Abstract
The aim of the current research was to fabricate, characterize, and compare physical, mechanical, antimicrobial, antioxidant, and release properties of whey protein isolate (WPI)-based films containing free or nanoencapsulated thyme (Thymus vulgaris) extract (TE) at concentrations of 0, 5, 10, and 15% w/w of WPI. Nanoliposomes with an average size of 350 nm were prepared using thin-film hydration and sonication method. The data obtained from FTIR reflected the occurrence of some new interactions between WPI and nanoliposomes. XRD results approved the negative effect of free TE on the crystallinity of WPI. Besides, SEM images showed that free TE caused the cracks and holes in the WPI matrix to increase. However, the encapsulated TE did not show these negative effects. The nanoliposome incorporation improved the mechanical stiffness, leading to a decrease in the water vapor permeability (WVP). The possible antimicrobial activity of the films containing TE-loaded nanoliposomes against Staphylococcus aureus and Escherichia coli was decreased in comparison to the free TE-incorporated films, probably due to the inhibition effect of the encapsulation preventing the release of TE from the matrix. In addition, the antioxidant potential of the films containing TE-loaded nanoliposomes was lower than that of free TE-incorporated films. Release studies indicated that the migration of TE in ethanol 95% simulant decreased significantly by the nanoencapsulation of TE. However, the release rate increased by an increase in temperature in both types of active films. Therefore, this work showed that there is a potential for the production of antioxidant and antimicrobial controlled-release nanoactive WPI-TE films for use in food packaging and medical fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most food products are perishable and sometimes associated with outbreaks of foodborne illnesses or quality loss due to the contamination by microbial pathogens or chemical/biochemical undesirable changes. Many synthetic and/or natural preservatives have been used as food additives to extend the shelf life of foods. The current major mode of application of food additives is direct introduction to the food system in free form (Devlieghere et al. 2004). However, undesirable interactions of these active agents with food components reduce their efficacy against pathogens and other spoilage effective factors, thus requiring the addition of larger preservative quantities to preserve the quality of food during storage and distribution (Campos et al. 2011). Moreover, the most bioactive compounds of natural preservatives are chemically reactive species, which can bring about considerable problems when being embedded into a complex food system, such as negative effects on the physical stability or integrity of the food chemistry as well as the degradation of the biological activity of bioactive compounds (Haghju et al. 2016).
One of the most promising systems to protect foods against oxidation and microbial or chemical deterioration, without conventional limitations, is to use active packaging (Decker et al. 2010). Active releasing packaging is a type of food preservation system in which an antioxidant or antimicrobial agent is incorporated into the package instead of being added at high levels directly unto the food. Biodegradable polymers such as starch, poly (lactic acid) (PLA), pectin, chitosan, gelatin, and cellulose derivatives offer alternative packaging options, advantageous to the synthetic packaging polymers because they do not contribute to the environmental pollution. Several researches have been conducted on using biopolymers for the preparation of active packaging of foods (Kuorwel et al. 2011).
Whey protein isolate (WPI), an abundant by-product in the cheese-making industry, has been successfully employed as a raw material for the preparation of biodegradable polymers (Ghanbarzadeh and Oromiehi 2008; Kadam et al. 2013). WPI has desirable film-forming properties, high barrier and mechanical properties, as well as good transparency and biodegradability (Bahram et al. 2014; Ramos et al. 2013). Apart from acting as selective barriers for moisture, gas, and solute migration, these films may be operated as carriers of many functional ingredients. Nowadays, great efforts are being made to prepare active packaging from WPI for food-packaging applications. Various additives such as antioxidants and antimicrobial agents, flavors, spices, and colorants have been added to WPI-based active films (Khwaldia et al. 2004).
Thyme (Thymus vulgaris L.), a member of the Lamiaceae family, is an aromatic and medicinal plant native to the western Mediterranean region of Europe (Lee et al. 2005). Thyme acts as an expectorant and spasmolytic agent for the bronchia, and it is part of herbal teas and infusions in folk medicine. Also, in nonmedicinal uses, thyme serves as preservative for foods and as an aromatic ingredient for seasoning various dishes (Díaz-Maroto et al. 2005). Seven major bioactive compounds of thyme essential oil are 1,8-cineole, linalool, α-terpineol, geraniol, transthujan-4-ol and terpinen-4-ol, thymol, and carvacrol. Caffeic, syringic, and genistic acids are the main phenolic acids in thyme (Grigore et al. 2010). Recent studies have shown that thyme extract and essential oil have strong antimicrobial (Imelouane et al. 2009; Rota et al. 2008), antifungal (Del Toro-Sánchez et al. 2010; Rasooli and Abyaneh 2004), and antioxidant activities (Alizadeh 2013; Roby et al. 2013).
Thyme extract (TE), as a natural preservative, can be used in the preparation of food active packaging. The protection of the active compounds against environmental factors (e.g., oxygen, light, moisture, and pH) is a major challenge of bioactive compound applications. Negative effects on the transparency and optical characteristics of films, poor miscibility and phase separation during the film-forming process, and the thermal or chemical degradation of the bioactive compounds during film production and food storage are the most common shortcomings of the direct addition of bioactive compounds into biodegradable active films (Haghju et al. 2016). The second problem of active packaging is controlling the migration of additives from the package to the food. Due to their low molecular weight and thus high diffusion coefficients, the release rates of most active compounds from films are potentially rapid, leading to the loss of prolonged activity of the active packaging during the shelf life of food products (Donsi et al. 2011). The encapsulation of TE might represent an alternative to overcome the problems related to the direct application of TE in food and/or active packaging. Liposomes are vesicles consisting of one or several bilayer membranes. In the food industry, liposomes have been investigated in terms of delivering proteins, enzymes, vitamins, antimicrobials, antioxidants, and flavors (Sherry et al. 2013). The choice of nanoliposomes has been preferred to liposomes, since they provide more surface area and have the potential to increase solubility, enhance bioavailability, improve controlled release, and enable precision targeting of the encapsulated material to a greater extent (Fathi et al. 2012; Gorjikhah et al. 2017; Mozafari 2010). The incorporation of nanoencapsulated essential oils (EOs) and herbal extracts in the form of nanoliposomes and nanoemulsions in various biopolymer-based active films such as alginate (Acevedo-Fani et al. 2015; Salvia-Trujillo et al. 2015), cornstarch and sodium caseinate (Jiménez et al. 2014), fish gelatin (Wu et al. 2015), and chitosan (Almasi et al. 2016; Haghju et al. 2016) has been described.
As the only research on the fabrication of WPI-based nano-active film by our other research group, Ghadetaj et al. (2018) prepared WPI active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. EO and reported that the nanoencapsulation improved the stability of the EO, resulting in the control of its release from the active film. Apart from this research, no former study used WPI film as the carrier of nanoencapsulated natural preservatives for the preparation of nano-active packaging materials. The aim of this work was to characterize morphological, barrier, mechanical, thermal, antimicrobial, antioxidant, and release properties of films cast from mixtures of WPI solution and various concentrations of TE-loaded nanoliposomes. Moreover, the influence of free and encapsulated TE was compared in order to establish a possible enhancement of TE functionality due to the encapsulation.
Materials and Methods
Materials
Whey protein isolate (WPI) (85% protein) was purchased from Arla Food Ingredient (Denmark). Hydroalcoholic extract of thyme (Thymus vulgaris L.) (concentration of 75%) was obtained from AdonisHerb Co. (Iran). To form nanoliposomes, phospholipid (l-α-lecithin granular, 99%) was obtained from Acros Organics (Geel, Belgium). Water used in the preparation of liposomes was purified and deionized using standard procedures. Glycerol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and other solvents and reagents (analytical grade) were purchased from Sigma-Aldrich (Chemie, Steinheim, Germany).
Preparation and Characterization of Nanoliposomes
Multi-lamellar vesicles (MLVs) were prepared according to our previous work (Haghju et al. 2016) using 60 mg lecithin and 10 mL thyme extract (TE) by thin-film hydration and sonication method. Lecithin and TE were mixed and their mixture was dissolved in dichloromethane/methanol (1:1) in a 100-mL round-bottom flask. Then, the resulting organic solvent was removed by a rotary evaporator for 6 h until a thin film was formed on the walls. After that, the lipid film was hydrated in 10 mL of distilled water and the mixture was stirred for 30 min at a temperature above the gel–liquid transition temperature (Tc) of the amphiphiles (~ 55 °C). In order to produce unilamellar nanovesicles, the liposomal suspensions were sonicated for 5 min at 20 kHz and 100 W through a probe-type sonicator (Sonics & Materials Vibracell, England). Then, the samples were cooled during the sonication using an ice-water bath to prevent heating. Finally, the suspension of nanoliposomes was stored in a refrigerator for further characterization and utilization.
The mean diameter and particle size distribution of the liposomes were determined using the dynamic light scattering (DLS) technique employing a Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK) considering the method of Zhang (Zhang et al. 2012). Prior to size measurement, the samples were diluted (1:100) with distilled water. All measurements were carried out at 25 °C in three replicates. The electrophoretic mobility or zeta potential of the nanoliposomes was measured in the aqueous dispersion by means of a Malvern Zeta Sizer Nano ZS (Malvern Instruments, Worcestershire, UK) at 25 °C. It determines the surface charge at the interface of the droplets dispersed in the aqueous solution. Dispersion was diluted to a particle concentration of 0.01% using distilled water.
Preparation of WPI Active Films
WPI films were prepared according to the method of Hassannia-Kolaee et al. (2016) with some modifications. First, 8 g WPI was dissolved in 100 mL distilled water and the solution’s pH was adjusted to 8 by using NaOH 0.1 N. Then, the solution was heated at 90 °C for 30 min and stirred with a magnetic stirrer. After cooling to room temperature, glycerol (40% w/w of WPI) was added as plasticizer. Afterwards, the free and encapsulated TE at three concentrations (5, 10, and 15% w/w of WPI) were mixed into the WPI solution and stirred at 300 rpm for 1 h. A constant amount (40 mL) of the filmogenic solutions was casted in a 10-cm-diameter Petri dish and dried for 48 h at ambient conditions (25 °C). Dried films with 0.12 ± 0.02 mm thickness, measured with an Alton M820–25 handheld micrometer (Beijing, China), were peeled and stored in a desiccator at 25 °C until analysis. The prepared films containing free extract and nanoliposomal encapsulated extract were coded as TE and TE.NL samples, respectively. Before the tests, all the samples were conditioned in a desiccator at 25 °C and 50% RH, by using magnesium nitrate-6-hydrate saturated solutions for 24 h. All the samples were prepared in triplicate.
Characterization of Films
Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) spectroscopic analyses of films were performed by a spectrophotometer (Shimadzu 4100, Thermo Nicolet) with a resolution of 2 cm−1 and a scanning range from 4000 to 400 cm−1. All the samples were analyzed using a KBr-pellet method. Three droplets of cast film solutions were dropped on a KBr pellet and dried gently, and then the KBr pellet was pressed into a small pellet, about 1 mm thick.
X-Ray Diffraction Analysis
X-ray diffraction (XRD) measurements were performed on the films, using a Bruker D8 Advance X-ray diffractometer (Karlsruhe, Germany) operating at a CuKα wavelength of 0.154 nm. The samples were exposed to the X-ray beam with the X-ray generator running at 40 kV and 40 mA. Scattered radiation was detected at ambient temperature in the angular region (2θ) of 2–40° at a rate of 1°/min and a step size of 0.02°.
Scanning Electron Microscopy
The morphologies of the surface and fracture surface of the films resulting from the tensile tests were studied with a scanning electron microscope (SEM) (Hitachi 4300S, Japan) at room temperature. The accelerating voltage applied was 5.0 kV. A gold coating of a few nanometers in thickness was coated on the fracture surfaces. For studying the cross section, the samples were viewed perpendicular to the fracture surface.
Mechanical Properties
Ultimate tensile strength (UTS), elongation at break (EB), and Young’s modulus (YM) of each film sample were measured at room temperature with a Universal Testing Machine (Model H100K-S, England) according to the standard test method ASTM D-882-10 (ASTM 2010). Dumbbell-formed test specimens with dimensions of 80 mm in length and 10 mm in width were used. The gauge length and the crosshead speed were 50 mm and 50 mm/min, respectively. UTS, EB, and YM were calculated by Eqs. (1), (2), and (3) respectively:
where Fmax is the maximum load (N), A is the cross-section area (m2), Lmax is the extension at the moment of rupture (m), L0 is the initial length of the specimen (m), F is force (N), and ∆L is change in length. All measurements were performed in three replicates.
Water Vapor Permeability
Water vapor permeability (WVP) was determined gravimetrically according to ASTM method E96-05 (ASTM 2005). Glass bottles, with a diameter of 20 mm and depth of 45 mm, were used to perform the test. The bottles were filled with 3 g of CaCl2 (for maintaining a relative humidity of 0% RH) and covered with a film specimen. The bottles covered with the film were placed in a container containing K2SO4 supersaturated solution (97% RH) at 25 °C. They were then weighed ten times at 3-h intervals. The water vapor transmission rate (WVTR) was determined from the slope of the mass change of the bottle versus time curve divided by the area of the glass bottle mouth (m2). Then, the WVP of the film was calculated using Eq. (4):
where WVTR is measured as the water vapor transmission rate (g/m2 s) through a film, L is the mean thickness of the film (m), and ∆P is the partial water vapor pressure difference (Pa) across the film. All measurements were performed in three replicates.
Measurement of Released TE
Film specimens were cut into 2 × 2 cm2 and were immersed in a glass vial containing 10 mL of 95% ethanol (v/v), as the simulant of fatty foodstuffs (Veraart 2010), under agitation (150 rpm) in a shaker at 4, 25, and 40 °C. At pre-determined time periods, an aliquot (2.0 mL) of the solution was removed, analyzed, and then returned into the vial. This procedure was repeated at time intervals during 168 h. The TE concentration in ethanol was determined using a UV/VIS spectrophotometer (Unico, S 2100 SUV, Dayton, NJ, USA) at 383 nm. All measurements per time and sample were performed in three replicates.
Antioxidant Activity of the Films
The antioxidant activities of the films were evaluated by the DPPH radical scavenging assay according to the method of Almasi (Almasi et al. 2014). First, approximately 100 mg of film was placed in a flask containing 2 mL of methanol and was stirred for 3 h at room temperature. The supernatant obtained was analyzed for DPPH radical scavenging activity. Then, 5 mL of supernatant was mixed with 0.2 mL of 1 mM methanolic solutions of DPPH. After that, the mixture was vortexed vigorously and left in the dark at room temperature for 30 min. The absorbance was measured at 517 nm against the corresponding blank solution (5 mL of methanol without the presence of the film) using a UV spectrophotometer (Unico, S 2100 SUV, Dayton, NJ). The percentage of DPPH radical scavenging activity was calculated using the following equation:
where Abscontrol and Abssample are the absorbance value at 517 nm of blank sample and DPPH assay solution, respectively. The measurements were performed in three replicates.
Antibacterial Activity
The antibacterial activities of the film samples were evaluated by the agar disk diffusion method with determination of inhibition zones (Almasi et al. 2016, Shahmohammadi Jebel and Almasi 2016). Two foodborne pathogenic bacteria, a Gram-positive bacterium (Staphylococcus aureus ATCC-19111) and a Gram-negative bacterium (Escherichia coli O157:H7 ATCC-11775), were used as testing organisms. First, the Gram-positive and Gram-negative bacteria were incubated separately in brain heart infusion (BHI) broth (Sigma-Aldrich Co., Ltd.) at 37 °C and in tryptic soy broth (TSB) (Sigma-Aldrich Co., Ltd.) at 30 °C, respectively, under aerobic conditions for 16 h. Then, old cultured broths (0.1 mL) from each bacterial strain were aseptically transferred to Eppendorf tubes containing 0.9 mL sterile water and were serially diluted twofold. After that, 1 mL of cells containing ~ 108 CFU mL−1 from the diluted broths was uniformly spread on TSB and BHI agar plates. Then, the film samples prepared in the form of a round disk of 5 mm diameter were placed carefully on the surface of an agar medium and subsequently incubated at 37 °C for 24 h. Finally, the zone of inhibition was estimated by measuring the diameter of the bacterial growth inhibition zone around the disk. All measurements were performed in three replicates.
Statistical Analysis
Statistical studies on a completely randomized design were performed with the analysis of variance (ANOVA) procedure in SPSS (Version 21, SPSS Inc., Chicago, IL) software. Duncan’s multiple range test (p ≤ 0.05) was used to detect differences among mean values of film properties. All data were expressed as means ± standard deviation (SD).
Results and Discussion
Characterization of Nanoliposomes
Before determining the effect of nanoliposomes on WPI film properties, it was necessary to ensure the success of nanoliposome production and the evaluation of produced nanocarriers’ diameter. According to the results of DLS, the produced nanoparticles have diameters in the range of 100 to 500 nm and the mean diameter of nanoliposomes was 355 nm. Therefore, the term “nano” liposome might be applied for them. This is because of the fact that in the production of nanocarrier systems, particles with dimensions less than 500 nm are considered as the carriers with nano dimensions (Sherry et al. 2013). Size measurements also revealed that the particle size distribution is monodisperse, proving the high efficiency of the method used in the production of the nanoliposomes.
The zeta potential values of free and TE-loaded nanoliposome-containing solutions were − 52.84 and − 52.46 mV, respectively. The liposome vesicles had charged surfaces with negative potentials in the absence or presence of encapsulated extract. The similarity of zeta potentials for free and TE-incorporated liposomes suggested that polyphenols were located inside the liposome structure rather than coating the surfaces (Haghju et al. 2016). If the absolute value of the zeta potential is between 30 and 60 mV, the nanoliposome system is stable (Wu et al. 2015). The absolute value of the zeta potential of nanoliposomes in this study was greater than 30 mV, showing that TE-containing nanoliposomes were stable enough to be incorporated with film-forming solution.
Characterization of the Films
FTIR Analysis
Figure 1 shows the results of the FTIR test on the active films. The pure WPI film showed several specific absorption peaks including the following: (1) The peaks ranging from 500 to 750 cm−1 were related to absorption bonds of glycerol; (2) the peaks ranging from 900 to 1150 cm−1 were related to N–H bonds and C–N stretching bonds (amide type III); (3) the peaks ranging from 1400 to 1550 cm−1 were related to bending groups of N–H (amide type II); (4) the peaks ranging from 1600 to 1700 cm−1 were related to vibratory stretching bonds of groups C=O and C–N (amide type I); (5) the peaks ranging from 2850 to 2980 cm−1 were related to the C–H stretching bond; and (6) the peaks ranging from 3000 to 3600 cm−1 were related to free O–H and N–H groups on protein strands (Ramos et al. 2013).
By adding free TE, some changes occurred in the spectrogram of the WPI film, the most important of which was the increase of the peaks’ intensity in the range of 2900 cm−1 related to the C–H bond as well as the emergence of a new peak in 3080 cm−1 related to –CH2 groups belonging to the phenolic compounds present in the TE. Besides, the intensity of the peak increased in 1634 cm−1 by adding the extract, attributable to the stretching vibration of the C=C bond in the allyl groups present in phenolic compounds. The intensity of these peaks increased with an increase in the concentration of the extract from 5 to 15%. Moreover, the fourth specified change due to the effect of TE addition was the decrease of the peak intensity in the range of 3400 cm−1, related to free O–H and N–H protein groups. The reduction in the intensity of these bonds and the emergence of new peaks indicated that the extract compounds were able to get linked with the relative protein strands and to stay with the chemical connections within the protein network.
The incorporation of nanoliposomes, however, changed the type of bonds. When the nanoliposomes were added to the WPI film, the intensity of the peak in the range of 2900 cm−1 fell again and the newly created peak in 3080 cm−1 disappeared. This shows that the creation of new connections and a change in the chemical structure of the extract happened by the presence of lecithin. In addition, the two peaks related to the new absorption bonds were observed in 1978 and 2504 cm−1 on the nanoliposome-loaded film. Lecithin in these wavenumbers showed an absorption bond (Jiménez et al. 2014). The intensity of these changes went up by an increase in the amount of nanoliposomes in the film. Another remarkable point in the films containing nanoliposomes was an increase in the intensity of the peak in the range of 3400 cm−1 compared to the films containing free extract. However, the intensity of the peak in the range of 1550–900 cm−1 reduced considerably, suggesting that the nature and the type of nanoliposome bonds with WPI are quite different from those of free extract bonds with these protein chains. However, the nanoliposomes containing TE, in any case, were able to stay in the network of the WPI film, not only by physical entrapment but also by the establishment of chemical bonds. Bahram et al. (2014) reported similar results about the effect of adding cinnamon essential oil on the structural properties of the WPI film.
XRD Analysis
XRD test is used to study the crystalline structure of biopolymer films. Figure 2 shows the X-ray diffractograms of pure WPI films and the films containing the highest concentration of free TE and nanoliposomes containing TE. Diffractograms of other samples have not been presented due to the similar effects of different concentrations on the structural properties of the films. As can be seen, pure WPI films show four specific peaks at 2θ of 10°, 20°, 28°, and the range of 35 to 40°, indicating that WPI has a relatively good crystalline degree; therefore, it can be counted as a semi-crystalline biopolymer. This type of behavior in the XRD test of WPI was similar to that reported by Li et al. (2011).
As specified in Fig. 2, the intensity of all peaks considerably decreased by the addition of the free TE and even some of the peaks, including the peak available in 2θ = 10°, disappeared entirely. This result shows the adverse effect of the extract on the crystalline nature of the WPI film. In fact, the compounds with low molecular weights available in the TE act as a plasticizer in the structure of the WPI film, preventing more compact and powerful connections between protein chains (Kavas et al. 2015; Tokur et al. 2016). Therefore, the structural density in the film network and consequently the crystalline degree reduced. Nevertheless, the crystalline nature of the film was completely preserved when the nanoliposome was added to the film structure, meaning that unlike the extract components, the double amphiphilic molecules of lecithin in nanoliposomes were able to create more and better connections with the hydrophilic protein matrix. Therefore, not only do nanocarriers not have negative interference in the structural density of the film but also they act as effective compounds in increasing the density and maintaining the crystalline nature in the film. These results show that high compatibility between biopolymer and the added active compound plays an important role in the creation of the crystalline structure of the film. As specified in Fig. 2, in the presence of 15% nanoliposome, in addition to the peaks related to WPI, a new specified peak at 2θ = 16° was created, probably related to the crystallization of lecithin molecules during the formation of the film.
Morphology Observation by SEM
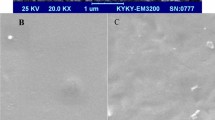
The morphology of WPI films was evaluated by using the SEM test. Figure 3 presents the cross-sectional images of the films after rupture in the tensile test. As it is obvious, the pure WPI film had a completely dense and compact cross section with low porosity. Furthermore, some cracks in the internal structure of the films were created; in addition, the amount of cavities and pores increased when free TE at the level of 15% was added. This would indicate a negative effect of the extract on the integrity of the films and the reduction of its structural density noted in the XRD test results. With the addition of nanoliposomes containing the TE, the amount of cracks as well as the amount of empty cavities in the inner surface of the film reduced, while both the density and integrity of the biopolymer matrix increased. In Fig. 3, it is clear that with the addition of nanoliposomes, the roughness of the internal structure of the films and its ripples increased, probably due to the presence of lecithin molecules in these films.
Figure 4 shows the SEM images from the surface of the films. Pure WPI film had a completely smooth, dense, and uniform surface. The roughness of the surface increased with the addition of the free TE. As it is clear, the sites of the extract appeared with a darker color compared to other parts of the film. TE was spread evenly on the bed of the WPI matrix, indicating relatively good adaptation of this hydroalcoholic extract and WPI protein film (which has active hydrophobic and hydrophilic groups). By comparing the images in Fig. 4, it will be undeniable that the surface roughness of a film like its cross section increased by adding the nanoliposome. This increase in roughness can be attributed to the presence of lecithin in the structure of the film. According to the figure, it is clear that the sites creating roughness are spread evenly, mostly accumulating around the locations containing the extract, confirming the role of lecithin in surrounding the phenolic compounds of the extract. According to the dimensions of nanoliposomes specified in Fig. 4, these images may be used to determine the mean diameter of nanoliposomes which will have similar results with those of the DLS test. The mean diameter of the nanoliposomes in SEM images was calculated as 400 nm. Generally, the results of the SEM test showed that the TE was able to spread evenly on the bed of the WPI film either in free or encapsulated forms. However, in a state of nanoliposome, its negative effect on the structural integrity and density of the film was less and did not cause any chaos on the order of the internal matrix of the film (Wu et al. 2015).
Mechanical Properties
Table 1 shows the mechanical properties of the WPI active film including ultimate tensile strength (UTS), Young’s modulus (YM), and elongation at break (EB). UTS and YM in WPI films were 7.07 and 83.19 MPa, respectively, proving a quite good mechanical strength of the WPI film. With the addition of free TE, both UTS and YM significantly decreased (p ≤ 0.05), and with an increase in the concentration of the extract, the amounts further reduced. Moreover, EB, in 5% concentration of extract, increased and fell again so that the film containing 15% free extract had the lowest UTS (2.09 MPa), the lowest YM (12.85 MPa), and the lowest EB (10.11%). The negative effect of TE on the structure of the WPI film was also proven by the results of SEM and XRD. The molecules of the extract caused a reduction in the mechanical resistance of the film by positioning in the space between the chains and reducing intermolecular connections in the protein matrix. Besides, flexibility due to the reduction of the molecular density was reduced in the high concentrations of the extract (Kavas et al. 2015). Thus, it could be concluded that TE acts like plasticizer and causes a reduction in the mechanical strength of WPI films. In the case of the effect of cinnamon essential oil (Bahram et al. 2014) and natamycin (Ramos et al. 2012), similar results have been reported on the properties of the WPI film.
However, as specified in Table 1, adding nanoliposome had a significant effect on the mechanical properties of the WPI film and UTS was increased significantly with the addition of 5% encapsulated TE. Furthermore, YM and EB increased 1.5 times more than pure WPI film. However, all three mechanical properties of the film reduced over again at higher concentrations. These results suggest that nanoliposomes at low concentrations are able to increase the amount of inter-chain connections by connecting lecithin to active groups of protein chains, leading to an increase in the mechanical strength and flexibility of film. At higher concentrations, with the saturation of the maximum number of possible chemical bonds, extra nanoliposomes like free TE acted as a plasticizer. In addition, being located between the chains and by the reduction of the molecular integrity, they reduced the tensile strength of the film again. Haghju et al. (2016) obtained similar results about the effect of nanoliposomes, carrying the nettle extract, on the mechanical properties of chitosan film. The effect of the other nanocarriers on mechanical properties of biopolymer films was also similar (Imran et al. 2012; Ma et al. 2016; Wu et al. 2015).
Water Vapor Permeability
For active packaging of food, water vapor permeability and preventing moisture from penetrating the food are considerably important. As shown in Fig. 5, the WVP of the control sample was 3.6 × 10−10 g/m h Pa. With the addition of the free TE, WVP increased significantly, and with the increase of the extract’s concentration, this increment was higher, the reason for which was the negative effect of the extract on inter-molecular connections. Therefore, increasing the free spaces between protein chains caused the free movement of water vapor molecules in the space between protein networks. The results showed that the active film containing 15% free extract had the maximum amount of WVP (5.9 × 10−10 g/m h Pa). However, the amount of WVP reduced by adding nanoliposomes. With an increase in the concentration of nanoliposomes in the film, WVP became less and the TE.NL.15 sample showed the minimum WVP (3.3 × 10−10 g/m h Pa). The structure of lecithin in the form of nanoliposome in TE.NL films caused more inter-chain interactions, less empty space and paths, and thus less WVP. These changes occurred because of better reaction of outer membrane polar groups of liposomes with whey protein chains. On the other hand, the presence of nanoliposomes in the structure of the film made the water molecules use a tortuous path in order to pass through the cross section of the film. This torsion reduced the rate of migration of water vapor molecules. The WVP reduction, by adding nanoliposomes containing the extract, had similarities with the results reported recently about the biopolymer-based edible films containing nanocarriers (Acevedo-Fani et al. 2015; Haghju et al. 2016; Imran et al. 2012; Ma et al. 2016; Wu et al. 2015).
Release Properties
To determine the effect of nanoencapsulation of TE on its release from the WPI matrix, the prepared films were kept in contact with ethanol 95% (as simulant of fatty foods). The kinetics of migration of TE from active film containing free and encapsulated extracts is shown in Fig. 6. The amount of the released extract from the films containing nanoliposome was less than that of the active films containing free extracts at all studied temperatures. On the other hand, the kinetics of TE release increased with an increase in the amount of extract at both films. TE release from nanoliposome-loaded film containing 15% extract occurred in the minimum speed and reached its highest amount at 40 °C after 168 h which was less than the released amount of other films, whereas the film containing 15% free extract showed the maximum release after 72 h at the same temperature. Thus, the nanoencapsulation of TE can be the main reason of release reduction to simulant in the films containing nanoliposome (Almasi et al. 2016; Jamshidian et al. 2012; Wu et al. 2015).
Similar results have been reported by Wu et al. (2015) who studied the diffusion of cinnamon essential oil in nanoliposome-loaded gelatin films. Ghadetaj et al. (2018) also reported a similar effect of nanoemulsion formation on the release kinetics of Grammosciadium ptrocarpum Bioss. EO from WPI films. Important factors in the release rate of the solid polymer phase to simulant liquid phase are the entrapment intensity inside the polymer matrix, the molecular weight of the active substance, and the amount of molecular bond with the polymer matrix. It can be concluded that the nanoliposomes added to the active films show less release rate due to properties such as high crystallization (approved by XRD results), high molecular weight, more structural density (approved by SEM images), and stronger connections with biopolymer chains (approved by the FTIR test). Nanoliposomes added to nano-active films in comparison with the active films containing free extract causes the creation of a film with a higher molecular weight of the migrant substance. A higher release rate of the extract in active films containing free TE is due to the low molecular weight and higher movement of TE compared to nanoliposome-loaded films, providing an easy pass from films to simulant.
Antioxidant Activity
The antioxidant activity of films was measured using the method of determination of inhibitory strength of the free radical DPPH, the results of which are shown in Table 2. The WPI control film showed low antioxidant activity (3.84%), probably because free radical reaction with the free amine group (NH2) remained to form macromolecule stable radicals. On the other hand, amine groups can create ammonium groups (\( {NH}_3^{+} \)) by the absorption of hydrogen ions from solution (Yen et al. 2008). The results showed that the antioxidant activity of the films increased significantly (p ≤ 0.05) by adding TE. With an increase in the amount of extract, the activity further increased. The film containing 15% free extract showed the utmost inhibitory activities on DPPH (36.94%). The reason for the antioxidant activity of thyme is the persistence of phenolic compounds in its structure, i.e., these compounds break the chain reactions of free radicals (Güder and Korkmaz 2012). Hydroxyl groups of phenolic compounds often act as hydrogen donors (Hamilton 1998). As shown in Table 1, the antioxidant activity of the films containing encapsulated TE is less than that of the films containing the free extract with a similar concentration. This may be due to a reaction between lecithin and amine groups or free hydroxyl whey protein, causing a reduction in the release of extract into the methanolic solution and finally a reduction of inhibitory activity of TE against free radicals. The chemical interactions between the whey protein macromolecules and liposomes were approved by FTIR analysis. The bonds between antioxidants and WPI matrix might be formed by means of hydrogen bonds, especially at the time of the film’s formation. Hence, it can be concluded that a small amount of antioxidants was extracted by methanol, while the majority of these antioxidants were extracted when free TE was added to the film. The low molecular weight of the free extract and low chemical bonds between bioactive compounds and whey protein chains in the film containing free TE, in comparison with the film containing nanoliposomes, are the reasons for the higher extraction rate and higher antioxidant activity of the films containing free TE (Almasi et al. 2016). Therefore, in order to get a film with high antioxidant properties and better inhibitory activity of free radicals, greater amounts of antioxidants should be added to the nanoliposome-loaded films.
The results of these tests are similar to those of previous studies of Moradi et al. 2012 and Siripatrawan and Harte 2010, who studied the effect of green tea extract and grape seed extract, respectively, on the chitosan film. Ghadetaj et al. (2018) observed similar trends in antioxidant activity of WPI films containing Grammosciadium ptrocarpum Bioss. EO emulsion and nanoemulsion.
Antimicrobial Activity
The extract and essential oil of thyme usually indicate a good antimicrobial property. Therefore, it was expected that active films have significant antimicrobial activity as well. Table 2 suggests the inhibitory strength of active films against the growth of Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus). As can be seen, the WPI control film has relatively lower antimicrobial property which is likely due to sulfhydryl and amide groups in its side chains (Fernández-Pan et al. 2012). By the addition of TE, the antimicrobial activity of the film increased considerably. Besides, with an increase in the concentration of the extract in both types of films containing free and encapsulated extracts, the antimicrobial activity increased.
The phenolic compounds in the thyme extract—particularly thymol and carvacrol—have significant antimicrobial activity (Imelouane et al. 2009; Fernández-Pan et al. 2012). For this reason, the film containing TE can inhibit the growth of test case microorganisms. As specified in Table 2, in a specific concentration, the antimicrobial activity of the extract encapsulated in the nanoliposome is significantly less than when the free extract is used. It occurs due to the creation of the chemical bonds and an increase in the extract’s molecular weight, reducing the speed of the migration of extract from the film to the surface of the culture; therefore, the antimicrobial activity of the film is reduced. In addition, the connection between the active compounds of the extract and lecithin molecules may reduce their antimicrobial activity.
The other remarkable point in Table 2 is that the antimicrobial activity of the TE on S. aureus in all concentrations is considerably higher compared to that on E.coli. It is because of the structural differences in the bacterial membrane. The cell wall of Gram-negative bacteria such as E. coli is more complex than that of Gram-positive bacteria such as S. aureus. It has a lipopolysaccharide layer in addition to the peptidoglycan layer, increasing the impermeability and resistance of Gram-negative bacteria against the penetration of active oxygen species and the destruction of the wall (Noori et al. 2018).
Conclusions
Thyme extract (TE)-incorporated WPI films were prepared by adding free and nanoliposomal forms of extract and were characterized and compared with pure WPI films. FTIR results indicated that some interactions occurred between WPI chains and nanoliposomes. Both barriers and mechanical properties of the films were significantly affected by the addition of different concentrations of free TE and nanoliposomes. The strong antimicrobial activity of the films containing TE-loaded nanoliposomes was observed against S. aureus in comparison to E. coli. However, the antimicrobial activity was diminished in nano-active films in comparison to free TE-loaded samples. A similar trend was observed for the antioxidant activity of films. Our results also indicated that the incorporation of TE to WPI film led to a sustained-release effect, which had potential for using the developed film as a release-controlled active film. The results indicated that the addition of TE in the nanoliposomal form to WPI film is able to slow down the release rate of the extract. More research will be needed to determine the efficacy of TE-loaded WPI-based active films in food packaging applications in order to explore their potential antimicrobial and antioxidant activities. The application of TE-loaded nano-active films as a novel food protection system has shown promising effect in the packaging of a variety of food products. This research indicated that the antimicrobial effect of TE-loaded WPI film is more than its antioxidant activity. Therefore, it would be suitable for those foods such as meat, products, cheese, nuts, fruits, and vegetables, suffering from microbial deterioration as their main problems. However, its antioxidant activity could be examined on the protection of edible oils, butter and other fatty foods. Further studies are required to investigate the effects of fabricated active films on the shelf-life extension of real food systems.
References
Acevedo-Fani, A., Salvia-Trujillo, L., Rojas-Graü, M. A., & Martín-Belloso, O. (2015). Edible films from essential-oil-loaded nanoemulsions: physicochemical characterization and antimicrobial properties. Food Hydrocolloids, 47, 168–177.
Alizadeh, A. (2013). Essential oil constituents, phenolic content and antioxidant activity in Iranian and British Thymus vulgaris L. International Journal of Agriculture and Crop Sciences, 6, 213–218.
Almasi, H., Ghanbarzadeh, B., Dehghannya, J., Entezami, A. A., & Khosrowshahi, A. A. (2014). Development of a novel controlled-release nanocomposite based on poly (lactic acid) to increase the oxidative stability of soybean oil. Food Additives & Contaminants: Part A, 31(9), 1586–1597.
Almasi, H., Zandi, M., Beigzadeh, S., Haghju, S., & Mehrnow, N. (2016). Chitosan films incorporated with nettle (Urtica Dioica L.) extract-loaded nanoliposomes: II. Antioxidant activity and release properties. Journal of Microencapsulation, 33(5), 449–459.
ASTM (2005). Standard test methods for water vapor transmission of material. E96–05 annual book of ASTM.
ASTM (2010). Standard test methods for tensile properties of thin plastic sheeting. D882–10 annual book of ASTM.
Bahram, S., Rezaei, M., Soltani, M., Kamali, A., Ojagh, S. M., & Abdollahi, M. (2014). Whey protein concentrate edible film activated with cinnamon essential oil. Journal of Food Processing and Preservation, 38(3), 1251–1258.
Campos, C. A., Gerschenson, L. N., & Flores, S. K. (2011). Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology, 4, 849–875.
Decker, E. A., Elias, R. J., Mcclements, D. J. (2010). Oxidation in foods and beverages and antioxidant applications: management in different industry sectors. Woodhead Publishing Limited, 125–134.
Del Toro-Sánchez, C., Ayala-Zavala, J., Machi, L., Santacruz, H., Villegas-Ochoa, M., Alvarez-Parrilla, E., & González-Aguilar, G. (2010). Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 67(3-4), 431–441.
Devlieghere, F., Vermeiren, L., & Debevere, J. (2004). New preservation technologies: possibilities and limitations. International Dairy Journal, 14(4), 273–285.
Díaz-Maroto, M. C., Díaz-Maroto Hidalgo, I. J., Sánchez-Palomo, E., & Pérez-Coello, M. S. (2005). Volatile components and key odorants of fennel (Foeniculum vulgare mill.) and thyme (Thymus vulgaris L.) oil extracts obtained by simultaneous distillation−extraction and supercritical fluid extraction. Journal of Agricultural and Food Chemistry, 53(13), 5385–5389.
Donsi, F., Annunziata, M., Sessa, M., & Ferrari, G. (2011). Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Science and Technology, 44(9), 1908–1914.
Fathi, M., Mozafari, M. R., & Mohebbi, M. (2012). Nanoencapsulation of food ingredients using lipid based delivery systems. Trends in Food Science & Technology, 23(1), 13–27.
Fernández-Pan, I., Royo, M., & Ignacio Maté, J. (2012). Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. Journal of Food Science, 77, 41–56.
Ghadetaj, A., Almasi, H., & Mehryar, L. (2018). Development and characterization of whey protein isolate active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. essential oil. Food Packaging and Shelf Life, 16, 31–40.
Ghanbarzadeh, B., & Oromiehi, A. (2008). Biodegradable biocomposite films based on whey protein and zein: barrier, mechanical properties and AFM analysis. International Journal of Biological Macromolecules, 43(2), 209–2015.
Gorjikhah, F., Azizi Jalalian, F., Salehi, R., Panahi, Y., Hasanzadeh, A., Alizadeh, E., Akbarzadeh, A., & Davaran, S. (2017). Preparation and characterization of PLGA-β-CD polymeric nanoparticles containing methotrexate and evaluation of their effects on T47D cell line. Artificial Cells, Nanomedicine, and Biotechnology, 45(3), 432–440.
Grigore, A., Paraschiv, I., Colceru-Mihul, S., Bubueanu, C., Draghici, E., & Ichim, M. (2010). Chemical composition and antioxidant activity of Thymus vulgaris L. volatile oil obtained by two different methods. Romanian Biotechnological Letters, 15, 5436–5443.
Güder, A., & Korkmaz, H. (2012). Evaluation of in-vitro antioxidant properties of hydroalcoholic solution extracts Urtica dioica L., Malva neglecta Wallr. and their mixture. Iranian Journal of Pharmaceutical Research: IJPR, 11(3), 913–923.
Haghju, S., Beigzadeh, S., Almasi, H., & Hamishehkar, H. (2016). Chitosan films incorporated with nettle (Urtica dioica L.) extract-loaded nanoliposomes: I. Physicochemical characterisation and antimicrobial properties. Journal of Microencapsulation, 33(5), 438–448.
Hamilton, R. J. (1998). Lipid analysis in oils and fats. Springer science & business media.
Hassannia-Kolaee, M., Khodaiyan, F., Pourahmad, R., & Shahabi-Ghahfarrokhi, I. (2016). Development of ecofriendly bionanocomposite: whey protein isolate/pullulan films with nano-SiO 2. International Journal of Biological Macromolecules, 86, 139–144.
Imelouane, B., Amhamdi, H., Wathelet, J. P., Ankit, M., Khedid, K., & El Bachiri, A. (2009). Chemical composition and antimicrobial activity of essential oil of thyme (Thymus vulgaris) from eastern Morocco. International Journal of Agriculture and Biology, 11, 205–208.
Imran, M., Revol-Junelles, A. M., René, N., Jamshidian, M., Akhtar, M. J., Arab-Tehrany, E., Jacquot, M., & Desobry, S. (2012). Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocolloids, 29(2), 407–419.
Jamshidian, M., Tehrany, E. A., & Desobry, S. (2012). Release of synthetic phenolic antioxidants from extruded poly lactic acid (PLA) film. Food Control, 28(2), 445–455.
Jiménez, A., Sánchez-González, L., Desobry, S., Chiralt, A., & Tehrany, E. A. (2014). Influence of nanoliposomes incorporation on properties of film forming dispersions and films based on corn starch and sodium caseinate. Food Hydrocolloids, 35, 159–169.
Kadam, D. M., Thunga, M., Wang, S., Kessler, M. R., Grewell, D., Lamsal, B., & Yu, C. (2013). Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. Journal of Food Engineering, 117(1), 133–140.
Kavas, G., Kavas, N., & Saygili, D. (2015). The effects of thyme and clove essential oil fortified edible films on the physical, chemical and microbiological characteristics of kashar cheese. Journal of Food Quality, 38(6), 405–412.
Khwaldia, K., Perez, C., Banon, S., Desobry, S., & Hardy, J. (2004). Milk proteins for edible films and coatings. Critical Reviews in Food Science and Nutrition, 44(4), 239–251.
Kuorwel, K. K., Cran, M. J., Sonneveld, K., Miltz, J., & Bigger, S. W. (2011). Antimicrobial activity of biodegradable polysaccharide and protein-based films containing active agents. Journal of Food Science, 76, 90–102.
Lee, S. J., Umano, K., Shibamoto, T., & Lee, K. G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chemistry, 91(1), 131–137.
Li, Y., Jiang, Y., Liu, F., Ren, F., Zhao, G., & Leng, X. (2011). Fabrication and charachterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocolloids, 25, 1098–1104.
Ma, Q., Zhang, Y., Critzer, F., Davidson, P. M., Zivanovic, S., & Zhong, Q. (2016). Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocolloids, 52, 533–542.
Moradi, M., Tajik, H., Rohani, S. M. R., Oromiehie, A. R., Malekinejad, H., Aliakbarlu, J., & Hadian, M. (2012). Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Science and Technology, 46(2), 477–484.
Mozafari, M. (2010). Nanoliposomes: Preparation and Analysis. Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers, 605, 29–50.
Noori, S., Zeynali, F., & Almasi, H. (2018). Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control, 84, 312–320.
Ramos, Ó. L., Silva, S. I., Soares, J. C., Fernandes, J. C., Poças, M. F., Pintado, M. E., & Malcata, F. X. (2012). Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Research International, 45(1), 351–361.
Ramos, Ó. L., Reinas, I., Silva, S. I., Fernandes, J. C., Cerqueira, M. A., Pereira, R. N., Vicente, A. A., Pocas, M. F., Pintado, M. E., & Malcata, F. X. (2013). Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids, 30(1), 110–122.
Rasooli, I., & Abyaneh, M. R. (2004). Inhibitory effects of thyme oils on growth and aflatoxin production by aspergillus parasiticus. Food Control, 15(6), 479–483.
Roby, M. H. H., Sarhan, M. A., Selim, K. A. H., & Khalel, K. I. (2013). Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Industrial Crops and Products, 43, 827–831.
Rota, M. C., Herrera, A., Martínez, R. M., Sotomayor, J. A., & Jordán, M. J. (2008). Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control, 19(7), 681–687.
Salvia-Trujillo, L., Rojas-Graü, M. A., Soliva-Fortuny, R., & Martín-Belloso, O. (2015). Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biology and Technology, 105, 8–16.
Shahmohammadi Jebel, F., & Almasi, H. (2016). Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydrate Polymers, 149, 8–19.
Sherry, M., Charcosset, C., Fessi, H., & Greige-Gerges, H. (2013). Essential oils encapsulated in liposomes: a review. Journal of Liposome Research, 23(4), 268–275.
Siripatrawan, U., & Harte, B. R. (2010). Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloids, 24(8), 770–775.
Tokur, B. K., Sert, F., Aksun, E. T., & Ozogul, F. (2016). The effect of whey protein isolate coating enriched with thyme essential oils on trout quality at refrigerated storage (4±2°C). Journal of Aquatic Food Product Technology, 25(4), 585–596.
Veraart, R. (2010). Compliance testing, declaration of compliance, and supporting documentation in the EU. In R. Rijk, & R. Veraart (eds.) Global Legislation for Food Packaging Materials. 199–201. Germany: Wiley-VCH Publishing
Wu, J., Liu, H., Ge, S., Wang, S., Qin, Z., Chen, L., Zheng, Q., Liu, Q., & Zhang, Q. (2015). The preparation, characterization, antimicrobial stability and in vitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocolloids, 43, 427–435.
Yen, M. T., Yang, J. H., & Mau, J. L. (2008). Antioxidant properties of chitosan from crab shells. Carbohydrate Polymers, 74(4), 840–844.
Zhang, H., Tehrany, E. A., Kahn, C., Ponçot, M., Linder, M., & Cleymand, F. (2012). Effects of nanoliposomes based on soya, rapeseed and fish lecithins on chitosan thin films designed for tissue engineering. Carbohydrate Polymers, 88(2), 618–627.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aziz, S.GG., Almasi, H. Physical Characteristics, Release Properties, and Antioxidant and Antimicrobial Activities of Whey Protein Isolate Films Incorporated with Thyme (Thymus vulgaris L.) Extract-Loaded Nanoliposomes. Food Bioprocess Technol 11, 1552–1565 (2018). https://doi.org/10.1007/s11947-018-2121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2121-6