Abstract
The changes in gel properties and myofibrillar protein structure of Trichiurus haumela surimi subjected to high pressure (300–450 MPa; 5 min) or high pressure synergistic heat (90 °C; 30 min) treatment were investigated. The results showed that high pressure below 400 MPa could improve the gel properties. The water holding capacity of the 350 MPa group increased by 27.40% compared with the control group. Moreover, the gel strength of surimi gel induced by high pressure of 300 MPa and 350 MPa increased by 38.08% and 40.00%, respectively, and continually increased by 17.86% and 21.00% after further heating. Both of high pressure and heating affected the composition of myofibrillar protein and partially degraded the actin and myosin heavy chain. High pressure reduced the contents of α-helix and β-turn, but increased β-sheet and random coil in the myofibrillar protein. Subsequent heat treatment converted the partial β-sheet into the α-helix structure. In addition, in the gels of surimi subjected to high pressure, the secondary structure of myofibrillar protein was significantly correlated with the whiteness, gel strength, and texture characteristics of the gels. Collectively, the results supported an optimum pressure of 300–350 MPa for the induction of hairtail surimi gel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hairtail (Trichiurus haumela) is an important catch fish with annual catch of 1,010,000 tons in China, and nearly one-third of the caught hairtail resources are small-sized fishes. These small-sized hairtail, with cheap prices and high yields, are important raw materials for the production of surimi. Myofibrillar protein is the main protein of surimi and plays an important role in the quality of surimi products because of its excellent gel-forming ability and water-holding capacity (WHC) (Cando et al. 2015). However, due to the low content of myofibrillar protein in the hairtail, the gel strength of heat-induced hairtail surimi gel is relatively low. Therefore, it is necessary to modify the structure of myofibrillar protein and improve its gel-forming ability.
High pressure processing (HPP) has received interest as an alternative technology to conventional thermal treatment, in terms of texture improvement, safety, and quality attributes in minimally processed food products (Li et al. 2019; Li et al. 2012). It was reported that HPP could change the structure of protein by different extents of unfolding and denaturation, which altered the functional properties of protein, such as coagulation, aggregation, or gelation (Ma et al. 2015). Meanwhile, Moreno et al. (2015) reported that HPP increased the springiness of flying fish surimi gel and the springiness reached a maximum at a pressure of 200 MPa, while the mechanical strength of surimi gel was independent of the applied pressure in the further study on the effect of pressure-heat synergy. Hence, while studying the HPP to improve the gel properties of the hairtail surimi, it is necessary to further understand the effect and modification mechanism of high pressure or high pressure synergistic heat on the myofibrillar protein and gel properties.
The present study was designed to evaluate the effects of high pressure and high pressure synergistic heat treatments on the myofibrillar conformation of hairtail surimi by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and circular dichroism (CD) spectroscopy, and to investigate the correlation between the surimi gel properties and the structure of myofibrillar protein.
Materials and Methods
Materials
Commercially frozen hairtail surimi (AA grade, cryoprotectants were 4% sucrose, 5% sorbitol, and 0.25% polyphosphate) was obtained from Ningbo Feiri Aquatic Industry Co., Ltd. (Ningbo, China). The surimi samples were vacuum packed in polyethylene (PE) bags (about 250 g per bag) and stored at − 80 °C until use. Moisture content was 74.3% and crude protein content was 15.0%.
All the chemicals used were of analytical grade. Bovine serum albumin (BSA) was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). The remaining chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
HPP Treatments
The Plunger Press system with a maximum operating pressure of 500 MPa (CQC2L-600, Beijing Speedway Zhongtian Co., Ltd., Beijing, China) was used to process the vacuum packed PE bags with surimi. The pressure come-up time was 1 min, adiabatic temperature was 18 ± 2 °C, and depressurization time was 5 s. The surimi samples were treated by different high pressure of 300, 350, 400, or 450 MPa for 5 min using water as a medium, at 18 ± 2 °C; for comparison, hairtail surimi in the control group without high pressure treatment and the corresponding pressure was 0.1 MPa.
Preparation of Surimi Gel
A 500 g of each pressure processed surimi was cut into small pieces, and chopped in a Stephan vertical vacuum cutter (Model UM 5, Stephan Machinery Co., Hamelin, Germany) for 2 min. Iced water was added to adjust the moisture content to 75%, then the sodium chloride (2.5 wt%) was added and continuously chopped for 8 min at a pressure of 0.5 bar to remove the air bubble generated during the chopping process. The entire chopping process was carried out below 10 °C. After chopping, each sample was individually poured into the plastic casing (2.5 cm in diameter) and the ends of the surimi sausages were tightened, respectively.
Half of the surimi sausages were cold-setting at 4 °C for 12 h to obtain the gels of P group which were subjected to simple high pressure. The other half of the surimi sausages were heat-setting at 90 °C for 30 min followed by cooling at 4 °C for 12 h to obtain the gels of PH group which were subjected to high pressure synergistic heat. Table 1 showed the sample codes, pressure, and treatment. All prepared gels were stored at 4 °C and all indicator analyses were performed within 24 h after sample preparation.
Determination of WHC
WHC was determined according to Barrera et al. (2002). The gels were manually cut into a thickness of 2 mm, weighed (W1), and set into two layers of filter paper. The samples were then centrifuged at 3000g for 15 min and immediately weighed again (W2). WHC was expressed as the ratio of gel weight after centrifugation (W2) to the initial gel weight (W1) and calculated according to flowing Eq. (1):
Determination of Whiteness
A colorimeter (CR-400, Konica Minolta, INC., Japan) was used to determine the L* (lightness), a* (redness/greenness), and b* (yellowness/blueness) values of the gels. The whiteness was then calculated using the following equation (Petcharat and Benjakul 2018) (2):
Determination of Textural Properties
The gels were equilibrated at room temperature (20 °C) for about 30 min before the tests of gel strength and texture profile analysis (TPA), and then cut to 2.5 cm in length and 2.5 cm in width. The textural properties of heat-induced gels were analyzed using a texture analyzer (TA. XT. Plus, Stable microsystems Co., UK.).
The gel strength was determined according to the method described by Jin et al. (2011). The gels were subject to a compression test using a spherical probe (P/0.5S, 1.0 mm/s depression speed) to evaluate the breaking force (g) and deformation (mm) of surimi gels. The gel strength (g·mm) was calculated as the product of the breaking force and deformation (Lanier 1996).
TPA tests of the gels were carried out according to the method of Kaewudom et al. (2013) with a slight modification. The gels were compressed by 50% of its original height using a cylindrical plunger (P/50) with a test speed of 5.0 mm/s. The TPA parameters of hardness, springiness, cohesiveness, and chewiness were determined by the resulting force-time curves.
Preparation of Myofibrillar Protein Solution
Myofibrillar protein solution (MPS) was prepared according to the method of Xiong et al. (2009). The gels were homogenized with 10 volumes (w/v) of Tris-maleate buffer (20 mmol/L, pH 7.0, containing 0.05 mol/L KCl). The homogenate was centrifuged at 10,000g for 10 min. The precipitation was then collected and homogenized with 10 volumes (w/v) of Tris-maleate buffer (20 mmol/L, pH 7.0, containing 0.6 mol/L KCl) and extracted for 1 h. The homogenate was again centrifuged at 10,000g for 10 min and the supernatant comprising the myofibrillar protein was collected. All the above operations were performed at 4 °C. The myofibrillar protein content was determined by Lowry method (Lv et al. 2018).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
SDS-PAGE was performed according to the method of Zhou et al. (2016). The concentration of MPS was adjusted to 20 mg/mL, mixed with bromophenol blue buffer in a ratio of 3:1, heated at 100 °C for 5 min, and loaded in gel comprised of 5% stacking gel and 15% resolving gel. Electrophoresis was performed at 80 V for the stacking gel and 120 V for the resolving gel. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 in 50% (v/v) methanol and 10% (v/v) acetic acid and detained with 30% (v/v) methanol and 10% (v/v) acetic acid until the elution solution was clarified. High- and low-molecular-weight markers were used to estimate the molecular weights of the proteins. Images were captured and analyzed using the Bio-Rad Gel Doc XR + system (Bio-Rad, Richmond, CA, USA).
Circular Dichroism Spectroscopy of Myofibrillar Protein
To determine the secondary structure of the myofibrillar protein, the MPS dialyzed at 4 °C for 12 h was diluted with water to a concentration of 0.2 mg/mL and tested using a CD spectropolarimeter (Jasco J-1500-150, Jasco Corp., Tokyo, Japan). A 300 μL of 0.2 mg/mL MPS was placed in a 1 mm quartz CD cell (Hellma, Muellheim, Baden, Germany) using the following parameters: 1.0 nm bandwidth, 200 mdeg/1.0 dOD of CD and FL scale, 1 s D.I.T., 0.1 nm/data step resolution, and 50 nm/min scan speed. The CD data were expressed in terms of the mean specific ellipticity [θ] (deg·cm2·dmol−1). The scanning range used was 190–250 nm, and Yang’s method (Yang et al. 1986) was used to evaluate the secondary structure of the myofibrillar protein.
Statistical Analysis
All experiments were performed in triplicate. Data were expressed as means and standard deviations (mean ± SD). The significance of the main effects was determined using one-way analysis of variance (ANOVA), and the significant differences (P < 0.05) among the groups were performed using Duncan’s Multiple Range Test. Correlation analysis was performed using Pearson method. All statistical analyses were performed using software SPSS (Version 21.0, SPSS Inc., Chicago, USA).
Results and Discussion
WHC
Changes in the WHC reflected the variations in protein-water interactions and gel structure (Zhang et al. 2018). The WHC of gels under different pressure was shown in Fig. 1. In the groups of P, high pressure increased the WHC and reached a maximum of 76.72% at 350 MPa. Zhang et al. (2015) reported that the pressure treatment at 200 MPa increased the WHC of myofibrillar protein gel from broilers and Cando et al. (2015) found that the WHC of Alaska Pollock surimi gel increased with the increasing of pressure (0.1–300 MPa). In the groups of PH, the application of HPP tended to a slight increase in the WHC (P > 0.05). Moreover, at the same pressure, the WHC values of P group were higher than those of the PH group. These results may be related to the fact that heating process reduced the number of water molecules linked to the surimi protein, resulting in the decrease of WHC. This effect was expected, as it had already been reported by Fernández-Martín et al. (1998) and Moreno et al. (2009).
Whiteness
Whiteness is an important indicator used to judge the color of surimi gel (Zhang et al. 2016). As shown in Table 2, the whiteness of the gels subjected to 300–450 MPa was significantly higher than that of P-0.1 group (P < 0.05). The results indicated that high pressure promote the polymerization of proteins. As pressure increased (300–450), a*and b* values of P group decreased (P < 0.05), the lightness and whiteness increased slightly, and the highest value of whiteness was about 56.74 obtained at a pressure of 350 MPa. These results indicated that appropriate pressure treatment can improve the whiteness of gel and correspond to the results of WHC. It was considered that gels with high WHC values had strong light-reflecting abilities that enhance the whiteness.
However, in the groups of PH, a*value increased and b*value decreased compared with the group of PH-0.1 (P < 0.05). The lightness and whiteness of gels decreased rapidly in the PH-400 and PH-450 (P < 0.05), whereas there was no significant difference between the groups of PH-350 and PH-0.1 (P > 0.05). It was suggested that the changes in whiteness of surimi gel induced by pressure or heating were attributed to the changes of myofibrillar protein structure and its WHC (Shie and Park 1999).
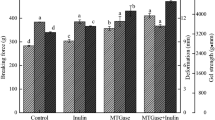
Gel Strength
Gel strength is an important index for evaluating the quality of surimi products because it directly affects customer acceptability. Changes in the gel strength under different high pressure are presented in Fig. 2. The gel strength of the PH-0.1 group was significantly higher than that of the P-0.1 group (P < 0.05), because the myofibrillar proteins in natural surimi are denatured and aggregated by heat induction to form a gel network structure (Nagano and Tokita 2011). In addition, whether in the group of P or PH, the gel strength revealed a tendency to increase first and then decrease with the increased pressure. The largest gel strength was in the group of P-350, which was up to 3746.12 g mm. However, when the pressure further increased to 400 MPa and 450 MPa, the gel strength began to decrease. A stronger gel network formed could imbibe more water (Kudre and Benjakul 2014). This result was essentially consistent with that of the WHC (Fig. 1). Guo et al. (2019) also reported that both the WHC and gel strength of golden threadfin bream myosin were enhanced under 100–300 MPa but reduced at 400–500 MPa. This phenomenon was related to changes in the composition and structure of protein (Cando et al. 2015). At the same pressure (except 0.1 MPa), the gel strength of PH group was weakened compared with P group. The reason may be that some denatured proteins caused by high pressure could only aggregate with random, disordered protein particles after further heating, which weakened the structure of gel network (Chakraborty et al. 2002). Moreover, when the pressure reached 400 MPa or above, the gel strength of the PH group was significantly reduced (P < 0.05). Therefore, controlling the pressure of less than 400 MPa is advantageous for the gel texture of hairtail surimi in actual production.
TPA Properties
In addition to the group of P-450, there was no significant difference in the hardness of P group (P > 0.05) as shown in Table 3. Compared with the group of P-0.1, the springiness, chewiness, and cohesiveness in the groups of P-300, P-350, P-400, and P-450 were significantly increased, which were consistent with the results of Le and Turgeon (2015). Meanwhile, four parameter values of TPA properties reached the maximum in the P-350 group. Cheng et al. (2011) used different methods to treat the gels with the same content of soybean dietary fiber, and found that the gels treated by the simple pressure were softer and more elastic.
Table 3 also presents that the hardness, springiness, and chewiness decreased but the cohesiveness increased after pressure induction in the groups of PH. Moreover, the springiness and chewiness decreased with the increasing of pressure. Compared with the groups of P at the same high pressure, four parameter values of TPA property in the groups of PH had a different degree of decline according to the Table 3. Moreno et al. (2015) found that simple pressure (40, 125, or 200 MPa) induction could improve the springiness and stability of the surimi gel, but the hardness of the gel in the PH group was higher than that in the P group. The changes of TPA property in this test were inconsistent with the Moreno’s, which may be related to different fish species, pressure settings, and heat treatment temperatures.
SDS-Page
Myofibrillar proteins play the most critical role during meat processing as they are responsible for the cohesive structure and firm texture of meat products. The most abundant proteins were myosin heavy chains (MHC) and actin (Zhang et al. 2014). As shown in Fig. 3, the myofibrillar protein mainly contained a 200 kDa of MHC, a 43 kDa of actin, and a 35 kDa of tropomyosin-T (TNT). It can be seen from lanes 1–5 that the number of electrophoretic bands was almost unchanged; this result probably indicated that high pressure had little effect on the composition of myofibrillar proteins (Qiu et al. 2013). However, a significant decrease in the amount of MHC and actin was observed after pressure and further heat treatment. Cando et al. (2016) also reported the heat-induced degradation of MHC in pollock surimi.
SDS-PAGE of myofibrillar protein from gels under different HPP (300–450 MPa). Lanes 1–5 and 6–10 corresponded to the groups of P-0.1, P-300, P-350, P-400, and P-450 and PH-0.1, PH-300, PH-350, PH-400, and PH-450, respectively. Positions of molecular weight standards are labeled on the right. MHC myosin heavy chain, TNT tropomyosin-T, M marker
Compared with the groups of P-300, P-350, and P-400, MHC in the group of P-450 had less degradation. Wang and Xiong (1998) reported that pressure treatment above 300 MPa can passivate myofibril-bound serine proteinases, resulting in a decrease in MHC degradation. Conversely, there was no significant difference in TNT content after high pressure. These results suggested that high pressure leads to degradation of actin and MHC in the myofibrillar protein, while had no effect on TNT.
CD Spectra
CD spectra in the far-ultraviolet wavelength region (190–260 nm) are used to characterize the conformation structure of myofibrillar protein under different HPP (Greenfield 2007), although the protein concentration required is quite low (about 0.1–0.3 mg/mL) (Liu et al. 2008). The CD spectra and secondary structure contents of myofibrillar protein in groups of P and PH were shown in Fig. 4 a, b, and c, respectively. The negative characteristic peaks of the α-helix in the protein appeared at 208 nm and 222 nm and the positive peak appeared at around 192 nm. The β-sheet had a negative peak at 216 nm and a positive spectrum at 185–200 nm, while β-turn had a positive peak at around 206 nm.
As shown in Fig. 4 a, the magnitude of CD spectra in the P groups was noticeably reduced after pressure induction. By calculation from the CD spectra (Fig. 4c), the high pressure reduced the contents of α-helix and β-turn, but increased the contents of β-sheet and random coil in the myofibrillar protein, which favored the gelling process (Liu et al. 2008). Meanwhile, the magnitude and the α-helix fraction gradually weakened with a rise of processing pressure, which can be considered that the secondary structure of the protein was clearly affected by high pressure treatment.
In the groups of PH (Fig. 4b), the shape and magnitude of each CD spectra were similar. Subsequent heat treatment converted the partial β-sheet into the α-helix structure while the WHC and gel strength decreased. Therefore, the myofibrillar protein in the gel induced by high pressure was stable and exhibits excellent gel properties (Xu et al. 2011).
Correlation Analysis
In the above results, the effects of HPP on the structure of myofibrillar protein and gel properties of hairtail surimi were determined. As it has been indicated that the functionality of proteins was mostly related to the structure (Wang et al. 2019). However, it was not clear if the secondary structure changes of myofibrillar protein would affect the properties of surimi gel. Therefore, the correlation between the structure of myofibrillar protein and properties of surimi gel was further demonstrated.
In Fig. 5, the α-helix content of myofibrillar protein in the groups of P was positively correlated with the gel strength and cohesiveness (P < 0.05). The hardness was negatively correlated with β-sheet content (P < 0.05) and positively correlated with chewiness (P < 0.05). These results were different from the result of Wang et al. (2019), which might be related to the different calculation methods and the content of NaCl. The WHC, increased after high pressure induction, was positively correlated with the random coil content. Compared with the α-helix, the random coils are relatively loose structures. The increase in random coils enhanced the interaction between the side chains and promoted the gel from forming a relatively stable three-dimensional structure (Imtiaz-Ul-Islam et al. 2011), this interaction is essential to the aggregation of myofibrillar proteins.
As shown in Fig. 5 (group PH), the β-sheet content was negatively correlated with the α-helix and the β-turn (P < 0.05), which resulted in the change of structure since the β-sheet was transferred to the α-helix and β-turn. Moreover, random coil content was negatively correlated with the gel strength and WHC. This was different from the results of P group. The probable reason was that high pressure synergistic heat induction accelerated the denaturation of surimi protein, affected the interaction between proteins, and destroyed the gel network structure that has been formed (Buamard and Benjakul 2018).
Conclusion
Simple high pressure treatments less than 400 MPa improved the WHC, whiteness, gel strength, chewiness, springiness, and cohesiveness of the gels, but had little effect on the hardness. Although the effect of HPP on the gel properties was practically masked by subsequent thermal processing, the results of SDS-PAGE indicated that both pressure and heat treatments can cause the degradation of actin and MHC in myofibrillar proteins. On the other hand, during formation of the gel which was subjected to simple high pressure, the α-helix and β-turn content decreased, while the β-sheet and random coil content increased. Compared with the gels which were subjected to simple high pressure (except 0.1 MPa), there were a higher proportion of random coil and a lower proportion of β-turn in the gels which were subjected to high pressure synergistic heat. Meanwhile, there was a significant correlation between secondary structure content and WHC, whiteness, gel strength, and texture characteristics when the gel was subjected to simple high pressure. In conclusion, it was confirmed that suitable high pressure can modify the structure properties of myofibrillar protein from hairtail surimi and adjust their unfolding and refolding characteristics during subsequent thermal treatment, which was responsible for the observed changes in various gel properties of hairtail surimi. These findings may provide a reference for the application of high pressure technology in the processing of hairtail surimi and its products.
References
Barrera, A. M., Ramírez, J. A., González-Cabriales, J. J., & Vázquez, M. (2002). Effect of pectins on the gelling properties of surimi from silver carp. Food Hydrocolloids, 16, 441–447.
Buamard, N., & Benjakul, S. (2018). Combination effect of high pressure treatment and ethanolic extract from coconut husk on gel properties of sardine surimi. LWT - Food Science and Technology, 91, 361–367.
Cando, D., Herranz, B., Borderías, A., & Moreno, H. (2015). Effect of high pressure on reduced sodium chloride surimi gels. Food Hydrocolloids, 51, 176–187.
Cando, D., Moreno, H. M., Borderas, A. J., & Skara, T. (2016). Combined effect of high hydrostatic pressure and lysine or cystine addition in low-grade surimi gelation with low salt content. Food and Bioprocess Technology, 9(8), 1391–1398.
Chakraborty, T., Jayaprakash, S., Srinivasu, P., Madhavendra, S., Ravi, S. A., & Kunwar, A. (2002). Furanoid sugar amino acid based peptidomimetics: well-defined solution conformations to gel-like structures. Tetrahedron, 58(14), 2853–2859.
Cheng, Z. Z., Yang, R. J., & Zhao, W. (2011). Gel properties of ribbonfish (Trichiurus haumela) surimi gels with soybean dietary fiber induced by high pressure and heating. International Journal of Food Engineering, 7(6), 39–47.
Fernández-Martín, F., Pérez-Mateos, M., & Montero, P. (1998). Effect of pressure/heat combinations on blue whiting (Micromesistius poutassou) washed mince: thermal and mechanical properties. Journal of Agricultural and Food Chemistry, 46(8), 3257–3264.
Greenfield, N. J. (2007). Using circular dichroism spectra to estimate protein secondary structure. Nature Protocols, 1(6), 2876–2890.
Guo, Z. B., Li, Z. Y., Wang, J. Y., & Zheng, B. D. (2019). Gelation properties and thermal gelling mechanism of golden threadfin bream myosin containing CaCl2 induced by high pressure processing. Food Hydrocolloids, 95, 43–52.
Imtiaz-Ul-Islam, M., Hong, L., & Langrish, T. (2011). CO2 capture using whey protein isolate. Chemical Engineering Journal, 171(3), 1069–1081.
Jin, S. K., Kim, I. S., Jung, H. J., Kim, D. H., Choi, Y. J., & Hur, S. J. (2011). Effect of cryoprotectants on chemical, mechanical and sensorial characteristics of spent laying hen surimi. Food and Bioprocess Technology, 4(8), 1407–1413.
Kaewudom, P., Benjakul, S., & Kijroongrojana, K. (2013). Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Bioscience, 1, 39–47.
Kudre, T. G., & Benjakul, S. (2014). Effects of bambara groundnut protein isolates and microbial transglutaminase on textural and sensorial properties of surmi gel from sardine (Sardinella albella). Food and Bioprocess Technology, 7(6), 1570–1580.
Lanier, T. C. (1996). Gelation of surimi pastes treated by high isostatic pressure. Progress in Biotechnology, 13(C), 357–362.
Le, X. T., & Turgeon, S. L. (2015). Textural and waterbinding behaviors of β-lactoglobulin-xanthan gum electrostatic hydrogels in relation to their microstructure. Food Hydrocolloids, 49, 216–223.
Li, W., Bai, Y., Mousaa, S. A. S., Zhang, Q., & Shen, Q. (2012). Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food and Bioprocess Technology, 5(6), 2233–2241.
Li, G., Chen, Y., Xuan, S., Lv, M., Zhang, J., Lou, Q., Jia, R., & Yang, W. (2019). Rheological properties and structure of myofibrillar protein extracted from Oratosquilla oratoria muscle as affected by ultra-high pressure. International Journal of Food Properties, 22(1), 1310–1321.
Liu, R., Zhao, S. M., Xiong, S. B., Xie, B. J., & Qin, L. H. (2008). Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Science, 80(3), 632–639.
Lv, M. C., Mei, K. L., Zhang, H., Xu, D. L., & Yang, W. G. (2018). Effects of electron beam irradiation on the biochemical properties and structure of myofibrillar protein from Tegillarca granosa meat. Food Chemistry, 254, 64–69.
Ma, X. S., Yi, S. M., Yu, Y. M., Li, J. R., & Chen, J. R. (2015). Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT - Food Science and Technology, 61(2), 377–384.
Moreno, H. M., Cardoso, C., Solas, M. T., & Borderías, A. J. (2009). Improvement of cold and thermally induced gelation of giant squid (Dosidicus gigas) surimi. Journal of Aquatic Food Product Technology, 18(4), 312–330.
Moreno, H. M., Bargiela, V., Tovar, C. A., Cando, D., Borderias, A. J., & Herranz, B. (2015). High pressure applied to frozen flying fish (Parexocoetus brachyterus) surimi: Effect on physicochemical and rheological properties of gels. Food Hydrocolloids, 48, 127–134.
Nagano, T., & Tokita, M. (2011). Viscoelastic properties and microstructures of 11S globulin and soybean protein isolate gels: magnesium chloride-induced gels. Food Hydrocolloids, 25(7), 1647–1654.
Petcharat, T., & Benjakul, S. (2018). Effect of gellan incorporation on gel properties of bigeye snapper surimi. Food Hydrocolloids, 77, 746–753.
Qiu, C. J., Xia, W. S., & Jiang, Q. X. (2013). Effect of high hydrostatic pressure (HHP) on myofibril-bound serine proteinases and myofibrillar protein in silver carp (Hypophthalmichthys molitrix). Food Research International, 52(1), 199–205.
Shie, J. S., & Park, J. W. (1999). Physical characteristics of surimi seafood as affected by thermal processing conditions. Journal of Food Science, 64(2), 287–290.
Wang, B. W., & Xiong, Y. L. (1998). Evidence of proteolytic activity and its effect on gelation of myofibrillar protein concentrate from bovine cardiac muscle. Journal of Agricultural and Food Chemistry, 46(8), 3054–3059.
Wang, J. Y., Li, Z. Y., Zheng, B. D., Zhang, Y., & Guo, Z. B. (2019). Effect of ultra-high pressure on the structure and gelling properties of low salt golden threadfin bream (Nemipterus virgatus) myosin. LWT - Food Science and Technology, 100, 381–390.
Xiong, G. Q., Cheng, W., Ye, L. X., Du, X., Zhou, M., Lin, R. T., et al. (2009). Effects of konjac glucomannan on physicochemical properties of myofibrillar protein and surimi gels from grass carp (Ctenopharyngodon idella). Food Chemistry, 116(2), 413–418.
Xu, X. L., Han, M. Y., Fei, Y., & Zhou, G. H. (2011). Raman spectroscopic study of heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristic. Meat Science, 87(3), 159–164.
Yang, J. T., Wu, C.-S. C., & Martinez, H. M. (1986). Calculation of protein conformation from circular dichroism. Methods in Enzymology, 130, 208–269.
Zhang, T., Xue, Y., Li, Z., Wang, Y., Yang, W., & Xue, C. (2014). Effects of ozone-induced oxidation on the physicochemical properties of myofibrillar proteins recovered from bighead carp (Hypophthalmichthys nobilis). Food and Bioprocess Technology, 8(1), 181–190.
Zhang, Z. Y., Yang, Y. L., Tang, X. Z., Chen, Y. J., & You, Y. (2015). Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chemistry, 188, 111–118.
Zhang, H., Wang, W., Zhang, S., Wang, H., & Ye, Q. (2016). Influence of 10-MeV E-beam irradiation and vacuum packaging on the shelf-life of grass carp surimi. Food and Bioprocess Technology, 9(5), 830–838.
Zhang, L., Li, Q., Shi, J., Zhu, B., & Luo, Y. (2018). Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: effects of different washing solutions. Food Hydrocolloids, 75, 116–124.
Zhou, H., Wang, C., Ye, J., Tao, R., Chen, H., & Cao, F. (2016). Effects of enzymatic hydrolysis assisted by high hydrostatic pressure processing on the hydrolysis and allergenicity of proteins from ginkgo seeds. Food and Bioprocess Technology, 9(5), 839–848.
Funding
This work was supported by the National Key Research and Development Program of China [2018YFD0901105], the Public Welfare Technology Research Project of Zhejiang Province [LGN18C200020], was sponsored by K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Xu, A., Yang, R. et al. Myofibrillar Protein Structure and Gel Properties of Trichiurus Haumela Surimi Subjected to High Pressure or High Pressure Synergistic Heat. Food Bioprocess Technol 13, 589–598 (2020). https://doi.org/10.1007/s11947-020-02416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02416-x