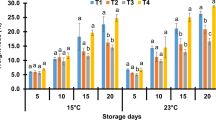
Abstract
Effect of ultrasound (US) treatment combined with carbon dots (CDs) coating on the microbial and physicochemical quality of fresh-cut cucumber was investigated. Cucumbers were dealt with ultrasound (226 W/cm2), CDs coating with CDs concentration of 4.5%, and their combination and then packaged and stored for 15 days at 4 °C. Results exhibited that US treatment combined with CDs coating markedly inhibited total bacterial count to 5.18 log CFU g−1, mold and yeast to 3.45 log CFU g−1 after 15 days of storage. US treatment combined with CDs coating also kept minimum respiration rate of 4.67 mg kg−1 h−1 CO2, weight loss of 8.54%, and malondialdehyde content of 2.24 μmol kg−1 and higher total soluble solids of 2.29 °Brix, firmness of 6.78 N, and ascorbic acid content of 0.0243 g kg−1; inhibited peroxidases activity to 139.83 U kg−1 s−1 and polyphenol oxidase activity to 137.17 U kg−1 s−1; preserved flavor and taste; and reduced the change of water status after 15 days of storage. These results illustrated that US treatment combined with CDs coating can effectively improve the microbial and physicochemical quality of fresh-cut cucumber.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fresh-cut products are in increasing demand, and the main focus is on the high quality and safety aspects of the products. Fresh-cut cucumber (Cucumis sativus L.) is welcomed in people’s life due to rich nutrients such as vitamins, nicotinic acid, and mineral substance (Mohammadi et al. 2016). However, fresh-cut cucumber suffers from deterioration in quality during preservation (Wu et al., 2012; Meng et al., 2014). Fresh-cut cucumber has a short storage period owing to tissue damage, nutritional loss, and microbial infection in cutting and processing (An et al., 2006; Pinheiro et al., 2013). Thus, the fresh-keep for fresh-cut products is critical.
Recently, the application of ultrasound (US) is increasing in food preservation. US is able to control the quality and microorganisms because of its cavitation effect (Sagong et al. 2011; Mothibe et al. 2011; Xin et al. 2013; Lagnika et al. 2013; Islam et al. 2014). Numerous researchers reported that US treatment is helpful to prolong the storage period of fresh products such as tomato (Pinheiro et al. 2015), kiwifruit (Vivek et al. 2016), and plum (Hashemi 2018). Yang et al. (2011) found that US and salicylic acid treatment delayed the decay and inhibited enzyme activity in peach fruit. Moreover, there were no obvious changes in quality. Combined treatment of US and oregano essential oil exhibited a synergetic effect on the inactivation of microorganisms (Millan-Sango et al. 2015). Gani et al. (2016) presented that US treatment exhibited low microbial load and good quality retention for strawberry, although several studies found that US treatment caused microbial inactivation and maintained the quality characteristic of fresh food. However, ultrasound treatment cannot completely control microbial and non-microbial spoilage at the storage period. Therefore, other preservative methods were needed to strengthen the preservative effect of US treatment.
Chitosan (CH) as a safe coating material can form a modified atmosphere to inhibit microorganisms and reduce respiratory metabolism, which effectively prolong the storage period of food (Huang et al. 2009; Carvalho et al. 2016). Many studies have exhibited that the CH coating treatment helped improve the microbial and physicochemical quality of vegetables and fruits such as papaya (González-Aguilar et al. 2009), guava (Hong et al. 2012), citrus (Arnon et al. 2014), and carrot (Song et al. 2017). Furthermore, the CH coating combined with nanoparticles can improve functional properties of chitosan-based coating. Eshghi et al. (2014) found that the nano-chitosan coating presented a good effect on preserving bioactive components and prolonging the storage period of strawberry. Carbon dots (CDs) are nanoparticles with the size of below 10 nm. The CDs have many good properties including low toxicity, good aqueous solubility, excellent biocompatibility, and easy modification (Xin et al. 2015). Therefore, the CDs were widely applied in many fields such as imaging, catalyst, and fluorescent sensor. The CDs can be modified and functionalized with some heteroatoms and compounds, so the CDs have good antibacterial properties. Yang et al. (2016) presented that the CDs exhibited good bacteriostatic ability, indicating that the CDs was promising to use for inhibiting microbial growth during storage of food products. The structure of CDs contains abundant hydrophilic functional groups, such as -OH, -COOH, and -NH2 (Li et al. 2018). The CH containing amino/acetamido group as well as both primary and secondary hydroxyl groups has good biocompatibility and non-toxicity, which prompts us to use it as a surface coating material for CDs nanoparticles (Harish et al. 2020). The CH modified the surface structure of CDs to improve safety in edible food coating application. Harish et al. (2020) found that the CDs nanoparticles surface was modified with chitosan, exhibiting a significant reduction of toxicity in cell. However, there was little scientific information on the application of CDs coating in the preservation of fresh fruit and vegetable. Therefore, kelp (Laminaria japonica) was used to prepare carbon dots in this work due to its low cost and being environment-friendly. This research starts to investigate the effect of US treatment combined with carbon dots from kelp coating on microorganism and physicochemical quality of fresh-cut cucumber.
Materials and Methods
Materials
Cucumbers with commercial maturity and kelp were purchased from agricultural trade market in Wuxi. Cucumbers were put into dark place at 4 °C for 6 h until the use. Cucumbers were washed using distilled water and left to dry. Fresh-cut cucumbers were obtained by cutting into 5-mm-thick slices using a steel knife. Fresh-cut samples were used in the following experiments.
Ultrasound and Carbon Dots Coating Treatment
Ultrasound (US) treatment was performed by using an ultrasound probe (JY98-IIDN, NingBo Scientz Biotechnology Co., Ningbo, China) with 15 mm diameter at 20 kHz. The ultrasound probe was immersed a depth of 2 cm in the beaker filled with distilled water of 1 L at 20 °C using constant water bath. A power intensity of 226 W/cm2 as the method calculated by Fonteles et al. (2016) was used to treat fresh-cut cucumber (100 g) for 10 min as the ultrasound optimal condition based on previous study (Fan et al. 2019b). The temperature increase was below 3 °C after US treatment. For carbon dots coating treatment, chitosan (CH) was added into 1% acetic acid and then whisked for 3 h at 60 °C. The solution was neutralized by sodium hydroxide to obtain 1.5% chitosan solution. Carbon dots (CDs) solution from kelp was obtained by using a hydrothermal method as described by Li et al. (2018). Size distribution of CDs was 0.54–0.83 nm by dynamic light-scattering using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) as shown in Fig. 1. The CDs solution was added into CH coating to achieve CDs coating solution with CDs concentrations of 4.5% based on previous work (Fan et al. 2019a). The experimental groups were carried out as follows: (1) control: samples without US and CDs coating treatment; (2) US treatment: samples were treated by US probe (20 kHz, 400 W) for 10 min; (3) CH coating treatment: samples were dipped into CH coating without CDs solution for 2 min and then drained at room temperature; (4) CDs coating treatment: samples were dipped into CDs coating solution for 2 min and then drained at room temperature; (5) US treatment combined with CDs coating (US+CDs): samples were treated by US probe for 10 min and then dipped into CDs coating solution for 2 min and drained at room temperature. After treatments, control and all treated samples were packaged in polyethylene/polyamide bag filled with 5% CO2, 5% O2, and 90% N2 and then stored in a refrigerator of 4 °C. Samples were measured for microbial and physicochemical quality at 3-day interval. The experiment was conducted in triplicates.
Microbial Measurement
The method of Wang et al. (2015) was used to measure total bacterial count and mold and yeast of samples. Twenty-five grams of samples were soaked in aseptic physiological saline (225 mL) and then shaken into the blender for 2 min. Series 10-fold dilution solutions were prepared by physiological saline. For total bacterial count, 1 mL of dilution solution and 15 mL of plate count agar were mixed into culture dish and then incubated for 48 h at 37 °C. For mold and yeast, 1 mL of dilution sample solution and 15 mL of Bangladeshi-red culture medium were mixed into culture dish, and then incubated for 96 h at 28 °C (Cao et al. 2010). Results of microbial measurement were represented as log CFU g−1.
Measurement of Physicochemical Quality
Respiration Rate and Weight Loss
Respiration rate of samples was determined according to Meng et al. (2014). One hundred grams of samples were placed in a 2-L airtight container and then placed at 4 °C for 30 min. Five milliliters of gas was collected and then tested by gas chromatograph. The parameter of detecting CO2 was set as the following: 80 °C of detector temperature, 50 °C of column temperature, and 0.5 mL s−1 of flow rate. Results of respiratory rate were represented as mg kg−1 h−1 CO2.
Samples were taken at 3-day interval and then weighed to test weight loss according to Lagnika et al. (2013). Results of weight loss were represented as %.
Total Soluble Solids and Firmness
The method of Aday and Caner (2014) was used to measure total soluble solids (TSS) by a refractometer (Aipli Co., Hangzhou, China). Results of TSS were represented as °Brix.
The method of Chen and Zhu (2011) was used to determine firmness at 3-day interval by texture instrument using a cylindrical probe (5 mm diameter). The speed of the probe was 0.5 mm s−1 during the pretest and penetration. Force of punctured pulp to 1 mm depth was considered as firmness (N).
Ascorbic Acid and Malondialdehyde Contents
Ascorbic acid content was tested by 2,6-dichloroindophenol titration (Cao et al. 2010; Lu et al. 2010). Ten grams of samples and 10 mL 3% metaphosphoric acid were ground, and the volume was added to 100 mL using metaphosphoric acid and then centrifuged under 3500×g for 15 min. Ten milliliters of supernatant was titrated by standard 2,6-dichloroindophenol. Results of ascorbic acid content were represented as g kg−1.
Malondialdehyde (MDA) is an index of membrane oxidation that can reduce the membrane integrity and increase the membrane permeability (Li et al. 2017). Malondialdehyde (MDA) content was tested according to Chen et al. (2018). One gram of a sample was ground with 5 mL 10% trichloroacetic acid and then centrifuged for 20 min. One milliliter of supernatant and 2 mL 0.67% 2-hiobarbituric acid were mixed and boiled for 30 min. The mixture was cooled and then centrifuged under 3500×g for 15 min. Supernatant was tested for the absorbance at 450 nm, 532 nm, 600 nm. MDA content was computed through the below formula:
Peroxidases and Polyphenol Oxidase Activities
One gram of a sample and phosphate buffer (5 mL, 0.05 mol L−1, pH 6.5) were blended and centrifuged under 3500×g for 20 min. Supernatant was collected and utilized for the below analysis.
Supernatant was used to test peroxidases (POD) activity according to Cao et al. (2018). Supernatant (0.5 mL) and 2.2 mL of 1% guaiacol as well as 0.2 mL of 1.5% hydrogen peroxide were blended. Supernatant was determined for the absorbance by a spectrophotometer at 470 nm.
Supernatant was used to test polyphenol oxidase (PPO) activity according to Eshghi et al. (2014). Supernatant (0.5 mL) and 0.01 mol L−1 catechol (3 mL) were mixed. Supernatant was determined for the absorbance by a spectrophotometer at 420 nm.
Flavor and Taste
Electronic nose (Ruifen Trading Co., Shanghai, China) containing 14 metal sensors (Table 1) was used to test the flavor of samples according to Chen et al. (2018). Five grams of samples were placed into the vial and then put in the thermostats (25 °C) for 120 min. Test was carried out as the below parameters: air flow of 1 L min−1, testing time of 150 s, and cleaning time of 150 s.
Electronic tongue (Intelligent Sensor Technology Co., Kanagawa, Japan) containing eight sensors (sourness, bitterness, astringency, aftertaste-B, aftertaste-A, umami, richness, and saltiness) was used to determine the taste of samples according to Feng et al. (2018). Fifty grams of samples and 100 mL of distilled water were ground and put into the centrifuge tube and then centrifuged at 3500×g for 20 min. Supernatants were used for the analysis by electronic tongue.
Water Status
Water status of samples during storage was tested according to Cheng et al. (2014). The LF-NMR (low-field nuclear magnetic resonance) instrument (Niumag Co., Shanghai, China) at 23 MHz was utilized for the experimental analysis. Samples were placed into a tube and then put into the magnet chamber. The measurement of T2 (transverse relaxation time) was conducted by Carre-Purcelle-Meiboome-Gill. MRI (magnetic resonance imaging) was utilized for observing proton density images of fan-shaped samples.
Statistical Analysis
Analysis of variance was performed by Duncan’s test using SPSS 19 statistics software. The results of data were represented as mean ± standard deviations. Difference was significant among treatments at P < 0.05.
Results and Discussion
Microbial Analysis
Figure 2 shows the change in total bacterial count and mold and yeast of fresh-cut cucumber at the storage period. Total bacterial count and mold and yeast of all samples exhibited an increasing trend with prolonging storage time. At the 15th day of storage, it can be seen from Fig. 2a that total bacterial count in samples was 6.72 log CFU g−1 for control, 5.88 log CFU g−1 for US, 5.96 log CFU g−1 for CH, 5.53 log CFU g−1 for CDs coating, and 5.18 log CFU g−1 for US+CDs. It was observed from Fig. 2b that mold and yeast in the samples were 4.71 log CFU g−1 for control, 4.02 log CFU g−1 for US, 4.16 log CFU g−1 for CH, 3.75 log CFU g−1 for CDs coating, and 3.45 log CFU g−1 for US+CDs. Total bacterial count and mold and yeast of samples treated by US, CH, CDs coating, and US+CDs were significantly (P < 0.05) lower compared with control. Especially, US+CDs treatment exhibited better bacteriostatic effect than other treatments. Hashemi (2018) found the reduction of bacteria and fungi number in plums treated with US. This may be because mechanical and chemical effects by US treatment helped reduce microbial load (Cao et al. 2010). Saxena et al. (2011) reported that chitosan coating in combination with modified atmosphere packaging can inhibit the microbial growth of fresh-cut jackfruit bulb at the storage period. The possible reason is that CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating helped improve the antimicrobial activity against total number of colonies, mold, and yeast based on the antimicrobial activity of CDs as reported by Fan et al. (2019a). Fan et al. (2019a) also reported the consistent results; they obtained that CDs coating exhibited good effect on inhibiting microbial growth of cucumber. Thus, CDs coating formed protective film to prevent microbial growth during storage. These results illustrated that US combined with CDs coating could effectively control microbial growth during storage.
Respiration Rate and Weight Loss
Respiration rate indicates the change in metabolic activities, which can reflect the quality deterioration of fresh-cut cucumber (Meng et al. 2012). Figure 3 a shows the change in respiration rate of samples with different treatments at the preservative period. Respiration rate of samples first raised and then descended during storage. After the third days of storage, respiration rate of samples presented the maximum value, which was 8.92 mg kg−1 h−1 CO2 for control, 8.47 mg kg−1 h−1 CO2 for US, 8.29 mg kg−1 h−1 CO2 for CH, 8.02 mg kg−1 h−1 CO2 for CDs coating, and 7.67 mg kg−1 h−1 CO2 for US+CDs, respectively. Respiration rate of samples through US, CH, CDs coating, and US+CDs treatments was significantly (P < 0.05) lower compared with control. Respiration rate (4.67 mg kg−1 h−1 CO2) of samples treated by US+CDs was markedly lower than that of other treatments, exhibiting that US+CDs treatment presented a superior effect on inhibiting respiration rate of cucumber. Cao et al. (2010) found that ultrasound treatment for 10 min was effective in reducing microbial numbers. This may be because US treatment could help limit water mobility and enzyme activity in the cucumber tissue, causing the reduction of respiratory rate (Li et al. 2017). Meng et al. (2013) also found that US combined with nano-ZnO coating effectively reduced carbon dioxide production during respiratory metabolism. Cosme Silva et al. (2017) presented that chitosan coating decreased respiration rate of mango during 15 days of storage. The possible reason is that CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating exhibited good film-forming properties, increasing surface crystallization degree, crack, and roughness (Chen et al. 2016). CDs coating formed micro atmosphere on the sample surfaces to modify the endogenous gas exchange, thereby reducing respiration rate (Meng et al. 2013; Cosme Silva et al. 2017). These results demonstrated that synergistic treatment of US and CDs coating suppressed respiration rate of the fresh-cut cucumber.
Weight loss relates to the loss of water and carbon dioxide production at the respiratory process (Shen et al. 2019). Figure 3 b shows the change in weight loss of fresh-cut cucumber at the preservative period. Weight loss of all samples gradually increased during storage. Compared with control, weight loss of samples subjected to US, CH, CDs coating, and US+CDs treatments presented a significant (P < 0.05) reduction. Weight loss of samples subjected to US+CDs treatment was the lowest value of 8.54% during 15 days of storage. According to the literature, Feng et al. (2018) also found consistent results that US decreased the loss of water in cucumber with controlled atmosphere during storage. This is because US treatment decreased metabolic rate causing the reduction of water loss (Fan et al. 2019b). Eshghi et al. (2014) found that nanochitosan coating displayed low weight loss of strawberry at the storage period of 20 days. Similarly, Muftuoğlu et al. (2010) presented that chitosan coating and modified atmosphere packaging reduced the loss of mass (below 1%). This is because CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating increased surface crystallization degree, which form good barrier function to decrease water vapor permeability (Gao et al. 2018). Additionally, CDs coating also helped reduce the respiration rate of samples (Feng et al. 2018). These results exhibited that US combined with CDs coating treatment limited water mobility and water vapor transmission of samples, thereby reducing weight loss.
TSS and Firmness
TSS reflects the change in soluble sugars of fresh-cut cucumber (Gani et al. 2016). Figure 4 a presents the change in TSS of samples during 15 days of storage. It can be seen that TSS in all samples generally decreased during storage. The decrease of TSS indicated that the product was aging. TSS participated in the carbohydrate metabolism of cells (Barman et al. 2014). Thus, the carbohydrate metabolism of fresh-cut cucumber caused the reduction of TSS. TSS value (2.29 °Brix) of samples treated by US+CDs was significantly (P < 0.05) higher compared with other treatments during 15 days of storage. Cao et al. (2010) also found that US treatment delayed the decrease of TSS in strawberry. This may be because the chemical and mechanical effects of US treatment inhibited enzyme activity related to carbohydrate metabolism, thereby maintaining high TSS level (Fan et al. 2019b). Eshghi et al. (2014) reported that nanochitosan-coated strawberry exhibited good effect on retarding the reduction of TSS. The reason is that CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating exhibited good barrier properties to reduce the gas exchange between the samples and the atmosphere, resulting in lower metabolic rates, causing a slowing of the hydrolysis of carbohydrates (Cosme Silva et al. 2017). These results indicated that US combined with CDs treatment helped decrease the metabolism of carbohydrates leading to a high TSS level.
Firmness is a critical quality parameter involving cell wall strength in fresh-cut cucumber (Chen and Zhu 2011). Figure 4 b exhibits the change in firmness of samples during 15 days of storage. It was observed that the firmness of all samples reduced with prolonging storage time. For control, firmness was 3.94 N, which was reduced by 59.04% at the 15th day of storage. Compared with control, firmness of samples treated by US, CH, CDs coating, and US+CDs was significantly (P < 0.05) higher. Firmness of US+CDs treated samples had maximum value of 6.78 N, which was reduced by 27.87% after 15 days of storage. Hashemi (2018) found that ultrasound treatment maintained higher level of firmness in plum fruit during 10 days of storage. This may be because US treatment reduced water mobility and enzyme activity, thereby delaying the loss of firmness (Feng et al. 2018). Zhang et al. (2016) also found that cherry tomato subjected to rice bran wax coating presented high firmness during 20 days of storage. This is because CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating increased surface crystallization degree crack and roughness, which helped form a semipermeable film to reduce O2 availability and metabolic rate, thereby maintaining the firmness of samples (Gao et al. 2018). These results demonstrated that US combined with CDs treatment is beneficial to maintain the higher firmness during storage.
Peroxidases and Polyphenol Oxidase Activities
Peroxidases (POD) and polyphenol oxidase (PPO) activities promote browning resulting in the short storage period of the products (Carvalho et al. 2016). Figure 5 shows the changes in POD and PPO activities of fresh-cut cucumber during storage. Figure 5 a indicates that POD activity of all samples first raised and then descended. Compared with control, POD activity for US, CH, CDs coating, and US+CDs treatments was significantly (P < 0.05) lower during storage. At the 6th day of storage, maximum value of POD activity was 187.67 U kg−1 s−1 for control. At the 9th day of storage, maximum value of POD activity was 172.2 U kg−1 s−1 for CH, 167.83 U kg−1 s−1 for CDs coating, 164.5 U kg−1 s−1 for US, and 155.67 U kg−1 s−1 for US +CDs, respectively. POD activity of samples subjected to US+CDs treatment had lower value of 139.83 U kg−1 s−1 compared with that of other treatments after the 15 days of storage. Wang and Fan (2019) reported that ultrasound treatment could decrease POD activity during 20 days of storage. The main reason for the inhibition in POD activity is that free radicals by US treatment may react with the amino acids of enzyme structure reducing POD activity (Wang et al. 2015). Gao et al. (2018) also found that cinnamaldehyde-chitosan coating improved POD activity of navel orange during 120 days of storage. The possible reason is that CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating increased barrier function to reduce the oxygen exchange, which restricted oxidation processes, thereby inhibiting the POD activity (Batista et al., 2018). These results exhibited that a combined treatment of mechanical and chemical effects by US and the formation of protective barrier by CDs coating reduced POD activity (Zhang et al. 2017; Batista et al., 2018).
As can be seen from Fig. 5b, PPO activity of all samples first raised and then descended during storage. For US +CDs treatment, PPO activity presented a significant (P < 0.05) reduction compared with control. At the 6th day of storage, maximum value of PPO activity was respectively 184.67 U kg−1 s−1 for control, 179.8 U kg−1 s−1 for CH, 176 U kg−1 s−1 for CDs coating treatment, 167.5 U kg−1 s−1 for US treatment, and 160.83 U kg−1 s−1 for US +CDs treatment. PPO activity of US+CDs treated samples had lower value of 137.17 U kg−1 s−1 compared with other treatments at the 15th day of storage. Li et al. (2017) presented that ultrasound treatment for 10 min lowered PPO activity of mushroom during storage. The main reason for the inhibition in PPO activity is that free radicals produced by US treatment cause the change of enzyme structure resulting in the inhibition of PPO activity (Wang et al. 2015). Galindo-Pérez et al. (2015) also found that xanthan gum nanoparticle coating exhibited good effect on inhibiting enzyme activities. This is because CDs coating may also play a role in inhibiting PPO activity. CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating increased good barrier properties, thereby reducing oxygen availability, leading to the reduction of oxidative reactions (Fan et al. 2019a). These results indicated that US affected the structure and activity of PPO, and CDs coating reduced the oxygen exchange to limit oxidation processes, thereby reducing PPO activity (Li et al. 2017; Batista et al., 2018).
Ascorbic Acid and MDA Contents
Figure 6 shows the change in ascorbic acid and MDA contents of fresh-cut cucumber at the storage period of 15 days. Figure 6 a indicates that ascorbic acid content gradually reduced during storage. Compared with control, ascorbic acid content in samples treated by US, CDs coating, and US+CDs was significantly (P < 0.05) higher. At the 15th day of storage, ascorbic acid content was reduced by 46.87% for control, 38.14% for CH, 35.84% for US, 31.83% for CDs coating, and 26.59% for US+CDs. Ascorbic acid content of US+CDs treated samples had higher value of 0.0243 g kg−1 compared with other treatments at the 15th day of storage. Hashemi (2018) reported that ultrasound treatment increased the retention of ascorbic acid content in Mirabelle plum. This may be because US treatment reduced microbial load of samples, thereby decreasing the loss of ascorbic acid (Cao et al. 2010). Souza et al. (2014) presented that nanomultilayer-coated fresh-cut mango maintained high ascorbic acid content during 14 days of storage. This is because CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating created good barrier film to limit gas exchange, which caused the reduction of ascorbic acid oxidation. In addition, CDs coating lowered respiration rate to reduce the consumption of ascorbic acid (Carvalho et al. 2016; Hashemi 2018). These results suggested that US combined with CDs coating treatment helped reduce microbial load and gas exchange, thereby reducing the loss of ascorbic acid.
Malondialdehyde (MDA) reflects lipid peroxidation related to the permeability and integrity of membrane (Sothornvit and Kiatchanapaibul 2009). Figure 6 b presents that MDA content raised as storage period prolonged. MDA content of samples treated by US, CH, CDs coating, and US+CDs was significantly (P < 0.05) lower compared with control during preservation. After storage of 15 days, MDA content of samples was 3.43 μmol kg−1 for control, 3.04 μmol kg−1 for US treatment, 2.95 μmol kg−1 for CH, 2.74 μmol kg−1 for CDs coating treatment, and 2.24 μmol kg−1 for US+CDs treatment, respectively. MDA content for US+CDs treatment had the lowest value. Li et al. (2017) found that ultrasound treatment could inhibit the increase of MDA content in mushrooms during storage. This may be because US treatment reduced water mobility and enzyme activity, thus reducing the accumulation of MDA content. Gao et al. (2018) reported that cinnamaldehyde-chitosan coating could repress the increase of MDA content of navel orange. The reason is that CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating created good barrier function to reduce membrane lipid peroxidation causing the reduction of MDA content (Meng et al. 2013). These results demonstrated that combined treatment of US and CDs coating can effectively reduce the accumulation of MDA content in samples during storage, exhibiting that US combined with CDs coating treatment maintained high membrane integrity and delayed the aging of fresh-cut cucumber.
Flavor and Taste
Figure 7 a shows the 14 sensors changes in flavor of fresh-cut cucumber during 15 days of storage. Figure 7 a presents that sensors S1 and S5 of all samples show more changes in comparison with other sensors. Sensors S1 and S5 displayed volatile compounds including aromatic compounds, alcohols, ketones, and aldehydes as shown in Table 1. Compared with control, S1 and S5 responding values for US, CH, CDs coating, and US+CDs treatments were significantly (P < 0.05) lower. Especially, US+CDs treatment had the lowest responding values of S1 and S5. This indicated that US+CDs treatment reduced the deterioration of volatile compounds including aromatic compounds, alcohols, ketones, and aldehydes after 15 days of storage. Hence, US combined with CDs coating can effectively decrease the degradation of flavor of fresh-cut cucumber during storage. Similar results were reported by Feng et al. (2018), who presented that US and modified atmosphere packaging can preserve the flavor of cucumber well. This may be because US treatment reduced microbial load, thereby preserving the flavor (Fan et al. 2019b). Additionally, CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating increased barrier properties, which helped form a micro-atmosphere environment to reduce the loss of flavor (Fan et al. 2019a).
Figure 7 b shows eight taste substances changes in fresh-cut cucumber by electronic tongue during storage of 15 days. It can be seen that the sourness and astringency of samples treated by US, CH, CDs coating, and US+CDs was significantly (P < 0.05) lower in comparison with control. Compared with other treatments, sourness and astringency for US+CDs was lower. Bitterness, astringency, aftertaste-A, aftertaste-B umami, richness, and saltiness for all treatments had no marked change in samples. Feng et al. (2018) found that ultrasound treatment preserved the characteristic taste of cucumber 25 days of during storage. This may be because US treatment reduced microbial load, thus reducing the loss of taste (Feng et al. 2018). Moreover, CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating formed good barrier function to reduce gas exchange and respiratory metabolism, which helped preserve the taste of samples (Arnon et al. 2015). These results illustrated that US combined with CDs coating treatment can effectively preserve the taste components during storage of fresh-cut cucumber.
Water Status
Water status of cucumber is presented in Fig. 8. It can be seen that T2 distribution curve presented three signal peaks. Relaxation time range of T21, T22, and T23 was respectively at 1–20 ms, 50–300 ms, and 400–1500 ms. According to the report of Feng et al. (2018), T21, T22, and T23 was respectively the state of bound water, water present in the cytoplasm, and free water. A21, A22, and A23 present the percentage of water fraction in the above three water status.
Table 2 shows the T2 and A2 of fresh-cut cucumber at the storage period of 15 days. T21, T22, and T23 values of all samples first raised and then descended during storage. There is significant difference (P < 0.05) in T21, T22, and T23 values for all treatments during 15 days of storage. After 15 days of storage, compared with other treatments, US+CDs treated samples had lower value for T21, T22, and T23. This may be due to the fact that US treatment reduced membrane permeability and enzyme activity in samples, thereby limiting the activity of metabolism (Fan et al. 2019b). In addition, CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating formed good barrier function, which formed a micro-atmosphere environment on the surface of samples to decrease the gas exchange and respiratory metabolism (Fan et al. 2019a). Such a micro-atmosphere environment reduced water mobility and metabolic activity of fresh-cut cucumber (Feng et al. 2018). As shown in Table 2, there is significant difference (P < 0.05) in A21, A22, and A23 values for all treatments during 15 days of storage. A21 and A22 first raised and then descended at the storage period. In addition, A23 first descended and then raised. These results demonstrated that US combined with CDs coating treatment helped reduce the change and redistribution of water status in fresh-cut cucumber during storage.
Figure 9 presents pseudo-color images of samples at the storage period. Pseudo-color images used different colors to indicate the density of hydrogen proton. The redder the color, the higher the proton density. Pseudo-color images can directly observe the water distribution of samples using MRI. As shown in Fig. 9, at the 15th day of storage, signal intensity of proton for US, CH, CDs coating, and US+CDs treated samples was higher compared with control. Especially, proton in samples treated by US+CDs exhibited higher signal intensity in comparison with other treatments. The higher the water content, the higher the proton density (Shen et al. 2019). Wang et al. (2018) found that signal intensity of carrot slice through ultrasound treatment is brighter using MRI. This may be because ultrasound treatment reduced the change of water status and retarded the aging (Fan et al. 2019b). Moreover, CDs containing abundant functional groups had excellent biocompatibility. CDs added to CH coating formed good barrier function to reduce the gas exchange and respiratory metabolism (Fan et al. 2019a). These results demonstrated that US+CDs treatment maintained higher moisture and reduced water mobility.
Conclusion
Ultrasound combined with carbon dots coating could improve microbial and physicochemical quality of fresh-cut cucumber including total bacterial count, mold and yeast, respiration rate, weight loss, total soluble solids, firmness, peroxidases activity, polyphenol oxidase activity, malondialdehyde content, ascorbic acid content, flavor, taste, and water status during storage for 15 days. Ultrasound combined with carbon dots coating exhibited superior preservation effect than either of the individual treatment. This indicated that ultrasound combined with carbon dots coating treatment is an effective method for maintaining the quality of fresh-cut cucumber during storage for 15 days.
References
Aday, M. S., & Caner, C. (2014). Individual and combined effects of ultrasound, ozone and chlorine dioxide on strawberry storage life. LWT - Food Science and Technology, 57(1), 344–351.
An, J., Min, Z., Lu, Q., & Zhang, Z. (2006). Effect of a prestorage treatment with 6-benzylaminopurine and modified atmosphere packaging storage on the respiration and quality of green asparagus spears. Journal of Food Engineering, 77(4), 951–957.
Arnon, H., Zaitsev, Y., Porat, R., & Poverenov, E. (2014). Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biology and Technology, 87, 21–26.
Arnon, H., Granit, R., Porat, R., & Poverenov, E. (2015). Development of polysaccharides-based edible coatings for citrus fruits: a layer-by-layer approach. Food Chemistry, 166, 465–472.
Barman, K., Siddiqui, M. W., Patel, V. B., & Prasad, M. (2014). Nitric oxide reduces pericarp browning and preserves bioactive antioxidants in litchi. Scientia Horticulturae, 171(171), 71–77.
Batista, S. W., Cosme Silva, G. M., Santana, D. B., Salvador, A. R., Medeiros, D. B., Belghith, I., da Silva, N. M., Cordeiro, M. H. M., & Misobutsi, G. P. (2018). Chitosan delays ripening and ROS production in guava (Psidium guajava L.) fruit. Food Chemistry, 242, 232–238.
Cao, S., Hu, Z., Pang, B., Wang, H., Xie, H., & Wu, F. (2010). Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control, 21(4), 529–532.
Cao, X., Cai, C., Wang, Y., & Zheng, X. (2018). The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innovative Food Science & Emerging Technologies, 45, 169–178.
Carvalho, R. L., Cabral, M. F., Germano, T. A., de Carvalho, W. M., Brasil, I. M., Gallão, M. I., Moura, C. F. H., Lopes, M. M. A., & de Miranda, M. R. A. (2016). Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharvest Biology and Technology, 113, 29–39.
Chen, Z., & Zhu, C. (2011). Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit ( Prunus salicina L.). Postharvest Biology and Technology, 61(2), 117–123.
Chen, H., Hu, X., Chen, E., Wu, S., McClements, D. J., Liu, S., Li, B., & Li, Y. (2016). Preparation, characterization, and properties of chitosan films with cinnamaldehyde nanoemulsions. Food Hydrocolloids, 61, 662–671.
Chen, H. Z., Zhang, M., Bhandari, B., & Guo, Z. (2018). Evaluation of the freshness of fresh-cut green bell pepper (Capsicum annuum var. grossum) using electronic nose. LWT - Food Science and Technology, 87, 77–84.
Cheng, X.-F., Zhang, M., Adhikari, B., & Islam, M. N. (2014). Effect of power ultrasound and pulsed vacuum treatments on the dehydration kinetics, distribution, and status of water in osmotically dehydrated strawberry: a combined NMR and DSC study. Food and Bioprocess Technology, 7(10), 2782–2792.
Cosme Silva, G. M., Silva, W. B., Medeiros, D. B., Salvador, A. R., Cordeiro, M. H. M., da Silva, N. M., Santana, D. B., & Mizobutsi, G. P. (2017). The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chemistry, 237, 372–378.
Eshghi, S., Hashemi, M., Mohammadi, A., Badii, F., Mohammadhoseini, Z., & Ahmadi, K. (2014). Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria x ananassa Duchesne) during storage. Food and Bioprocess Technology, 7(8), 2397–2409.
Fan, K., Zhang, M., Fan, D., & Jiang, F. (2019a). Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified atmospheric packaged fresh-cut cucumber. Journal of the Science of Food and Agriculture, 99(13), 6032–6041.
Fan, K., Zhang, M., & Jiang, F. (2019b). Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: influence on microbial inhibition and storage quality. Ultrasonics Sonochemistry, 54, 162–170.
Feng, L., Zhang, M., Adhikari, B., & Guo, Z. (2018). Effect of ultrasound combined with controlled atmosphere on postharvest storage quality of cucumbers (Cucumis sativus L.). Food and Bioprocess Technology, 11(7), 1328–1338.
Fonteles, T. V., Leite, A. K., Silva, A. R., Carneiro, A. P., Miguel, E. C., Cavada, B. S., Fernandes, F. A., & Rodrigues, S. (2016). Ultrasound processing to enhance drying of cashew apple bagasse puree: influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrasonics Sonochemistry, 31, 237–249.
Galindo-Pérez, M. J., Quintanar-Guerrero, D., Mercado-Silva, E., Real-Sandoval, S. A., & Zambrano-Zaragoza, M. L. (2015). The effects of tocopherol nanocapsules/xanthan gum coatings on the preservation of fresh-cut apples: evaluation of phenol metabolism. Food and Bioprocess Technology, 8(8), 1791–1799.
Gani, A., Baba, W. N., Ahmad, M., Shah, U., Khan, A. A., Wani, I. A., Masoodi, F. A., & Gani, A. (2016). Effect of ultrasound treatment on physico-chemical, nutraceutical and microbial quality of strawberry. LWT - Food Science and Technology, 66, 496–502.
Gao, Y., Kan, C., Wan, C., Chen, C., Chen, M., & Chen, J. (2018). Quality and biochemical changes of navel orange fruits during storage as affected by cinnamaldehyde -chitosan coating. Scientia Horticulturae, 239, 80–86.
González-Aguilar, G. A., Valenzuela-Soto, E., Lizardi-Mendoza, J., Goycoolea, F., Martínez-Téllez, M. A., Villegas-Ochoa, M. A., Monroy-García, I. N., & Ayala-Zavala, J. F. (2009). Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol’. Journal of the Science of Food and Agriculture, 89(1), 15–23.
Harish, R., Nisha, K. D., Prabakaran, S., Sridevi, B., Harish, S., Navaneethan, M., Ponnusamy, S., Hayakawa, Y., Vinniee, C., & Ganesh, M. R. (2020). Cytotoxicity assessment of chitosan coated CdS nanoparticles for bio-imaging applications. Applied Surface Science, 499, 143817.
Hashemi, S. M. B. (2018). Effect of pulsed ultrasound treatment compared to continuous mode on microbiological and quality of Mirabelle plum during postharvest storage. International Journal of Food Science & Technology, 53(3), 564–570.
Hong, K., Xie, J., Zhang, L., Sun, D., & Gong, D. (2012). Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Scientia Horticulturae, 144, 172–178.
Huang, L. L., Zhang, M., Yan, W. Q., Mujumdar, A. S., & Sun, D. F. (2009). Effect of coating on post-drying of freeze-dried strawberry pieces. Journal of Food Engineering, 92(1), 107–111.
Islam, M. N., Zhang, M., Adhikari, B., Cheng, X., & Xu, B. G. (2014). The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. International Journal of Refrigeration, 42(3), 121–133.
Lagnika, C., Zhang, M., & Mothibe, K. J. (2013). Effects of ultrasound and high pressure argon on physico-chemical properties of white mushrooms (Agaricus bisporus) during postharvest storage. Postharvest Biology and Technology, 82(4), 87–94.
Li, N., Chen, F., Cui, F., Sun, W., Zhang, J., Qian, L., Yang, Y., Wu, D., Dong, Y., & Jiang, J. (2017). Improved postharvest quality and respiratory activity of straw mushroom (Volvariella volvacea) with ultrasound treatment and controlled relative humidity. Scientia Horticulturae, 225, 56–64.
Li, L.-S., Jiao, X.-Y., Zhang, Y., Cheng, C., Huang, K., & Xu, L. (2018). Green synthesis of fluorescent carbon dots from Hongcaitai for selective detection of hypochlorite and mercuric ions and cell imaging. Sensors and Actuators B: Chemical, 263, 426–435.
Lu, D., Zhang, M., Wang, S., Cai, J., Xiang, Z., & Zhu, C. (2010). Nutritional characterization and changes in quality of Salicornia bigelovii Torr. during storage. LWT - Food Science and Technology, 43(3), 519–524.
Meng, X., Zhang, M., & Adhikari, B. (2012). Extending shelf-life of fresh-cut green peppers using pressurized argon treatment. Postharvest Biology and Technology, 71, 13–20.
Meng, X., Zhang, M., & Adhikari, B. (2013). The effects of ultrasound treatment and nano-zinc oxide coating on the physiological activities of fresh-cut kiwifruit. Food and Bioprocess Technology, 7(1), 126–132.
Meng, X., Zhang, M., Zhan, Z., & Adhikari, B. (2014). Changes in quality characteristics of fresh-cut cucumbers as affected by pressurized argon treatment. Food and Bioprocess Technology, 7(3), 693–701.
Millan-Sango, D., McElhatton, A., & Valdramidis, V. P. (2015). Determination of the efficacy of ultrasound in combination with essential oil of oregano for the decontamination of Escherichia coli on inoculated lettuce leaves. Food Research International, 67, 145–154.
Mohammadi, A., Hashemi, M., & Hosseini, S. M. (2016). Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innovative Food Science and Emerging Technologies, 33, 580–588.
Mothibe, K. J., Zhang, M., Nsoratindana, J., & Wang, Y. C. (2011). Use of ultrasound pretreatment in drying of fruits: drying rates, quality attributes, and shelf life extension. Drying Technology, 29(14), 1611–1621.
Muftuoğlu, F., Ayhan, Z., & Esturk, O. (2010). Modified atmosphere packaging of kabaaşı apricot (Prunus armeniaca L. ‘Kabaaşı’): effect of atmosphere, packaging material type and coating on the physicochemical properties and sensory quality. Food and Bioprocess Technology, 5(5), 1601–1611.
Pinheiro, J., Alegria, C., Abreu, M., Gonçalves, E. M., & Silva, C. L. M. (2013). Kinetics of changes in the physical quality parameters of fresh tomato fruits (Solanum lycopersicum , cv. ‘Zinac’) during storage. Journal of Food Engineering, 114(3), 338–345.
Pinheiro, J., Alegria, C., Abreu, M., Gonçalves, E. M., & Silva, C. L. M. (2015). Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum , cv. Zinac) quality and microbial load during storage. Ultrasonics Sonochemistry, 27, 552–559.
Sagong, H.-G., Lee, S.-Y., Chang, P.-S., Heu, S., Ryu, S., Choi, Y.-J., & Kang, D.-H. (2011). Combined effect of ultrasound and organic acids to reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. International Journal of Food Microbiology, 145(1), 287–292.
Saxena, A., Saxena, T. M., Raju, P. S., & Bawa, A. S. (2011). Effect of controlled atmosphere storage and chitosan coating on quality of fresh-cut jackfruit bulbs. Food and Bioprocess Technology, 6(8), 2182–2189.
Shen, X., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biology and Technology, 149, 159–165.
Song, Z., Li, F., Guan, H., Xu, Y., Fu, Q., & Li, D. (2017). Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control, 74, 34–44.
Sothornvit, R., & Kiatchanapaibul, P. (2009). Quality and shelf-life of washed fresh-cut asparagus in modified atmosphere packaging. LWT - Food Science and Technology, 42(9), 1484–1490.
Souza, M. P., Vaz, A. F. M., Cerqueira, M. A., Texeira, J. A., Vicente, A. A., & Carneiro-da-Cunha, M. G. (2014). Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food and Bioprocess Technology, 8(3), 647–654.
Vivek, K., Subbarao, K. V., & Srivastava, B. (2016). Optimization of postharvest ultrasonic treatment of kiwifruit using RSM. Ultrasonics Sonochemistry, 32, 328–335.
Wang, J., & Fan, L. (2019). Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrasonics Sonochemistry, 58, 104631.
Wang, W., Ma, X., Zou, M., Jiang, P., Hu, W., Li, J., Zhi, Z., Chen, J., Li, S., & Ding, T. (2015). Effects of ultrasound on spoilage microorganisms, quality, and antioxidant capacity of postharvest cherry tomatoes. Journal of Food Science, 80(10), C2117–C2126.
Wang, L., Xu, B., Wei, B., & Zeng, R. (2018). Low frequency ultrasound pretreatment of carrot slices: effect on the moisture migration and quality attributes by intermediate-wave infrared radiation drying. Ultrasonics Sonochemistry, 40, 619–628.
Wu, Z. S., Zhang, M., & Wang, S. (2012). Effects of high pressure argon treatments on the quality of fresh-cut apples at cold storage. Food Control, 23(1), 120–127.
Xin, Y., Zhang, M., & Adhikari, B. (2013). Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.). Journal of Food Engineering, 119(3), 640–647.
Xin, T. Z., Ananthanarayanan, A., Luo, K. Q., & Peng, C. (2015). Glowing graphene guantum dots and carbon dots: properties, syntheses, and biological applications. Small, 11(14), 1620–1636.
Yang, Z., Cao, S., Cai, Y., & Zheng, Y. (2011). Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innovative Food Science and Emerging Technologies, 12(3), 310–314.
Yang, J., Zhang, X., Ma, Y. H., Gao, G., Chen, X., Jia, H. R., Li, Y. H., Chen, Z., & Wu, F. G. (2016). Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Applied Materials & Interfaces, 8(47), 32170–32181.
Zhang, L., Chen, F., Zhang, P., Lai, S., & Yang, H. (2016). Influence of rice bran wax coating on the physicochemical properties and pectin nanostructure of cherry tomatoes. Food and Bioprocess Technology, 10(2), 349–357.
Zhang, Z., Niu, L., Li, D., Liu, C., Ma, R., Song, J., & Zhao, J. (2017). Low intensity ultrasound as a pretreatment to drying of daylilies: impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrasonics Sonochemistry, 36, 50–58.
Funding
This study received financial support from National Key R&D Program of China (No. 2018YFD0700303), Yangzhou City Agricultural Key R&D Program (No.YZ2019034), the 111 Project (BP0719028), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX19_1802), Jiangsu Province (China) Key Project in Agriculture (Contract No. BE2015310217), Jiangsu Province (China)“Collaborative Innovation Center for Food Safety and Quality Control”Industry Development Program, and National First-class Discipline Program of Food Science and Technology (No. JUFSTR20180205), all of which enabled us to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, K., Zhang, M. & Chen, H. Effect of Ultrasound Treatment Combined with Carbon Dots Coating on the Microbial and Physicochemical Quality of Fresh-Cut Cucumber. Food Bioprocess Technol 13, 648–660 (2020). https://doi.org/10.1007/s11947-020-02424-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02424-x