Abstract
The main aim of this study was to evaluate the effect of the application of an alginate–chitosan (A–Ch) coating on the bioactive compounds and the antioxidant capacity of fresh figs (Ficus carica), collected at two maturity stages (referred to as stages III and IV), and stored for 15 days at 6 °C. The composition of the internal atmosphere of the figs, as well as the polyphenol content and antioxidant capacity, was analyzed at 0, 3, 6, 9, 12, and 15 days, respectively. The sensory quality of coated and uncoated figs, stored for 15 days, was also assessed. Fresh figs were used as a reference in the sensory quality evaluation. The A–Ch coating caused considerable modifications in the internal atmosphere of the figs at the two maturity stages evaluated. The ripening process was delayed as O2 was reduced and CO2 concentrations were increased. Further, the total polyphenol content of the figs and, also, identified individual polyphenols, were preserved by the application of the A–Ch coating. Anthocyanins, in particular cyanidin-3-O-rutinoside, were the most abundant bioactive compound. Uncoated figs also exhibited higher antioxidant capacity than coated figs at maturity stage III, whereas in coated figs antioxidant capacity was kept constant along storage period regardless of their maturity stage. Interestingly, the coated figs stored for 15 days at 6 °C showed a high acceptability in the sensory evaluation, being similar to fresh figs. Therefore, the A–Ch coating could be an excellent post-harvest technology useful in preserving not only the organoleptic and sensory attributes but also bioactive components of figs during storage at low temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Figs (Ficus carica) have long been associated with longevity and health benefits due to their high content of vitamins, amino acids, antioxidants, and dietary fibers (Martínez-García et al. 2013; Solomon et al. 2006; Turan and Celik 2016). This fruit, in particular the dark or purple varieties, represents an important source of bioactive compounds, in particular high amounts of anthocyanins (Dueñas et al. 2008; Çalişkan and Aytekin Polat 2011; Kamiloglu and Capanoglu 2015); the content being similar to that of black plums (Usenik et al. 2009), blueberries (Bunea et al. 2013), sweet cherries, and strawberries (Wu et al. 2006). This high content of anthocyanins has made figs an attractive fruit to consumers, since it has been observed that their intake can inhibit inflammatory processes and heart failure, in addition to promoting anticarcinogenic and hypoglycemic activities (Szymanowska et al. 2015; Castro-Acosta et al. 2016). Besides, figs contain large amounts of the flavonoid quercetin-3-O-rutinoside (Del Caro and Piga 2008; Kamiloglu and Capanoglu 2015) which has been associated with different health benefits, such as the reduction of oxidative stress (Nishimura et al. 2016) and anti-inflammatory activity (Mascaraque et al. 2014).
Generally, figs are harvested at two different maturity stages, either physiological maturity (stage III) or commercial maturity (stage IIV) (Crisosto et al. 2010; Owino et al. 2004). Figs in maturity stage IV are completely mature, being more susceptible to overripe, microbial infection, and loss of the organoleptic properties (Villalobos et al. 2014) whereas figs at physiological maturity tend to show long shelf life since the biochemical and physiological changes continue after harvesting (Marei and Crane 1971).
Commercially, a very large percentage of figs are consumed dried in cookies or bars, and to a lesser extent, figs are also eaten in fresh form (Reyes-Avalos et al. 2016). However, dehydration affects the sensorial, nutritional, and functional fruit quality (Kamiloglu and Capanoglu 2015; Martínez-García et al. 2013), while fresh figs are highly sensitive to microbial growth even when stored at low temperatures; therefore, it may be useful to evaluate alternative processes to extend the shelf life of this fruit (Martínez-García et al. 2013). A feasible alternative could be the application of edible coatings, which is a preservation method used not only to improve food appearance, but also to maintain the quality of different fruits and vegetables (Meighani et al. 2015; Arnon et al. 2015).
Edible films can modify the internal atmosphere (O2 and CO2) of coated products, delaying the metabolic changes associated with the ripening process of fruits and, therefore, extending the shelf life (Meza-Velázquez et al. 2013; Tzoumaki et al. 2009). Previously, we characterize an alginate–chitosan bilayer edible film and evaluated the effectiveness of this film on the quality and shelf life of figs stored at low temperature (Reyes-Avalos et al. 2016). Interestingly, this edible film demonstrated excellent water vapor and gas barrier properties which contributed to decreasing the transpiration and respiration rates of stored figs. Further, figs coated with the film exhibited a better resistance to fungal contamination than uncoated figs, improving the overall quality and also extending shelf life during low-temperature storage. However, the information about possible effects of this coating on bioactive compounds, such as antochyanins, is very scarce. Thus, the aim of this study was to evaluate the effect of the application of this coating on the bioactive compounds and the antioxidant capacity of figs (Ficus carica) var. Mission, harvested at two maturity stages, during storage at low temperature. In particular, internal atmosphere gas, polyphenol content, antioxidant capacity, and the sensorial quality of figs were analyzed in this study.
Material and Methods
Chemical Reagents
Sodium alginate was acquired from CYTECSA, S.A. de C.V. (México, D.F.), soy lecithin from Golden Bell (México, D.F.), and olive oil was purchased at a local store. J.T. Baker (Phillipsburg, NJ, USA) supplied water, phosphoric acid, ammonium diacid phosphate, and acetonitrile HPLC grade. Chitosan, sodium carbonate, lactic acid and calcium chloride, Folin−Ciocalteu and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ABTS diammonium salt (2, 2-azinobis-3-ethylben zothiazoline-6-sulphonic acid) reagents, potassium persulfate, 2,4,6-Tri-(2-pyridyl)-s-triazine (TPTZ), hydrochloric acid, ethyl alcohol, iron (III) chloride hexahydrate, sodium acetate 3-hydrate, glacial acetic acid; CO2, oxygen standard grade, gallic acid, chlorogenic acid, quercetin-3-O-rutinoside, cyanidin-3-O-glucoside, and cyanidin-3-O-rutinoside were all purchased from Sigma-Aldrich Company (St. Louis MI, USA).
Plant Material
The figs used in this study were collected in August–September in Cd. Lerdo (Durango, Mexico; 25° 32′ 10″ N/103° 31′ 28″ W) as previously described in Reyes-Avalos et al. (2016). Fruits free of any physical damage were selected and washed using 0.02% sodium hypochlorite solution. Finally, according to their maturity stage, either stage III or stage IV, the fruits were divided into two batches before applying any treatment. Each treatment was repeated four times (n = 4).
The first batch was selected as a control group (without coating application), while the second batch was coated with the alginate–chitosan (A–Ch) film. The preparation and application of the A–Ch coating has previously been described in Reyes-Avalos et al. (2016). All figs, coated and uncoated, were stored at 6 °C and 95% RH for 15 days.
The internal CO2 and O2 concentrations, total phenolic, polyphenol profile, and antioxidant capacity were evaluated at 0, 3, 6, 9, 12 and 15 days of storage. Sensory quality of coated and uncoated figs was determined at 15 days of storage; fresh figs were used for comparison purposes. Approximately, 15 g of coated and uncoated figs from each storage period was homogenized and lyophilized at − 20 °C for 72 h in a Labconco FreeZone Triad Cascade Benchtop Freeze drier (Kansas City, MO, USA) for subsequent analysis.
Internal CO2 and O2
Two milliliters of gas from the internal atmosphere of the fruit was extracted with a syringe inserted through the fruit blossom ends. The CO2 and O2 concentrations in the gas sample were determined using an Agilent 6820 gas chromatograph (Agilent Technology, Palo Alto, CA, USA) equipped with a TCD detector, two 6 ft × 1/4 in Alltech CTR I columns (Alltech Associates, Inc., Deerfield, IL, USA) as previously described in Reyes-Avalos et al. (2016). The internal gas sample was isothermally separated at 95 °C while the injector and detector temperatures were 70 °C and 170 °C, respectively. All determinations were measured in triplicate. CO2 and O2 concentrations were expressed as μM (micromoles of gas/L air).
Polyphenol Compound Analysis
Extraction
Polyphenol compounds were extracted according to the method proposed by Eim et al. (2013) with slight modifications. Approximately, 0.5 g of lyophilized figs was homogenized with 10 mL of methanol (MeOH) using an Ultra-Turrax IKA T25D (IKA® Works, Inc., Wilmington, USA) at 13,500 rpm for 1 min. The samples were stored in darkness and mechanically stirred for 16 h at 4 °C. The samples were centrifuged (ALC 4218, Milano, Italy) at 1750g for 15 min and filtered through a Whatman no. 4 filter paper. The supernatants were stored at 4 °C for later analysis. All extractions were performed in duplicate.
Total Phenolic Content
Total soluble polyphenols were spectrophotometrically measured in accordance with the Folin–Ciocalteu method, using 96-well microplates, as previously described by González-Centeno et al. (2014) with some modifications. Briefly, 95 μL of distilled water was placed in each well, and 10 μL of the extract was added, followed by 5 μL of the Folin–Ciocalteu reagent. Then, the mixture was incubated for 5 min and 80 μL of 7.5% (w/v) Na2CO3 solution was added. A Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Vanda, Finland) was used to incubate the mixture for 30 min, in the absence of light, at 25 °C before measuring the absorbance at 765 nm. Gallic acid (0–200 ppm) was used as standard for calibration and the phenolic content results were expressed as milligram of gallic acid per 100 g of dry matter (dm). Each of the given values is the mean of six experimental determinations.
Identification and Quantification of Polyphenol Compounds by HPLC–DAD
The polyphenolic compounds were extracted and analyzed by HPLC–DAD according to the method described by Del Caro and Piga (2008) with slight modifications. Approximately, 2 g of lyophilized figs was homogenized with 10 mL of MeOH (HPLC grade) and stirred for 16 h at 4 °C. Then, the samples were centrifuged at 4000g for 5 min at 4 °C and concentrated to 5 mL at 30 °C in vacuum conditions. The concentrated samples were then diluted to 10 mL with Milli-Q water and filtered through a ∅ 0.45-μm PTFE filter prior to HPLC analysis. The chromatographic analysis was carried out using an HPLC Agilent 1200 (Agilent Technology, Palo Alto, CA, USA) equipped with a diode array detector (DAD), a quaternary pump, and two LiChrospher C18 5-μm (4 mm × 150 mm) columns (Phenomenex) connected in series. The temperature, flow rate, and injection loop were of 25 °C, 0.5 mL/min, and 20 μL, respectively. The mobile phase was comprised of (A) 50 mM ammonium diacid phosphate solution brought to 2.6 pH with phosphoric acid, (B) 80% acetonitrile and 20% phase A, and (C) 200 mM phosphoric acid. The mobile phase gradient was of 100% A at 5 min, 92% A and 8% B at 8 min, 14% B and 86% C at 20 min, 16.5% B and 83.5% C at 25 min, 21.5% B and 78.5% C at 35 min, 50% B and 50% C at 70 min, 100% A at 75 min, and 100% A at 80 min. The polyphenol compounds were analyzed at four different wavelengths: 280 nm for catechins and benzoic acids, 316 nm for hydroxycinnamic acids, 365 nm for flavonols, and 520 nm for anthocyanins. High purity standards of gallic acid, chlorogenic acid, quercetin-3-O-rutinoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-glucoside were used not only to identify but also quantify these polyphenolic compounds.
Evaluation of the Antioxidant Capacity
The effect of the A–Ch bilayer edible film and, also, of the maturity stage on the antioxidant capacity of figs was also tested. The 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and ferric reducing antioxidant power (FRAP) assays were used as described by González-Centeno et al. (2012). For both antioxidant assays, an automated microplate reader, in particular a Multiskan Spectrum (Thermo Scientific, Waltham, MA USA) was used.
Sensory Quality
The sensory analysis of coated and uncoated (control) fig fruits was carried out following the methodology previously described by Gol et al. (2013) with some modifications. Specifically, the sensory quality was evaluated from the following parameters: appearance, color, odor, firmness, flavor, and overall acceptability for a total of 3 samples; one sample corresponding at 0 day (fresh) and 2 samples (coated and uncoated) stored for 15 days. All figs were labeled with 4-digit code numbers and randomly provided to the panelists. The samples were evaluated by a panel of 50 volunteers aged between 19 and 53 years old, who were habitual consumers of figs. Drinking water was provided to panelists for eliminating the residual taste between samples. Each attribute was scored on a structured hedonic scale labeled from “extremely unpleasant” (0) to “extremely pleasant” (9).
Data Analysis
All data were statistically analyzed using analysis of variance (ANOVA). Further, the post hoc analysis was carried out using Fisher’s least significant difference (LSD) test with a significance level of 0.05. All calculations were carried out using Minitab 18 statistical software (Minitab Inc. 2018).
Results and Discussion
Internal Concentrations of CO2 and O2 of Figs
Regardless of the maturity stage of the evaluated figs, the application of the alginate–chitosan (A–Ch) coating caused important changes in the composition of the internal atmosphere of figs during storage at low temperature (p < 0.05). Thus, coated figs exhibited a higher CO2 concentration than uncoated figs (Fig. 1), whereas O2 concentration was higher in uncoated figs (Fig. 2).
Internal CO2 concentration (μM) of coated figs with an A–Ch coating and uncoated (control) figs, collected at two maturity stages and stored for 15 days at 6 °C. Bars over the mean results stand for ± standard deviation (n = 4). Different lowercase letters indicate significant differences in coated and uncoated figs according to the LSD test (p < 0.05) (n = 4)
Internal O2 concentration (μM) of coated figs with an A–Ch coating and uncoated (control) figs, collected at two maturity stages and stored for 15 days at 6 °C. Bars over the mean results stand for ± standard deviation (n = 4). Different lowercase letters indicate significant differences in differences in coated and uncoated figs according to the LSD test (p < 0.05) (n = 4)
As can be observed in Fig. 1, the CO2 concentration of coated figs decreased during storage, from ~ 1600 to ~ 710 μM, for figs at stage III, and from ~ 1850 to ~ 740 μM, for figs at stage IV; whereas in uncoated figs, the CO2 concentration decreased from ~ 600 to less than 200 μM, regardless of the maturity stage.
However, the internal O2 of uncoated figs exhibited a slight but significant increase during storage, from ~ 5890 to 7650 μM for figs of both stages of maturity (III and IV), whereas in coated figs internal O2 increased from ~ 3100 to ~ 3990 μM for figs stage III, and from ~ 2900 to ~ 3250 μM for figs at stage IV (Fig. 2). As can be observed, CO2 concentration decreased during the first days of storage, which could be attributed to the low respiration rate as consequence of low temperature. In the subsequent days of storage, the A–Ch coating promoted the CO2 accumulation inside of fruit as consequence to the low gas permeability of coating (Reyes-Avalos et al. 2016).
The composition of the internal atmosphere of coated figs showed a similar behavior to other coated fruits since it has been observed that coated fruits and vegetables tend to exhibit a CO2-rich internal atmosphere (Cisneros-Zevallos and Krochta 2002; Meza-Velázquez et al. 2013). Several authors have also observed that edible coatings are able to decrease the respiratory rate of figs, which in turn may lead to the modification of the internal atmosphere (Reyes-Avalos et al. 2016; Bourbon et al. 2011; Fabra et al. 2012). It is well known that the modification of the internal atmosphere, by increasing CO2 and decreasing O2 concentration, is one of the key factors in delaying the ripening process of fruits (Díaz-Mula et al. 2011; Majidi et al. 2014; Chaudhary et al. 2015).
Total Polyphenols
The influence of the A–Ch coating on the total polyphenol content (TPC) of figs at the two maturity stages (III and IV) stored at 6 °C can be observed in Fig. 3. Here, TPC was influenced by both the application of the coating and also the maturity stage of the figs. Thus, in uncoated figs, TPC decreased as the maturity stage increased (p < 0.05), whereas in coated figs TPC remained almost unchanged, accounting for about 206 mg GAE/100 g dm, regardless of the maturity stage and storage time (p > 0.05). In fact, only uncoated figs at stage III exhibited a slight but significant increase in the TPC as storage time increased, achieving a maximum of 246.15 ± 8.84 mg GAE/100 g dm at 12 days of storage (p < 0.05) (Fig. 3). Interestingly, these results are concomitant with those from other studies performed in sweet cherry fruits coated with the sodium alginate film (Díaz-Mula et al. 2012), and in blue berries coated with the A–Ch film (Chiabrando and Giacalone 2015), showing that the TPC of coated fruits was lower than in uncoated fruits. Several authors have suggested that lower TPC in coated samples could be the result of the hypoxia and the inhibition of the phenylalanine ammonia-lyase promoted by the lower O2 and higher CO2 concentration which result from the coating (Imahori et al. 2008; Wang et al. 2015).
Total polyphenol concentration (mg of GAE/100 g dm) of coated figs with an A–Ch coating and uncoated (control) figs, collected at two maturity stages and stored for 15 days at 6 °C. Bars over the mean results stand for ± standard deviation (n = 4). Different lowercase letters (a, b, c, d) indicate significant differences in differences in coated and uncoated figs according to the LSD test (p < 0.05) (n = 4)
Identification and Quantification of Anthocyanins, Flavonols, and Polyphenol Acids of Figs by HPLC
In order to gain more insight into the different polyphenolic compounds present in figs, methanolic extracts were subjected to HPLC–DAD analysis. The chromatograms from the HPLC–DAD analysis of the methanolic extracts from figs are shown in Fig. 4.
HPLC chromatogram of the individual polyphenols present in the methanolic extracts of fig samples. GA gallic acid (280 nm; 17.08 min), CA chlorogenic acid (316 nm; 32.39 min), Q3R quercetin-3-rutinoside (365 nm; 49.03 min), C3G cyanidin-3-glucoside (520 nm; 37.40 min), C3R cyanidin-3-rutinoside (520 nm; 38.50 min)
As can be seen, five different polyphenolic compounds were identified at different wavelengths (280–520 nm). In particular, gallic acid (GA) and chlorogenic acid (CA) were identified at 280 and 316 nm, respectively. The flavonol quercetin-3-O-rutinoside (Q3R) was also found at 365 nm whereas at 520 nm two anthocyanins were identified: cyanidin-3-O-glucoside (C3G) and cyanidin-3-O-rutinoside (C3R). Interestingly, C3R, with around 90%, was the predominant compound followed by Q3R, whereas C3G, CA, and GA were found in minor amounts (listed in order of abundance).
The influence of the application of the A–Ch film on each of these polyphenolic compounds is described below.
Effects of the A–Ch Coating on Anthocyanins
The results from the quantification of two anthocyanins, cyanidin-3-O-glucoside (C3G) and cyanidin-3-O-rutinoside (C3R), found in the methanolic extracts obtained from the different samples are shown in Table 1.
As can be observed, the application of the film significantly affected the concentration of C3G and C3R at maturity stage III (p < 0.05) whereas at maturity stage IV it was not significant (p > 0.05). Interestingly, uncoated figs at maturity stage III showed a significant increase from 4.8 to 13 mg/100 g dm for C3G and from 53 to 117 mg/100 g dm for C3R during the first 9 days of storage (p < 0.05). After this period, these anthocyanins, C3G and C3R, exhibited a significant decrease (p < 0.05), up to 5.84 mg/100 g dm for C3G and to 63.81 mg/100 g dm for C3R.
The coated figs at maturity stage III did not exhibit a clear trend, and in this case C3G and C3R anthocyanin concentrations during the storage period varied from ~ 2.5 to ~ 5 mg/100 g dm and from ~ 35 to ~ 65 mg/100 g dm, respectively (p > 0.05).
Similar results have been reported by other authors for plums coated with alginate (Valero et al. 2013) and blackberries coated with chitosan (Oliveira et al. 2014). The lower anthocyanin content in the coated fruits could be a consequence of the internal atmosphere modification (high CO2–low O2), since it has been observed that the biochemical reactions involved in the anthocyanin synthesis can be regulated by modified atmosphere (Díaz-Mula et al. 2011; Selcuk and Erkan 2014).
Effect of the A–Ch Coating on Quercetin-3-O-Rutinoside
The quercetin-3-O-rutinoside (Q3R) was the most abundant flavonoid found in the methanolic extracts of figs. Table 2 shows the concentration of this compound in coated and uncoated figs, at the two maturity stages, during low-temperature storage.
Interestingly, the application of the A–Ch coating on figs at stage III did not show any significant effect on the Q3R concentration. However, when the A–Ch coating was applied on figs at stage IV, the concentration of Q3R was slightly higher than in uncoated figs, in particular at the end of the storage period evaluated. Overall, the Q3R content ranged from ~ 20 to ~ 30 mg/100 g dm in figs at stage III; whereas for figs at stage IV, it varied from ~ 15 to ~ 25 mg/100 g dm. These results are in agreement with Rößle et al. (2011), who observed that the application of an alginate film did not affect the content of Q3R in apple samples. Nevertheless, it should be emphasized that information relating to the effect of edible coatings on the content of Q3R is very scarce.
Effect of the A–Ch Coating on Phenolic Acids
The influence of the A–Ch coating on the content of chlorogenic acid (CA) and gallic acid (GA) of figs, at two maturity stages (III and IV), stored at low temperature (6 °C) is presented in Table 3.
As can be seen, significant changes were observed in the CA content when figs, at stage III, were coated with A–Ch coating (p < 0.05), whereas the application of A–Ch coating in figs at stage IV did not cause such changes (p > 0.05). Interestingly, the CA content increased, both for uncoated and coated figs, as storage time increased (p < 0.05). In particular, for uncoated figs, CA increased from 1.21 ± 0.16 to 3.14 ± 0.45 mg/100 g dm while a similar increase from 1.31 ± 0.2 to 3.62 ± 0.14 mg/100 g dm was observed for coated figs.
The application of the A–Ch coating changed GA content significantly at the end of the storage period (p < 0.05). In particular, coated figs exhibited higher GA levels than those found in uncoated samples. Thus, an increment in GA content in figs at stage III, from ~ 1.7 to ~ 2.8 mg/100 g dm, was caused by the coating application, whereas for uncoated samples the GA content remained almost constant. However, no significant changes in the GA content were observed for coated figs at stage IV, while for uncoated figs, this phenolic acid decreased during storage period. These results suggested that CA could be more affected by the internal atmosphere modification as consequence of the application of the A–Ch coating than GA. It is important to note that most of polyphenol compounds showed high variability along storage period, in particular those in maturity stage III, which could be attributed to different biochemical changes involved in ripening process. Again it is important to emphasize that there is very limited information in the scientific literature relating to the influence of coatings on this type of phenolic compounds. Further studies focused in the biochemical mechanisms involved in the synthesis of this type of bioactive compounds during storage at low temperatures should be carried out in order to explain this phenomenon.
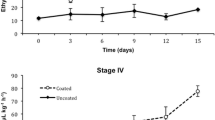
Effect of the Application A–Ch Coating on the Antioxidant Capacity of Fig Fruits
The results corresponding to the antioxidant capacity, determined by the ABTS and FRAP assays, of uncoated figs and, also, of figs coated with an A–Ch film stored at 6 °C during 15 days are presented in Table 4.
Interestingly, significant differences were observed in the antioxidant capacity, measured by ABTS and FRAP, as a consequence of the application of the A–Ch coating (p < 0.05). Thus, in general, the antioxidant capacity of uncoated figs was higher than in coated figs. Further, the maturity stage of figs was also an important parameter since figs at stage IV exhibited lower antioxidant capacity than fruits collected at stage III (see Table 4). For instance, the antioxidant capacity of uncoated figs at stage III increased during storage, either for ABTS (from 1930.7 ± 39.0 to 2140.1 ± 18.2 μM TE/100 g dm) or FRAP assay (1928.8 ± 41.4 and 2322.5 ± 82.4 μM TE/100 g dm). On the contrary, no significant differences (p > 0.05) were observed in coated figs at this maturity stage, accounting for a mean value of 1672.2 ± 34.9 μM TE/100 g dm for ABTS, and 1726.0 ± 55.4 μM TE/100 g dm for the FRAP assay. It is important to point out that the maximum antioxidant capacity of uncoated figs at maturity stage III, for both ABTS and FRAP assays, was observed after 9 days of storage, decreasing afterwards.
On the other hand, no significant changes (p > 0.05) in the antioxidant capacity of coated figs at stage IV were observed, accounting for a mean value of 1505.9 ± 26.3 and 1432.3 ± 41.4 μM TE/100 g dm for ABTS and FRAP assays, respectively. However, uncoated figs at the same maturity stage exhibited a significant decrease in the antioxidant capacity as storage time increased. In the case of the antioxidant capacity measured by ABTS, a decrease from 1860.8 ± 65.3 to 1500.0 ± 98.7 μM TE/100 g dm was observed, while a decrease from 1861.9 ± 61.1 to 1427.9 ± 76.8 μM TE/100 g dm was determined by the FRAP method.
Interestingly, the antioxidant capacity of figs, measured for both ABTS and FRAP assays, showed a good correlation with the C3R content (R2 = 0.80).
Several authors have also observed that the lower antioxidant capacity exhibited by the coated fruits could be associated with the delay of the ripening process (Díaz-Mula et al. 2012; Wang and Gao 2013). Thus, these results suggested that the ripening delay caused by the A–Ch coating promotes a high preservation of the antioxidant capacity of figs during storage at low temperature (6 °C), regardless of the maturity stages (III and IV) evaluated.
Sensory Quality Evaluation
The results obtained after the sensory quality evaluation of fresh figs as well as coated and uncoated figs stored for 15 days at 6 °C are shown in Fig. 5.
Color, texture, appearance, odor, taste, and general acceptability were the main sensory attributes assessed. Overall, the results clearly show that coated figs did not present significant differences, for the different sensory attributes evaluated, in comparison with fresh figs (p > 0.05), the score being higher than 7 for all attributes.
On the contrary, uncoated figs exhibited a significant reduction of the score related to the sensory attributes evaluated, taking the fresh figs as a reference (p < 0.05), and obtaining a score lower than 6 for all attributes.
Generally, the sensory attributes of fruits undergo a significant reduction as the time of storage increases, reducing the overall quality of fruits. Similar results have been observed for strawberries coated with a chitosan-based film (Valenzuela et al. 2015) and for pineapple coated with the alginate film (Mantilla et al. 2013) during storage at low temperature (< 6 °C). Thus, these results showed that the application of the A–Ch coating could reduce the loss of the sensory characteristics of figs, retaining its main sensory attributes at least during 15 days of storage at 6 °C.
Conclusions
The effect of the application of an alginate–chitosan (A–Ch) coating on the internal atmosphere, the bioactive compounds, antioxidant capacity, and sensorial quality of figs, collected at two stages of maturity (III and IV), has been evaluated. Thus, the A–Ch coating modified the internal atmosphere (increasing CO2 and decreasing O2 content) of figs, delaying the ripening process. In particular, the application of the A–Ch coating promoted, on the one hand, a controlled synthesis of polyphenol compounds ensuring a better retention of these compounds, in particular of C3R and Q3R, whereas for GA was not only retained but also increased. Further, during the storage period evaluated, the antioxidant capacity and CA of coated figs were preserved. Finally, it should be highlighted that the application A–Ch coating caused a much more efficient preservation of the overall sensory quality of the fruit during storage, especially more noticeable when figs at maturity stage IV were coated.
Therefore, the application of A–Ch coating can be a potential and effective alternative to preserve not only the bioactive compounds, but also, the organoleptic characteristics assuring the shelf life of the fruit during storage at low temperature.
References
Arnon, H., Granit, R., Porat, R., & Poverenov, E. (2015). Development of polysaccharides-based edible coatings for citrus fruits: a layer-by-layer approach. Food Chemistry, 166, 465–472.
Bourbon, A. I., Pinheiro, A. C., Cerqueira, M. A., Rocha, C. M. R., Avides, M. C., Quintas, M. A. C., & Vicente, A. A. (2011). Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. Journal of Food Engineering, 106(2), 111–118.
Bunea, A., Rugină, D., Sconţa, Z., Pop, R. M., Pintea, A., Socaciu, C., Tăbăran, F., Grootaert, C., Struijs, K., & VanCamp, J. (2013). Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry, 95, 436–444.
Çalişkan, O., & Aytekin Polat, A. (2011). Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Scientia Horticulturae, 128(4), 473–478.
Castro-Acosta, M. L., Smith, L., Miller, R. J., McCarthy, D. I., Farrimond, J. A., & Hall, W. L. (2016). Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. The Journal of Nutritional Biochemistry, 38, 154–161.
Chaudhary, P. R., Jayaprakasha, G. K., Porat, R., & Patil, B. S. (2015). Influence of modified atmosphere packaging on ‘Star Ruby’ grapefruit phytochemicals. Journal of Agricultural and Food Chemistry, 63(3), 1020–1028.
Chiabrando, V., & Giacalone, G. (2015). Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. International Journal of Food Sciences and Nutrition, 66(3), 248–253.
Cisneros-Zevallos, L., & Krochta, J. M. (2002). Internal modified atmospheres of coated fresh fruits and vegetables: understanding relative humidity effects. Journal of Food Science, 67(8), 2792–2797.
Crisosto, C. H., Bremer, V., Ferguson, L., & Crisosto, G. M. (2010). Evaluating quality attributes of four fresh fig (Ficus carica L.) cultivars harvested at two maturity stages. HortScience, 45(4), 707–710.
Del Caro, A., & Piga, A. (2008). Polyphenol composition of peel and pulp of two Italian fresh fig fruits cultivars (Ficus carica L.). European Food Research and Technology, 226(4), 715–719.
Díaz-Mula, H. M., Serrano, M., & Valero, D. (2012). Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food and Bioprocess Technology, 5(8), 2990–2997.
Díaz-Mula, H. M., Zapata, P. J., Guillén, F., Valverde, J. M., Valero, D., & Serrano, M. (2011). Modified atmosphere packaging of yellow and purple plum cultivars. 2. Effect on bioactive compounds and antioxidant activity. Postharvest Biology and Technology, 61(2–3), 110–116.
Dueñas, M., Pérez-Alonso, J. J., Santos-Buelga, C., & Escribano-Bailón, T. (2008). Anthocyanin composition in fig (Ficus carica L.). Journal of Food Composition and Analysis, 21(2), 107–115.
Eim, V. S., Urrea, D., Rosselló, C., García-Pérez, J. V., Femenia, A., & Simal, S. (2013). Optimization of the drying process of carrot (Daucus carota v. Nantes) on the basis of quality criteria. Drying Technology, 31(8), 951–962.
Fabra, M. J., Talens, P., Gavara, R., & Chiralt, A. (2012). Barrier properties of sodium caseinate films as affected by lipid composition and moisture content. Journal of Food Engineering, 109(3), 372–379.
Gol, N. B., Patel, P. R., & Rao, T. V. R. (2013). Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biology and Technology, 85, 185–195.
González-Centeno, M. R., Jourdes, M., Femenia, A., Simal, S., Rosselló, C., & Teissedre, P.-L. (2012). Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). Journal of Agricultural and Food Chemistry, 60(48), 11850–11858.
González-Centeno, M. R., Knoerzer, K., Sabarez, H., Simal, S., Rosselló, C., & Femenia, A. (2014). Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.) – a response surface approach. Ultrasonics Sonochemistry, 21(6), 2176–2184.
Imahori, Y., Takemura, M., & Bai, J. (2008). Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biology and Technology, 49(1), 54–60.
Kamiloglu, S., & Capanoglu, E. (2015). Polyphenol content in figs (Ficus carica L.): effect of sun-drying. International Journal of Food Properties, 18(3), 521–535.
Majidi, H., Minaei, S., Almassi, M., & Mostofi, Y. (2014). Tomato quality in controlled atmosphere storage, modified atmosphere packaging and cold storage. Journal of Food Science and Technology, 51(9), 2155–2161.
Mantilla, N., Castell-Perez, M. E., Gomes, C., & Moreira, R. G. (2013). Multilayered antimicrobial edible coating and its effect on quality and shelf-life of fresh-cut pineapple (Ananas comosus). LWT - Food Science and Technology, 51(1), 37–43.
Marei, N., & Crane, J. C. (1971). Growth and respiratory response of fig (Ficus carica L. cv. Mission) fruits to ethylene. Plant Physiology, 48(3), 249–254.
Martínez-García, J. J., Gallegos-Infante, J. A., Rocha-Guzmán, N. E., Ramírez-Baca, P., Candelas-Cadillo, M. G., & González-Laredo, R. F. (2013). Drying parameters of half-cut and ground figs (Ficus carica L.) var. Mission and the effect on their functional properties. Journal of Engineering, 2013, 8.
Mascaraque, C., Aranda, C., Ocón, B., Monte, M. J., Suárez, M. D., Zarzuelo, A., Marín, J. J. G., Martínez-Augustin, O., & de Medina, F. S. (2014). Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacological Research, 90, 48–57.
Meighani, H., Ghasemnezhad, M., & Bakhshi, D. (2015). Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. Journal of Food Science and Technology, 52(7), 4507–4514.
Meza-Velázquez, J. A., Alanís-Guzmán, G., García-Díaz, C. L., Fortis-Hernandez, M., Preciado-Rangel, P., & Esparza-Rivera, J. R. (2013). Efecto de una película de hidroxipropilmetil celulosa-parafina en melón Cantaloupe (Cucumis melo) almacenado en frío. Revista Mexicana de Ciencias Agrícolas, 4, 259–271.
Minitab 18 Statistical Software (2018). State College, PA: Minitab, Inc. http://www.minitab.com. Accessed 1 Feb 2018.
Nishimura, M., Ohkawara, T., Sato, Y., Satoh, H., Suzuki, T., Ishiguro, K., Noda, T., Morishita, T., & Nishihira, J. (2016). Effectiveness of rutin-rich Tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: a randomised, double-blind, placebo-controlled study. Journal of Functional Foods, 26, 460–469.
Oliveira, D. M., Kwiatkowski, A., Rosa, C. I. L. F., & Clemente, E. (2014). Refrigeration and edible coatings in blackberry (Rubus spp.) conservation. Journal of Food Science and Technology, 51(9), 2120–2126.
Owino, W. O., Nakano, R., Kubo, Y., & Inaba, A. (2004). Alterations in cell wall polysaccharides during ripening in distinct anatomical tissue regions of the fig (Ficus carica L.) fruit. Postharvest Biology and Technology, 32(1), 67–77.
Reyes-Avalos, M. C., Femenia, A., Minjares-Fuentes, R., Contreras-Esquivel, J. C., Aguilar-González, C. N., Esparza-Rivera, J. R., & Meza-Velázquez, J. A. (2016). Improvement of the quality and the shelf life of figs (Ficus carica) using an alginate–chitosan edible film. Food and Bioprocess Technology, 9(12), 2114–2124.
Rößle, C., Brunton, N., Gormley, R. T., Wouters, R., & Butler, F. (2011). Alginate coating as carrier of oligofructose and inulin and to maintain the quality of fresh-cut apples. Journal of Food Science, 76(1), H19–H29.
Selcuk, N., & Erkan, M. (2014). Changes in antioxidant activity and postharvest quality of sweet pomegranates cv. Hicrannar under modified atmosphere packaging. Postharvest Biology and Technology, 92, 29–36.
Solomon, A., Golubowicz, S., Yablowicz, Z., Grossman, S., Bergman, M., Gottlieb, H. E., Altman, A., Kerem, Z., & Flaishman, M. A. (2006). Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). Journal of Agricultural and Food Chemistry, 54(20), 7717–7723.
Szymanowska, U., Złotek, U., Karaś, M., & Baraniak, B. (2015). Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chemistry, 172, 71–77.
Turan, A., & Celik, I. (2016). Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. International Journal of Biological Macromolecules, 91, 554–559.
Tzoumaki, M. V., Biliaderis, C. G., & Vasilakakis, M. (2009). Impact of edible coatings and packaging on quality of white asparagus (Asparagus officinalis L.) during cold storage. Food Chemistry, 117(1), 55–63.
Usenik, V., Štampar, F., & Veberič, R. (2009). Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chemistry, 114(2), 529–534.
Valenzuela, C., Tapia, C., López, L., Bunger, A., Escalona, V., & Abugoch, L. (2015). Effect of edible quinoa protein-chitosan based films on refrigerated strawberry (Fragaria × ananassa) quality. Electronic Journal of Biotechnology, 18(6), 406–411.
Valero, D., Díaz-Mula, H. M., Zapata, P. J., Guillén, F., Martínez-Romero, D., Castillo, S., & Serrano, M. (2013). Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biology and Technology, 77, 1–6.
Villalobos, M. D. C., Serradilla, M. J., Martín, A., Ruiz-Moyano, S., Pereira, C., & Córdoba, M. D. G. (2014). Use of equilibrium modified atmosphere packaging for preservation of ‘San Antonio’ and ‘Banane’ breba crops (Ficus carica L.). Postharvest Biology and Technology, 98, 14–22.
Wang, S. Y., & Gao, H. (2013). Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT- Food Science and Technology, 52(2), 71–79.
Wang, X., Kong, D., Ma, Z., & Zhao, R. (2015). Effect of carrot puree edible films on quality preservation of fresh-cut carrots. Irish Journal of Agricultural and Food Research, 54(1), 64–71.
Wu, X., Beecher, G. R., Holden, J. M., Haytowitz, D. B., Gebhardt, S. E., & Prior, R. L. (2006). Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry, 54(11), 4069–4075.
Funding
This work was financially supported by the Programa Integral de Fortalecimiento Institucional (PIFI) of the Mexican Government and the Spanish Government (MICINN) (AGL 2012–4627). The author M.C. Reyes-Avalos received funding from the Consejo Nacional de Ciencia y Tecnología (CONACYT) of México and R. Minjares-Fuentes from the Goverment of the Balearic Islands, the research fellowship (FPI/1477/2012) of the “Conselleria d’Educació, Cultura i Universitats,” and the European Social Fund (FSE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reyes-Avalos, M., Minjares-Fuentes, R., Femenia, A. et al. Application of an Alginate–Chitosan Edible Film on Figs (Ficus carica): Effect on Bioactive Compounds and Antioxidant Capacity. Food Bioprocess Technol 12, 499–511 (2019). https://doi.org/10.1007/s11947-018-2226-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2226-y