typical ambient temperature. An appropriate means to preserve and hence prolong the shelf life of the fruit is therefore needed (López et al., 2010; Wang et al., 2013; Zhang et al., 2006, 2017a).
Hot air drying, microwave drying, and infrared drying can be used to dry blueberry. Compared with freeze drying, however, these drying methods tend to more extensively reduce the sensory quality and nutritional values of the fruit; freeze drying is therefore an attractive alternative for the preservation of blueberry (Huang, Zhang, Yan et al., 2009; Kim & Kerr, 2013; Pei et al., 2014; Saxena et al., 2012; Zielinska et al., 2015). An important problem related to freeze drying of blueberry nevertheless exists. Since blueberry epidermis is covered with a layer of wax, water transport from inner to outer parts of the fruit is restricted. This results in prolonged drying time and increased energy consumption. Moreover, when the outward movement of internally sublimed water, which takes place when the fruit temperature reaches the phase transition temperature of water, is restricted, there would be a pressure build-up within the fruit. At a point when such a pressure exceeds the strength of the epidermis, the fruit skin would crack or even burst to release the pressure. This leads to collapse and deformation, which adversely affect the appearance of the dried fruit. Internal juice may also leak out, sometimes adhering to the surface of the fruit (Zielinska et al., 2015). Studies have nevertheless confirmed that appropriate pretreatments to modify the skin structure can effectively improve the water transport capability through the fruit skin (Shi et al., 2008). Currently utilized pretreatments for blueberry include chemical and mechanical pretreatments. Chemical residue and possible alteration of the taste of the fruit are among the limitations of chemical pretreatments (Ketata et al., 2013). Mechanical pretreatment, on the other hand, involves the use of no chemicals, so it is more health and environmentally friendly than chemical pretreatment. However, mechanical pretreatment still has limitations, including juice loss from the fruit.
Among alternative pretreatment methods for blueberry, use of carbon dioxide-based laser is an attractive one. This is because the laser beam emitted at the working wavelength of CO2 (10.6 μm) is strongly absorbed by water; the laser can also produce spots (micropores) on the surface of the fruit in a much controllable manner. In addition to CO2-based laser, ultrasonic pretreatment is also of interest (Fan et al., 2020; Zhang et al., 2019). Ultrasound has been shown to help modify the skins (or peels) of an array of fruit, hence improving its water transport behavior (Kek et al., 2013). Nowacka et al. (2014), for instance, observed that after 10 min of ultrasonic pretreatment, cell walls of kiwifruit slices started to exhibit microscopic channels that promoted water transport. However, no study is so far available on comprehensive comparison of CO2-based laser and ultrasound to modify the skin structure of blueberry to enhance its freeze drying rate and to reduce skin rupture and hence internal juice leakage upon drying.
The purpose of this study was to compare the effects of CO2-based laser perforation and ultrasonic skin pretreatments on drying rate and selected quality characteristics of dried frozen blueberries prepared by infrared freeze drying (IRFD), which has been shown to be capable of accelerating the rate of conventional freeze drying (Hnin et al., 2019). Freezing–thawing pretreatment was also conducted and compared with laser and ultrasonic pretreatments. Shrinkage, color, rehydration capacity, as well as total anthocyanin and phenolic contents were determined; the contents of these bioactive compounds were determined as they are well recognized to be the key compounds responsible for the health benefits of blueberry. Fourier transform infrared (FTIR) spectra were also determined to identify possible chemical structure alterations and moisture adsorption of the fruit upon pretreatments.
Materials and Methods
Materials and Chemical Reagents
Individually quick frozen blueberries were supplied by Shanghao Biotech Co., Ltd. (Qingdao, China). Blueberries with uniform size and diameter between 12 and 17 mm were stored in a cryogenic refrigerator (Haier, DM-86L626, Qingdao, China) at −65 °C until the time of an experiment. A diagram of the whole experimental program is shown in Fig. 1. Note that the initial moisture content of blueberries was 89.54% (w.b.) and the total soluble solids content of the frozen fruit was 12.94 oBrix.
Methanol, hydrochloric acid, folinol, sodium carbonate, potassium chloride, sodium acetate, gallic acid monohydrate, ferrous sulfate heptahydrate, salicylic acid, and 30% hydrogen peroxide solution were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Skin Pretreatments
CO2 laser device (Yiwang Laser Equipment, CO2-30L, Wuxi, China) was used for laser skin pretreatment. The emission wavelength was noted to be 10.64 μm, while the marking speed, frequency, and power were 100 mm/s, 20 kHz, 30 W, respectively. Perforations were made in a square grid pattern with the dimensions of 2.0 × 2.0 mm; 25 perforations were made per one blueberry fruit. The distance between the laser head and blueberry surface was maintained at 150 mm; a fruit sample was perforated in its frozen state. After perforation, the sample was returned to the freezer and stayed for at least 12 h prior to subsequent drying. The reason for the refreezing process is that the pretreatment caused some ice crystals in the fruit to melt, so refreezing was needed prior to subsequent IRFD.
In the case of ultrasonic pretreatment, individually frozen blueberries were first thawed at 4 °C for 5 h. The sample was introduced into an ultrasonic bath (Ultrasonic Instruments, KQ-300VDV, Kunshan, China) for 20 min; the frequency and specific power of ultrasound were 45 kHz and 20 W/g, respectively. Temperature during ultrasonic pretreatment was noted to be lower than 30 °C at all times. After the treatment, excess water on the surface of the sample was removed by a stream of cool air. The sample was then returned to the freezer and stayed for at least 12 h prior to subsequent drying.
In the case of freezing–thawing pretreatment, individually frozen blueberries were thawed at 4 °C for 4 h, and then refrozen in the same cryogenic refrigerator. The refreezing time was again at least 12 h prior to subsequent drying.
Amount of 60 g of individual fruit was treated in each case. All the pretreatment experiments were performed in triplicate. Individually frozen blueberries without any pretreatment and dried by IRFD were used as the control sample.
Infrared Freeze Drying
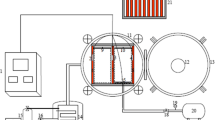
Infrared freeze drying system (Changzhou One Step Drying Equipment, Changzhou, China) shown in Fig. 2 was used for the experiments. Blueberry sample was loaded into the drying chamber where the infrared radiator was set to maintain the drying temperature at 60 °C. The cold trap temperature was maintained at −40 °C, while the pressure within the drying chamber was 80 Pa. Each drying experiment was performed in triplicate.
Drying Kinetics Determination
The moisture and dry matter contents of frozen, dried, and rehydrated blueberries were gravimetrically determined by vacuum drying at 70 °C as per the ISO 1026–1982 standard. The moisture ratio was then calculated as per Eq. (1):
where Mt, M0, and Me are the instantaneous, initial, and equilibrium moisture contents of the sample, respectively. Evolution of the sample moisture content is shown in terms of the drying curve.
Physical Property Determination
Shrinkage Determination
The solid displacement method of Huang, Zhang, Mujumdar et al. (2009) was used to determine the shrinkage of a sample. Shrinkage was calculated as:
where V0 and V1 are the volumes of the sample before and after drying, respectively. Each measurement was performed in triplicate.
Color Determination
Color of a sample was measured using a spectrophotometer (Konica Minolta, CM-3600d, Tokyo, Japan). The meter was calibrated against a standard white plate prior to each measurement using D65 as the light source at 10° standard observer. Lightness (L*), redness/greenness (a*), and yellowness/blueness (b*) values were measured. Total color difference (∆E) was then calculated as per Eq. (2). Each measurement was performed in triplicate.
Rehydration Capacity Determination
The rehydration capacity is defined by Lewicki (1998) as the ratio of the mass of water adsorbed during rehydration to the dry mass of the fruit. Two grams of dried blueberries was placed in 40 g distilled water at 37 °C to reconstitute; the sample was kept in the water for 2, 7, 17, 32, and 52 min (Feng et al., 1999). After rehydration, the sample was wiped with filter paper to remove excess water and then weighed. A rehydration curve of each sample was prepared by plotting the amount of adsorbed water per unit dry matter against time. Each measurement was performed in triplicate.
Bioactive Compound Content Determination
Blueberry powder (0.1 g) (or 5 g fresh mass) was accurately weighed and placed in a 25-mL tube. Then, 25 mL of 80% (v/v) methanol was added; 1% (v/v) HCl was also added to ensure that the pH of the solution was in the range of 2–3. Extraction was performed under ultrasonic condition (frequency of 45 kHz and specific power of 12 W/g sample + solvent) for 30 min. Clarified extract was obtained by filtering through a filter paper; extraction was performed in triplicate for each sample. The extract was stored in a refrigerator at 4 °C until further analysis (but not longer than 24 h).
Total Anthocyanin Content Determination
pH differential assay as suggested by Giusti and Wrolstad (2001) was used to determine the total anthocyanin content (TAC) of a sample extract. One milliliter of crude extract was diluted to 10 mL, either with a buffer solution of pH 1.0 or pH 4.5, and stored in dark for 15 min. One milliliter of distilled water and 9 mL of the corresponding buffer solutions were used as blanks. Absorbances were measured at 520 nm and 700 nm using a spectrophotometer (Unico Instruments, UV-2600, Shanghai, China). Monomeric anthocyanins content is expressed as mg cyanidin 3-glucoside equivalent (C-3-G equivalent) and was calculated as follows:
where V is the total volume of the extract (mL), DF is the dilution factor, MW is the relative molecular weight of cyanidin-3-glucoside (449.2), ε is the extinction coefficient (26,900), and m is the mass of the blueberry powder (g). TAC is expressed in terms of mg cyanidin 3-glucoside equivalent per g dry fruit (mg C-3-G equivalent/g, d.b.). Each measurement was performed in triplicate.
Total Phenolic Content Determination
Total phenolic content (TPC) of a sample was determined by the Folin–Ciocalteu assay as recommended by Lao et al. (2019). One milliliter of crude extract was combined with 5 mL of Folin–Ciocalteu reagent in a test tube; 4 mL of sodium carbonate (7.5% v/v) was then mixed. After 1 h of incubation at room temperature, the absorbance was measured at 765 nm using the same spectrophotometer. TPC is expressed in terms of mg gallic acid equivalent per g of dry fruit (mg GAE/g, d.b.). Each determination was performed in triplicate.
Fourier Transform Infrared Spectroscopy
FTIR spectra of freeze-dried powdery samples were obtained using an FTIR spectrometer (Thermo Fisher Scientific, Nicolet™ iS™ 10, Waltham, MA, USA). The measurement was performed at 4000–400 cm−1 with a resolution of 2 cm−1 and scan speed of 128. Twenty milligrams of a sample was ground with 2 g of potassium bromide and made into a tablet prior to each measurement.
Statistical Analysis
The experimental data were subject to one-way analysis of variance (ANOVA) and are reported as mean values with standard deviations. Significances among mean values were established using Duncan’s new multiple range tests at a confidence level of 95%. SPSS 23 (IBM, Chicago, IL, USA) was used for all statistical computations.
Results and Discussion
Drying Curves of Blueberries
Figure 3 shows the drying curves in terms of the moisture ratio of blueberries undergone different pretreatments. The moisture ratio not only indicates the remaining moisture inside a raw material but also reflects the drying rate (Vega-Galvez et al., 2012; Wu et al., 2020). The moisture content (wet basis) of the samples right after CO2 laser perforation, ultrasonic, and freezing–thawing pretreatments were 89.04%, 91.52%, and 88.45%, respectively. The typical decreasing trend of moisture with time was observed in all cases.
CO2 laser perforation and ultrasonic pretreatments could, in descending order, help accelerate the drying process when compared with freezing–thawing pretreatment. CO2 laser perforation created pores on the skin of the fruit. This provided channels for water transport from inside of the fruit to its exterior. Munzenmayer et al. (2020) arrived at a similar conclusion; these investigators observed that laser perforation could reduce the freeze drying time of blueberries from 17 to 13 h. In the case of ultrasonic pretreatment, the enhancement is attributed to the cavitation and mechanical effects of ultrasound on the tissue structure of blueberries. This is in accordance with the results of Ziying et al. (2018) who reported that the drying rate of ultrasonically pretreated apple was 1.25 times higher than that of the control sample. Gao et al. (2018) reported indeed that ultrasonically induced cavitation bubbles helped destroy the stratum corneum structure of the epidermis.
Freezing–thawing treatment, while might being capable of reducing the skin thickness and damaging the internal structure of blueberries (Zielinska et al., 2015), did not exert any significant enhancement effect in this case. This is probably because ice crystals produced by rapid freezing (− 65 °C) were smaller, thus resulting in negligible damage to blueberry structure during thawing.
Physical Properties of Blueberries
Color of Blueberries
It is seen in Table 1 that, compared with the control sample, ultrasonic pretreatment exhibited a significant impact on the color of blueberries; L*, a*, and b* values of the fruit increased when ultrasonic pretreatment was applied. On the other hand, no significant differences in color were observed among the control sample and those undergone CO2 laser perforation and freezing–thawing pretreatments. Note that L*, a*, and b* values of the frozen blueberries were 26.35 ± 0.67, 3.51 ± 0.52, and − 1.27 ± 0.23, respectively.
Compared with frozen blueberries, L* value of all dried samples decreased, except for those undergone ultrasonic pretreatment. It has been reported that shrinkage and structural deformation during drying may have shifted photons or absorbed more light, thus resulting in the decreased L* values (da Silva Júnior et al., 2018). Ultrasonically pretreated dried blueberries became lighter, as shown in Fig. 4. ΔE value of freeze-dried blueberries that undergone different pretreatments could be summarized as follows: ultrasonically treated > CO2 laser perforation treated > freezing–thawing treated > control. Except for the ultrasonically treated fruit, blueberries rather retained their original color. This observation was related to the loss of anthocyanins during the ultrasonic pretreatment process. This observation is in accordance with that of Siucińska et al. (2016) who reported that ΔE value of dried sour cherries that undergone ultrasonic pretreatment was higher than that of the untreated sample. Cavitation-induced damage was noted to be responsible for such a change.
Shrinkage of Blueberries
Shrinkage of the samples ranged from 25.45 to 56.67%. Samples undergone ultrasonic, freezing–thawing, and CO2 laser perforation suffered shrinkage in ascending order; control sample suffered most extensive shrinkage (see Table 1 and Fig. 4). Pretreatment could reduce the degree of shrinkage when comparing the pretreated samples with the control one. This is due to the fact that pretreatments could help enhance the moisture transport capability of the fruit during drying. Ultrasonic pretreatment maintained the original volume of blueberries to the highest extent, with the degree of shrinkage of 25.45%. This trend of results is in accordance with that of Guo et al. (2020) who observed that ultrasonic pretreatment not only changed the internal tissue structure of the berries to form microporous channels but also destroyed the fruit cell walls to increase the rate of water transport during the drying process, thereby reducing the deformation of the fruit during drying. In the case of freezing–thawing pretreatment, Tatemoto et al. (2016) reported that this pretreatment could help prevent carrot tissue deformation and shrinkage during drying. Laser-perforated blueberries, on the other hand, suffered more extensive shrinkage. Mayor and Sereno (2004) suggested that shrinkage of plant tissues during drying is caused by the imbalance of pressures inside and outside of the tissues. The more rapidly the water is removed, the higher the moisture gradients and the more extensive shrinkage. The higher rate of drying caused by the laser-perforation pretreatment might be responsible for the observed more extensive shrinkage.
Rehydration Capacity of Blueberries
Rehydration capacity is an important quality attribute of a dried product; rehydration characteristics of dried foods are associated with changes in the cellular structure of the foods induced by pretreatment or dehydration process (Xie et al., 2017). Figure 5 shows the rehydration kinetics of dried blueberries that undergone different pretreatments. The rehydration capacities of blueberries expectedly increased with the rehydration time; the rate of increase nevertheless decreased with time. Rehydration capacities of differently pretreated samples were noted to be significantly different. The rehydration rate of ultrasonically pretreated blueberries was the highest; the rehydration curve tended to reach the plateau only after 10 min of rehydration, indicating that the water adsorption had reached the maximum. Rapid and complete rehydration is the desired property of a dried product as faster rehydration and higher rehydration ratio illustrate the superior water reconstitution characteristics of the product; such superiority indicates less damage of the cellular structure of the product (Wang et al., 2018).
The aforementioned rehydration behavior is believed to be related to the blueberry tissue structure. Ultrasonic cavitation exhibited significant effect on the blueberry tissue and resulted in microporous structure prior to drying. This led to increased water transport (reconstitution) capability of the dried sample. This finding is consistent with that of Alolga et al. (2020) on garlic slices. The trend of the rehydration capacity was opposite to that of shrinkage. Less extensive shrinkage implies that a dried sample suffers less damage and can easier adsorb water and hence rehydrate more rapidly. On the contrary, a sample with a higher degree of shrinkage would exhibit a lower rehydration capability. Jayaraman et al. (1990) also observed that the contraction of capillaries in plant tissues led to the restricted rehydration of cauliflowers.
Exception to the abovementioned argument was observed in the case of CO2 laser perforation. Although shrinkage of the laser-treated sample was more extensive than that of the sample treated by freezing–thawing pretreatment, the rehydration capability of the former was higher. This is most probably ascribed to the pores that formed on the skin of the fruit. Perforation is indeed often used as an effective method to improve the rehydration capacity of a dried product.
Bioactive Compound Contents
Total Anthocyanin Content
Figure 6 shows the TAC of the samples that undergone different pretreatments. As expected, fresh and dried blueberries had significantly different TAC, indicating that anthocyanins in the blueberries degraded during drying; anthocyanins are indeed known to be sensitive to heat (Wojdylo et al. 2014). Comparing with the control sample, only the CO2 laser perforation treatment significantly reduced TAC; there was no significant difference among the ultrasonically treated, freezing–thawing treated, and control samples. Note that TACs of mature blueberry peels were 7 and 192 times of those of the whole fruit and pulp, respectively (Ribera et al., 2010). Heat involved in the laser treatment of the blueberry epidermis is then believed to result in the extensive loss of anthocyanins.
Although not significantly different, the content of anthocyanins in the sample treated with ultrasound was slightly higher than that of the control sample. Zhang et al. (2020) observed similar results; the content of anthocyanins in ultrasonically treated strawberries was higher than that in the untreated control sample. This may be related to the extraction of anthocyanins from the treated blueberries. Tiwari et al. (2010) illustrated that ultrasound could promote dissolution of anthocyanins; anthocyanin extraction (for quantification purpose) was therefore more efficient. In the case of freezing–thawing pretreatment, ice crystals might have damaged the cellular structure of the fruit, resulting in juice leakage and hence the slightly more extensive loss of anthocyanins, which is water-soluble in nature (Nowak et al., 2019).
Total Phenolic Content
The effects of different pretreatments on the TPC of blueberries are shown in Fig. 7. Phenolics are among the major health beneficial compounds in blueberries; phenolics are also sensitive to heat and oxygen. TPC of blueberries significantly reduced upon drying. No significant differences were noted among the control sample as well as those undergone CO2 laser perforation and ultrasonic pretreatment; freezing–thawing pretreatment, however, exerted a significant adverse influence on TPC of the sample. TPC of blueberries treated with ultrasound was significantly higher than that treated with freezing–thawing pretreatment, again probably because ultrasonic pretreatment helped promote release of phenolic compounds from the matrix of blueberries.
Among the three pretreatment methods, CO2 laser perforation and ultrasound could effectively prevent bursting of blueberries during drying. Interestingly, at the end of drying, except for the sample pretreated by ultrasound, surface of blueberries appeared sticky; the sticky appearance was due to sugar crystallization. Similar phenomenon was observed by Zielinska et al. (2015) who reported that the form and number of crystals in blueberries were different when different drying methods, which led to different degrees of sugar crystallization, were employed.
FTIR Spectra
Figure 8 shows FTIR spectra of the powders prepared from dried blueberries undergone different pretreatments. The main characteristic peaks of the samples appeared at 3400 cm−1, 2927 cm−1, 1640 cm−1, 1734 cm−1, and 1060 cm−1. The strong absorption bands of blueberries were between 3000 and 3500 cm−1, which correspond to the tensile vibrations of O–H and N–H (Radzki et al., 2016). The peak at 2927 cm−1 is related to the C-H stretching vibration (Li et al., 2010), while the absorption peak near 1640 cm−1 represents the stretching vibration of C = C bond of the aromatic group of anthocyanins (Beullens et al., 2006). The peak at 1734 cm−1 is related to the stretching vibration of C = O (Tao et al., 2017), while the one at 1060 cm−1 is related to the stretching vibration of C-O. The peak at 950 ~ 750 cm−1 is mainly the absorption peak of polysaccharides, while the absorption peaks of inorganic elements should be at wavenumbers lower than 700 cm−1.
Peak positions were similar for both the control and pretreated samples, indicating that the pretreatments did not alter the molecular structure of the fruit powder. The peaks of the infrared spectrum were normalized at 1424 cm−1 and then semi-quantitatively analyzed. The ratio of each peak is shown in Table 2. There were differences in the absorption peak intensities belonging to the control and pretreated samples, indicating that the pretreatments exhibited some effects on the vibration intensity of the original functional groups of blueberries. Note that the O–H stretching vibration was more intensive than the C-H stretching one, indicating that blueberries contained a relatively large number of OH group (Tang et al., 2019). It is indeed generally believed that hygroscopicity is related to the number of OH group (Bichot et al., 2020). Tang et al. (2019), for example, illustrated that hygroscopicity of eosinophilic pollen was determined by the OH group of organic compounds contained within such a pollen; FTIR spectra revealed good correlation between hygroscopicity of the pollen and its number of OH group. In our case, it can be said that the pretreatments are beneficial to the alleviation of the hygroscopicity of blueberries. Further research is required to determine the moisture adsorption characteristics of blueberries, however.
Conclusion
The effects of CO2 laser perforation, ultrasonic, and freezing–thawing pretreatments on the drying characteristics and selected properties of infrared freeze-dried individually quick frozen blueberries were investigated. Laser perforation was noted to effectively improve the drying rate of blueberries by causing physical damage to the skin of the fruit. While exerting no significant impact on the color of the dried product, laser perforation helped reduce shrinkage, increase rehydration capability, and enhance total phenolics content of the dried fruit. Ultrasonic pretreatment, on the other hand, significantly affected the color of dried blueberries but, at the same time, reduced shrinkage, improved rehydration capability and enhanced total anthocyanins content of the dried fruit. The three pretreatments may have the potential to reduce hygroscopicity of freeze-dried blueberries. Interestingly, however, only ultrasonic pretreatment was capable of preventing blueberries from cracking during drying, thus avoiding undesirable adhesion between individually dried fruit and hence its enhanced appearance and prolonged storage life. Ultrasonic pretreatment is therefore recommended as the most appropriate pretreatment for blueberries prior to infrared freeze drying. Further study on how to avoid or reduce the loss of blueberry skin color during ultrasonic pretreatment is recommended.
Data Availability
The data are available from the corresponding author upon suitable request.
References
Alolga, R. N., Osae, R., Essilfie, G., Saalia, F. K., Akaba, S., & Chikari, F. (2020). Sonication, osmosonication and vacuum-assisted osmosonication pretreatment of Ghanaian garlic slices: Effect on physicochemical properties and quality characteristics. Food Chemistry, 343, 128535.
Aranaz, P., Romo-Hualde, A., Zabala, M., Navarro-Herrera, D., de Galarreta, M. R., Gil, A. G., Martinez, J. A., Milagro, F. I., & González-Navarro, C. J. (2017). Freeze-dried strawberry and blueberry attenuates diet-induced obesity and insulin resistance in rats by inhibiting adipogenesis and lipogenesis. Food & Function, 8, 3999–4013.
Beullens, K., Kirsanov, D., Irudayaraj, J., Rudnitskaya, A., Legin, A., Nicolaï, B. M., & Lammertyn, J. (2006). The electronic tongue and ATR-FTIR for rapid detection of sugars and acids in tomatoes. Sensors and Actuators B: Chemical, 116, 107–115.
Bichot, A., Lerosty, M., Radoiu, M., Mechin, V., Bernet, N., Delgenes, J. P., & Gárcia-Bernet, D. (2020). Decoupling thermal and non-thermal effects of the microwaves for lignocellulosic biomass pretreatment. Energy Conversion & Management, 203, 112220.
da Silva Júnior, E. V., de Melo, L. L., de Medeiros, R. A. B., Pimenta Barros, Z. M., & Azoubel, P. M. (2018). Influence of ultrasound and vacuum assisted drying on papaya quality parameters. LWT - Food Science and Technology, 97, 317–322.
Fan, K., Zhang, M., & Chen, H. (2020). Effect of ultrasound treatment combined with carbon dots coating on the microbial and physicochemical quality of fresh-cut cucumber. Food and Bioprocess Technology, 13, 648–660.
Feng, H., Tang, J., Mattinson, D. S., & Fellman, J. K. (1999). Microwave and spouted bed drying of frozen blueberries: The effect of dryingand pretreatment methods on physical properties and retention of flavor volatiles. Journal of Food Processing and Preservation, 23, 463–479.
Gao, R., Ye, F., Lu, Z., Wang, J., Shen, X. L., & Zhao, G. (2018). A novel two-step ultrasound post-assisted lye peeling regime for tomatoes: Reducing pollution while improving product yield and quality. Ultrasonics Sonochemistry, 45, 267–278.
Giusti, M. M., & Wrolstad, R. E. (2001). Characterization and measurement of anthocyanins by UV-Visible spectroscopy. Current Protocols in Food Analytical Chemistry, 00, F1.2.1–F1.2.13.
Guo, Y., Wu, B., Guo, X., Ding, F., Pan, Z., & Ma, H. (2020). Effects of power ultrasound enhancement on infrared drying of carrot slices: Moisture migration and quality characterizations. LWT - Food Science and Technology, 126, 109312.
Hnin, K. K., Zhang, M., Devahastin, S., & Wang, B. (2019). Influence of novel infrared freeze drying of rose flavored yogurt melts on their physicochemical properties, bioactive compounds and energy consumption. Food and Bioprocess Technology., 12, 2062–2073.
Huang, L. L., Zhang, M., Mujumdar, A. S., Sun, D. F., Tan, G. W., & Tang, S. (2009). Studies on decreasing energy consumption for a freeze-drying process of apple slices. Drying Technology, 27, 938–946.
Huang, L. L., Zhang, M., Yan, W. Q., Mujumdar, A. S., & Sun, D. F. (2009). Effect of coating on post-drying of freeze-dried strawberry pieces. Journal of Food Engineering, 92, 107–111.
Jayaraman, K. S., Das Gupta, D. K., & Rao, N. B. (1990). Effect of pretreatment with salt and sucrose on the quality and stability of dehydrated cauliflower. International Journal of Food Science & Technology, 25, 47–60.
Kek, S. P., Chin, N. L., & Yusof, Y. A. (2013). Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food and Bioproducts Processing, 91, 495–506.
Ketata, M., Desjardins, Y., & Ratti, C. (2013). Effect of liquid nitrogen pretreatments on osmotic dehydration of blueberries. Journal of Food Engineering, 116, 202–212.
Kim, M., & Kerr, W. L. (2013). Vacuum-belt drying of rabbiteye blueberry (Vaccinium ashei) slurries: Influence of drying conditions on physical and quality properties of blueberry powder. Food and Bioprocess Technology, 6, 3227–3237.
López, J., Uribe, E., Vega-Gálvez, A., Miranda, M., Vergara, J., Gonzalez, E., & Di Scala, K. (2010). Effect of air temperature on drying kinetics, vitamin C, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O´Neil. Food and Bioprocess Technology, 3, 772–777.
Lao, Y., Zhang, M., Devahastin, S., & Ye, Y. (2019). Effect of combined infrared freeze drying and microwave vacuum drying on quality of kale yoghurt melts. Drying Technology, 38, 621–633.
Lewicki PP . (1998). Some remarks on rehydration of dried foods. Journal of Food Engineering, 36, 81–87.
Li, Y., Lu, C., Meng, X., Ma, Y., & Feng, Y. (2010). Structural identification of malvidin-3-galactoside from St Cloud Blueberry Fruits. . Food Science, 31, 14–17.
Mayor, L., & Sereno, A. M. (2004). Modelling shrinkage during convective drying of food materials: A review. Journal of Food Engineering, 61, 373–386.
Munzenmayer, P., Ulloa, J., Pinto, M., Ramirez, C., Valencia, P., Simpson, R., & Almonacid, S. (2020). Freeze-drying of blueberries: Effects of carbon dioxide (CO2) laser perforation as skin pretreatment to improve mass transfer, primary drying time, and quality. Foods, 9, 211.
Nowacka, M., Tylewicz, U., Laghi, L., Dalla Rosa, M., & Witrowa-Rajchert, D. (2014). Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chemistry, 144, 18–25.
Nowak, K. W., Zielinska, M., & Waszkielis, K. M. (2019). The effect of ultrasound and freezing/thawing treatment on the physical properties of blueberries. Food Science and Biotechnology, 28, 741–749.
Pei, F., Yang, W. J., Shi, Y., Sun, Y., Mariga, A. M., Zhao, L. Y., et al. (2014). Comparison of freeze-drying with three different combinations of drying methods and their influence on colour, texture, microstructure and nutrient retention of button mushroom (Agaricus bisporus) slices. Food and Bioprocess Technology, 7, 702–710.
Radzki, W., Ziaja-Sołtys, M., Nowak, J., Rzymowska, J., Topolska, J., Sławińska, A., Michalak-Majewska, M., Zalewska-Korona, M., & Kuczumow, A. (2016). Effect of processing on the content and biological activity of polysaccharides from Pleurotus ostreatus mushroom. LWT - Food Science and Technology, 66, 27–33.
Ribera, A. E., Reyes-Diaz, M., Alberdi, M., Zuñiga, G. E., & Mora, M. L. (2010). Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. Journal of Soil Science & Plant Nutrition, 10, 509–536.
Saxena, A., Maity, T., Raju, P. S., & Bawa, A. S. (2012). Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying. Food and Bioprocess Technology, 5, 672–679.
Shi, J., Pan, Z., McHugh, T. H., Wood, D., Zhu, Y., Avena-Bustillos, R. J., & Hirschberg, E. (2008). Effect of berry size and sodium hydroxide pretreatment on the drying characteristics of blueberries under infrared radiation heating. Journal of Food Science, 73, E259–E265.
Siucińska, K., Konopacka, D., Mieszczakowska-Frąc, M., & Połubok, A. (2016). The effects of ultrasound on quality and nutritional aspects of dried sour cherries during shelf-life. LWT - Food Science and Technology, 68, 168–173.
Tang, M., Gu, W., Ma, Q., Li, Y. J., Zhong, C., Li, S., et al. (2019). Water adsorption and hygroscopic growth of six anemophilous pollen species: The effect of temperature. Atmospheric Chemistry and Physics, 19, 2247–2258.
Tao, Y., Wang, P., Wang, J., Wu, Y., Han, Y., & Zhou, J. (2017). Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technology, 311, 77–87.
Tatemoto, Y., Mibu, T., Yokoi, Y., & Hagimoto, A. (2016). Effect of freezing pretreatment on the drying characteristics and volume change of carrots immersed in a fluidized bed of inert particles under reduced pressure. Journal of Food Engineering, 173, 150–157.
Tiwari, B. K., Patras, A., Brunton, N., Cullen, P. J., & O’Donnell, C. P. (2010). Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrasonics Sonochemistry, 17, 598–604.
Vega-Galvez, A., Lara, E., Flores, V., Di Scala, K., & Lemus-Mondaca, R. (2012). Effect of selected pretreatments on convective drying process of blueberries (var. O’neil). Food and Bioprocess Technology, 5, 2797–2804.
Wang, J., Law, C. L., Nema, P. K., Zhao, J. H., Liu, Z. L., Deng, L. Z., et al. (2018). Pulsed vacuum drying enhances drying kinetics and quality of lemon slices. Journal of Food Engineering, 224, 129–138.
Wang, Y., Zhang, M., Mujumdar, A. S., & Mothibe, K. J. (2013). Microwave-assisted pulse-spouted bed freeze-drying of stem lettuce slices-effect on product quality. Food and Bioprocess Technology, 6, 3530–3543.
Wojdyło, A., Figiel, A., Lech, K., Nowicka, P., & Oszmiański, J. (2014). Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food and Bioprocess Technology, 7, 829–841.
Wu, X. F., Zhang M., Ye Y., & Yu D. (2020). Influence of ultrasonic pretreatments on drying kinetics and quality attributes of sweet potato slices in infrared freeze drying (IRFD). LWT - Food Science and Technology, 131, 109801.
Xie, L., Mujumdar, A. S., Fang, X. M., Wang, J., Dai, J. W., Du, Z. L., et al. (2017). Far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD) of wolfberry (Lycium barbarum L.): Effects on drying kinetics and quality attributes. Food and Bioproducts Processing, 102, 320–331.
Zhang, L., Liao, L., Qiao, Y., Wang, C., Shi, D., An, K. & Hu, J. (2020). Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chemistry, 303,125386
Zhang, M., Chen, H., Mujumdar, A. S., Tang, J., Miao, S., & Wang, Y. (2017a). Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Critical Reviews in Food Science and Nutrition, 57, 1239–1255.
Zhang, Z., Niu, L., Li, D., Liu, C., Ma, R., Song, J., & Zhao, J. (2017b). Low intensity ultrasound as a pretreatment to drying of daylilies: Impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrasonics Sonochemistry, 36, 50–58.
Zhang, M., Tang, J., Mujumdar, A. S., & Wang, S. (2006). Trends in microwave-related drying of fruits and vegetables. Trends in Food Science & Technology, 17, 524–534.
Zhang, X. T., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effect of combined ultrasonication and modified atmosphere packaging on storage quality of pakchoi (Brassica chinensis L.). Food and Bioprocess Technology, 12, 1573–1583.
Zielinska, M., Sadowski, P., & Blaszczak, W. (2015). Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). LWT - Food Science and Technology, 62, 555–563.
Ziying, R., & Yaxiang B. (2018). Ultrasound pretreatment of apple slice prior to vacuum freeze drying. Proceedings of the 2nd International Conference on Material Science, Energy and Environmental Engineering (MSEEE 2018), Atlantis Press, 112–117.
Funding
This study is financially supported by the National Key R&D Program of China (Contract No. 2017YFD0400901), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX20_1957), Jiangsu Province Key Laboratory Project of Advanced Food Manufacturing Equipment and Technology (No. FMZ202003), as well as Special Funds for Taishan Industry Leading Talents Project, all of which enabled us to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, F., Zhang, M., Devahastin, S. et al. Comparative Evaluation of the Properties of Deep-Frozen Blueberries Dried by Vacuum Infrared Freeze Drying with the Use of CO2 Laser Perforation, Ultrasound, and Freezing–Thawing as Pretreatments. Food Bioprocess Technol 14, 1805–1816 (2021). https://doi.org/10.1007/s11947-021-02677-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02677-0