Abstract
Thyme essential oil (TO) is a good antimicrobial agent, however, its high volatility and reactivity limits its application as food preservative. β-cyclodextrin (β-CD) is able to encapsulate organic molecules, forming host–guest complexes with hydrophobic and volatile molecules such as TO constituents, controlling volatility and reactivity. In addition, controlled released of the β-CD trapped compounds could be possible by exposing the capsules to high relative humidity (RH). With this in mind, the controlled release of antifungal volatiles throughout exposure of TO:β-CD capsules to high relative humidity was studied. Thymol (TOL) was the major constituent of TO, detected by gas chromatography before and after encapsulation. Capsules of the 8:92 ratio (TO:β-CD) showed the highest TOL content. Hydrogen bonds and hydrophobic interactions were detected between the oil constituent and β-CD by IR and 1H NMR spectroscopy. During moisture sorption, the TO capsules showed a lower water uptake compared with free β-CD. Similar behavior was observed during water desorption. In all cases, a hysteresis process was observed when comparing sorption and desorption isotherms. At high RH, TOL is displaced and almost 76% is released to the headspace. The growth of Alternaria alternata was inhibited significantly by the addition and exposure to TO:β-CD as measured by both the agar dilution and the headspace method, respectively. Therefore, the encapsulation of antifungal volatile compounds as TO in β-CD, could be an alternative to control the release of natural antimicrobials that can be of interest to the agricultural area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, the interest in natural antimicrobial compounds to preserve food quality has increased, and numerous studies on the antimicrobial activity of a wide range of natural compounds have been reported [1–3]. There has been an increasing concern of consumers on foods free or with lower levels of synthetic chemical preservatives, because they could be toxic for humans and the environment [3]. Concomitantly, consumers have also demanded foods with long shelf-life and without pathogens that cause food borne diseases. This has led to a continuous search for effective natural antimicrobial compounds to preserve fresh-cut produce quality without detriment of its sensorial attributes.
Several essential oils (EOs), such as garlic, cinnamon, thyme, oregano, clove, basil, coriander, citrus peel, laurel, ginger, rosemary, and peppermint, among others, have been studied as antimicrobial natural products against both bacteria and molds [3–14]. The inherent aroma and antimicrobial activity of EOs are related to the chemical configuration of their components, the proportions in which they are present, and the interactions among them, affecting their bioactive properties [15]. For the application of EOs as food preservatives, their impact on acceptability should be considered [12, 16]. Thyme (Thymus vulgaris) can be used as inhibitor of Aspergillus spp., Alternaria spp., Fusarium spp. and Penicillium spp., some of the most important agents of food borne diseases and/or spoilage molds [17, 18]. Thymol (a monoterpene phenol) is the major constituent of some thyme varieties, like T. vulgaris and Thymus zigis [19, 20]. Thymol exhibits strong antimicrobial and antioxidant activity [21]. Thyme essential oil (TO) is a good antimicrobial agent, however, its high volatility and reactivity limits its application as a food preservative.
The chemical reactivity of natural antimicrobial agents with the food and package matrix could significantly affect the sensorial properties of produce [1]. In addition, reactions with lipids, proteins, carbohydrates, and other additives could bring about an overall decrease in the activity of the antimicrobial compound [12]. The ability of most antimicrobials, including EOs, to inhibit microorganisms can be lost on extended storage [1]. Depending on the time and storage temperature, these antimicrobials can be volatilized or become inactive [12, 22]. Normally, direct application of antimicrobials to food must be done at high concentrations to achieve good antimicrobial activity against target microorganisms in food produce meant to be stored for an extended period of time [1]. Obviously, antimicrobial compounds that possess high antimicrobial activity, but negatively affect flavor and odor acceptability would be unacceptable and not considered for these applications.
Encapsulation in β-cyclodextrin (β-CD) is one method to control the odor and reactivity of active compounds throughout the release of natural antimicrobial compounds [14]. β-CD is a cyclic molecule made up of seven D-glucose monomers linked by α(1,4) bonds, shaped as a truncated hollow cone. It possesses a hydrophobic cavity, whereas the external face is hydrophilic [14]. These properties have made β-CD an option for the encapsulation of several organic and inorganic compounds. Encapsulation in β-CD is considered a molecular complex formation, where the hydrophobic active constituents of the EO could interact within the hydrophobic cavity of β-CD, highlighting that when the capsule is formed the molecular exterior attains a hydrophilic character [1, 23]. Considering the external properties of the capsule, its conformation could be affected by high RH in the atmosphere, and the EO volatiles could be released to the environment. This model is supported by a previously published hypothesis stating that the controlled release of antimicrobial volatile compounds from EOs could be achieved using the high in-package RH of fresh-cut fruits and vegetables [1].
Considering the possibility of the natural preservation offered by TO with the controlled release offered by β-CD capsules when exposed to high RH, the main goal of this study was to design and characterize a controlled release system of antifungal TO volatiles encapsulated in β-CD. Design and characterization included: optimization of the encapsulation process, gas chromatography analysis (GC-MS, GC-FID) before and after encapsulation, IR, 1H NMR spectroscopy, moisture sorption isotherms, release of encapsulated TO during RH increments, and assessment of the antifungal activity of the designed system.
Materials and methods
Materials
TO and TOL were obtained from Sigma–Aldrich Co, β-CD (Cavamax W7 food grade) was supplied by Wacker Biochem, USA. All other reagents used in this work were of analytical grade.
Preparation of the capsules
TO: β-CD capsules were prepared using the precipitation method previously reported [14]. Ten grams of β-CD were dissolved in ethanol:water (1:2) at 55 °C. Then, TO oil (0, 0.4. 0.8 1.2, and 1.4 g) dissolved in ethanol (10% w/v) was added to β-CD to reach TO:β-CD ratios of 0:100, 4:96, 8:92, 12:88, and 14:86 (%w/w), respectively. The amount of recovered capsules was calculated by subtraction of its moisture content from the total weight, which was determined by drying a sample (3–4 g) in a vacuum oven at 70 °C for 24 h, under pressure <6.7 kPa (AOAC, 1990). The percentage of moisture was calculated comparing the initial and the final sample weight of the pure β-CD and the oil inclusion complex. Each sample was done in triplicate.
Gas chromatography (GC-MS, GC-FID) analysis
Identification of the volatile constituents of TO before and after the encapsulation process was accomplished by GC-MS, using a Varian GC-3400 Cx, equipped with a Saturn 2100T Mass selective detector (Varian, Mexico) and a DB-5 capillary column (30 m × 0.25 mm, film thickness 0.25 μm). Column temperature was raised from 55 to 65 °C at a 1 °C/min rate, and held for 3 min, then the temperature was raised from 65 to 290 °C at a 10 °C/min rate, and held at this final temperature during 10 min. Helium was the carrier gas, at a flow rate of 1 mL/min. For GC-MS detection, an electron ionization system with ionization energy of 70 eV was used. Injector and MS transfer line temperatures were set at 100 and 290 °C, respectively. Identification of the compounds was based on the comparison of their mass spectra with those of Saturn library and NIST 98 library data of the GC-MS system. Quantification was done using a Varian Star 3400-Cx chromatograph equipped with a FID detector (Varian Mexico) and a DB-5 capillary column (30 m × 0.25 mm, film thickness 0.25 μm). Injector and detector temperatures were set at 220 and 290 °C, respectively. Column temperature program was the same as that used for the GM-MS analysis. Nitrogen was used as the carrier gas, at a flow rate of 1 mL/min. One microlitre of the extract was injected manually in the split-less mode. EO was diluted with dichloromethane to a concentration of 100 μg/mL. EO from the capsules was extracted by ultrasound. For this, 10 mg of solid capsules was dissolved in 1 mL of deionised water and 2 mL of dichloromethane was added, sonicated during 30 min and the organic phase was recovered. This step was repeated twice and the total volume of the organic phase was considered for the quantification of encapsulated compounds. The main constituent of the EO was quantified using a standard calibration curve.
Moisture sorption isotherms
Moisture adsorption–desorption isotherms of free β-CD and TO capsules (1 g, initial weight) were determined using the gravimetric static method of saturated salt solutions (diedrite, CsF, MgCl2, NaBr, K2SO4), with different relative humidity (0, 3, 33, 66, 96%, respectively) at 20 °C. Initial moisture was recorded, and after equilibrium (three weeks) samples were weighed again. After adsorption, samples were saturated at 100% of relative humidity (RH) with pure water, and the experiment was carried out again to determine desorption isotherms. Data are reported as grams of adsorbed or desorbed water/gram of solid. Triplicate determinations were made for each sample.
IR spectroscopy
IR spectra of free and encapsulated oil, as well as free β-CD, were recorded using an infrared spectrophotometer FTIR Nicolet, Protege 460 (Nicolet, Madison, WI). Scanning conditions were as follows: wave number range, from 4,000 to 400 cm−1; resolution, 4 cm−1; number of scans, 64; scan speed, 0.63 cm−1; detector, DTGS. β-CD, physical mixture of TO and β-CD, and capsules TO:β-CD were recorded using KBr pellets.
1H NMR spectroscopy
The inclusion complexes, TO and TOL, in CDs were characterized by 1H NMR spectroscopy using a Bruker AVANCE 400 spectrometer (Bruker Instruments Inc. USA). The working frequency was 400 MHz applied to samples diluted in D2O (1 mM) in NMR tubes (5 mm diameter) at T = 23.0 ± 0.1 °C. The solvent peak at 4.72 ppm was used as the internal reference. Results were based on chemical shift of cyclodextrin protons before and after encapsulation.
Controlled release assay
TO volatiles released from capsules were measured during 2 weeks of exposure at different humidity (0, 3, 33, 66, 96, 100%, respectively) at 20 °C. Capsules (10 mg) were taken from the high RH environment and the residual volatiles were extracted with HPLC water (1 mL) and methylene chloride (2 mL), as previously reported [14]. Once TOL, as the main TO capsule constituent, had been quantified as previously described it was used to follow the percentage of release
Antifungal activity
Antifungal potential of free TO and of the capsules with the highest volatile content was tested against Alternaria alternata using both the agar dilution and headspace methods. The antifungal activity was determined according to the agar dilution method. For this, 5.3 × 10−5 and 8.5 × 10−5 g/mL of TO and 0.005, 0.015, and 0.025 g/mL of capsules were added to Petri dishes containing potato dextrose agar, and then inoculated in the center with the fungi. The efficiency of the treatments was evaluated daily during 3 days at 25 °C. Antifungal activity was measured by the headspace method placing small beakers containing 0.02 and 0.28 g of free TO, and 0.04, 0.08, and 0.12 g of capsules of TO, respectively, in a 1-L container where an Alternaria alternate inoculated plate had been set over the beakers and sealed. The system was incubated at 5 °C to reduce fungal growth and enhance the action of the treatments. Controls for both assays were pure agar inoculated with the fungi and without exposure to any compound. Mycelial diameter was recorded (cm) in triplicate every 7 days during 21 days.
Experimental design and statistical analysis
This experiment was based on a completely randomized design with equal replications. Analysis of variance for the treatments was done using NCSS statistical software. Mean comparisons of the studied parameters among treatments were done using the least significant difference test at a 5% level (p < 0.05).
Results and discussion
Recovery of thyme inclusion complex
The weights of the recovered TO capsules were lower than the amount of β-CD and TO originally used (Table 1). There was a significant increase (p < 0.05) in the recovery of the 8:92, 12:88, and 14:86 ratio capsules as compared to that from the 4:96 ratio. No significant differences (p > 0.05) amongst the 8:92, 12:88, and 14:86 ratios were observed. These data indicate that ratios of TO:β-CD greater than 8:92 did not significantly affect (p > 0.05) the amount of TO recovered from capsules. This result could suggest that the maximum inclusion of TO in β-CD had been reached.
For the preparations at 8:92, 12:88, and 14:86 ratios, there was a 92 to 98% increase in the amount of recovered product relative to the initial oil:β-CD weights. At these greater concentrations of TO in the starting solution, there is a greater recovery of oil components to form the capsules. This statement was verified by gas chromatography assays detailed in the next section. Similar patterns have been previously observed for garlic oil encapsulated in β-CD, where a saturation occurred at the 12:88 ratio, however, for cinnamon leaf oil it was found that no saturation occurred up to the 14:86 ratio [14].
Other factors could contribute to a low recovery of EO capsules, such as EO constituents remaining in the solution after forming capsules, and the evaporation that could occur during the encapsulation process [14]. However, it has to be remarked that a saturation of the cyclodextrin matrix is reached, and the optimization of the process can be established with respect to the highest recovery, which, in this case, for TO capsules, the optimal recovery ratio was 8:92.
Volatiles of thyme oil before and after encapsulation
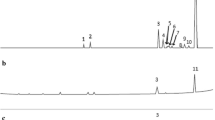
TO volatiles were identified and quantified by GC-MS and GC-FID, respectively, (Fig. 1) before (a) and after (b) the encapsulation process. Thymol (TOL) was the major constituent of T. vulgaris (53%). Other prevalent compounds present in the oil were ρ-cymene (31%), γ-terpinene (9.75%), and linalool (5.1%). The rest of the volatile compounds were found at concentrations lower than 1% (α-terpineol, borneol, and bornyl acetate). Variations in oil composition can occur due to different factors, such as species, chemotype, season, and geographical origin.
Hudaib and Aburjai (2007) studied the volatile components of T. vulgaris L. from wild-growing and cultivated plants in Jordan [19]. The oil was characterized by marked levels of phenolic monoterpenoids (mainly TOL and carvacrol) in the 70.8–89.0% range and high levels of the monoterpenoid hydrocarbons ρ-cymene (3.4–8.2%) and γ-terpinene (1.6–7.7%) were also observed. However, the main components of the volatile fraction of the dichloromethane extract of the aerial parts of a Spanish T. vulgaris L. were 1,8-cineole and linalool (42%) [24]. In some plants from Catalonia (Spain), linalool was the predominant volatile (40–70%) [25]. The volatile ρ-cymene (40–50%) was the most important component of T. vulgaris L. in Central Otago, New Zealand [26]; and γ-terpinene, p-cymene and TOL probably defines the chemotype of the EO from Italian T. vulgaris L. [27]. Our results are very similar in the main four compounds sequence (TOL > ρ-cymene > γ-terpinene > linalool) to Thymus zygis proposed previously [20].
Figure 1b shows the proportions of volatile constituents of the encapsulated TO. Regarding the chromatogram of free TO, γ-terpinene proportion decreased only 2% whereas linalool increased the same percentage. Similar proportions were maintained by the rest of the components. This similarity in composition for the main volatiles between the free and the encapsulated oil is likely to be due to the small size and polarity characteristics of all the volatile molecules, which made them easy to be included in the β-CD [14]. However, the concentration of all TO compounds decreased when the capsules were formed.
Comparing the relative absorption of the main constituent of TO, in relation to the ratio TO:β-CD, when increasing the oil amount added to the β-CD the absorption of major constituents decreased. This decrement could be caused by the decrement of free β-CD that had been occupied by the oil constituents and saturation occurred. This can be observed and ratified in the volatile load of TO capsules, where the saturation started at the ratio of 8:92. The recovered free β-CD decreased while the amount of added oil increased, which is in agreement with the proposed saturation process (data not shown).
The content of TOL in the different TO:β-CD ratios are shown in Fig. 2. All capsules showed significant differences (p < 0.05). The 8:92 ratio (TO:β-CD) presented the highest TOL content (2.08%, w/w). This shows that the capsules obtained with the highest TO:β-CD ratio (which shows the highest yield), not necessarily retained the main volatile content, measured by the TOL content. This behavior can be explained considering that, within the different TO:β-CD ratios (0:100, 4:96, 8:92, 12:88, and 14:86 percent weight ratios), as the proportion of TO increased and the β-CD decreased, the absorption of the major constituent decreased. This decrease could be explained considering that a saturation process of the β-CD cavities occurred as the concentration of EOs increased. From these results, it is possible to consider that equilibrium had been reached at the 8:92 ratio. Therefore, this ratio was considered the optimum and was used to perform the IR, 1H RMN, sorption–desorption isotherms, controlled release, and antifungal capacity assays.
IR spectroscopy characterization of TO capsules
Molecular interactions between TO components and the β-CD in the capsules were confirmed to occur by IR spectroscopy analysis. Figure 3 shows the IR spectra of: (a) free TO, (b) free β-CD, (c) physical mixture of TO with β-CD, and (d) TO:β-CD capsules. IR spectral analysis revealed differences in some band positions in the capsules with respect to the initial compounds. These differences in the IR spectra are typical of β-CD solid state complexes, due to the loss of vibrating and bending of the guest molecule during complex formation [14]. Free TO exhibited two bands at 3,434 and 3,533 cm−1 in the OH vibrations region, which probably correspond to the hydroxyl group of TOL, since this compound represents 53% of the total volatiles inTO. In the spectrum of TO:βCD capsules (Fig. 3d), the OH bands corresponding to TOL are almost completely reduced, whereas the intense and broad β-CD bands are hardly influenced by molecular complex formation. To confirm the above, we can also observe that the centroid of this complex band in the spectrum is shifted in the range of the OH vibrations by about 18 cm−1 towards lower frequencies compared to the position of the similar band in the β-CD spectrum. This indicates that an interaction has occurred between TO and β-CD via hydrogen bonding, and that the complex is not a physical mixture. On the other hand, in the 2,962–2,870 range, there are three bands that correspond to three different C–H bonds that could be expected in TO, whereas in the β-CD spectrum there is only one band at 2,929 cm−1. In the complex, this band is shifted towards lower frequencies by 4 cm−1. The 1,619 cm−1 band presents only in the IR spectrum of TO, assigned to the conjugated double bond of the ring. The bands between 1,450 and 1,600 cm−1 may be assigned to stretching of –CH2– group while CH2=CH– stretching is shifted to 1,290 cm−1. Physical mixture was similar to those of pure TO and β-CD, exhibiting the respective bands, thus offering an indication that no inclusion occurred by simple mixing. Finally, the bands in the 1157, 1082 and 1027 cm−1 (C–O) regions of β-CD have the same intensity, shape, and position as those of the β-CD complex, indicating that this conformation has not been changed during the formation of the complex.
1H NMR spectroscopy characterization of TO capsules
NMR spectroscopy is one of the most useful tools for the structural analysis of inclusion complexes, this is because of selective line broadening and/or chemical shift displacements of 1H NMR signals when a molecule is included inside the β-CD cavity. Table 2 reveals significant variations in the chemical shift of host molecules, the main shifts are observed on the resonance of H–C(3) and H–C(5) of β-CD, indicating that the guest molecules are inside the hydrophobic cavity [28, 29]. Figure 4 shows the 1H NMR spectra of the complexes with different TO–β-CD ratios in D2O. In this figure, it is possible to observe not only the resonances in the range of 3.2–5 ppm attributed by the protons of β-CD, but also the resonances of TO, providing direct evidence for complex formation. In addition, the relationship between protons host/guest changed in all the formulations and the 1H NMR signal of the guest shifted downfield. In fact, it is known that the inclusion of a guest molecule in a β-CD ring shifts the 1H NMR signals of the included guest downfield and those of the affected CD protons upfield [30], as a consequence of the hydrophobic interaction between host and guest molecules [31]. Our results confirm the formation of complexes between β-CD and at least one TO component.
Moisture sorption isotherms of TO capsules
Figure 5 shows the sorption isotherms (grams of adsorbed water/gram of solid) of free β-CD and encapsulated TO. Free β-CD showed a constant uptake from 33 to 97% RH with a total of ≈0.1–0.2 g of water absorbed per gram at 97% RH. This water uptake of free β-CD corresponded to <20% weight change at 97% RH. These results agree with the reported water content (14%) of cyclodextrins at normal conditions [32]. A further 3% increase of RH (100%) resulted in a drastic increase in moisture sorption. When the adsorption isotherm of the TO:β-CD complex was analysed, it was possible to observe a type II isotherm, in which, at RH below 60%, the complex practically does not adsorb water. However, at higher RH adsorbed water increased. Interestingly, no significant differences (p < 0.05) were found between free β-CD and TO capsules at 100% RH. These results accord with those previously reported for garlic and cinnamon leave β-CD complexes [14].
It is noteworthy that β-CD and β-CD:TO exhibit different adsorption–desorption profiles, indicating that the presence of TO in the capsules affects moisture uptake. Considerable hysteresis was observed on both free β-CD and encapsulated TO. At all RHs, free β-CD retained more water than the complex. These results may be explained considering that during the encapsulation of the EO by β-CD, the oil constituents are positioned in the hydrophilic sites of the β-CD molecule and, consequently, the capacity of capsules to adsorb water is reduced [14]. These results could sustain the theory that TO constituents are interacting with the β-CD molecule through hydrogen bonds as has been showed with the interactions of other EO compounds [14].
Controlled release
TOL content in the capsules (8:92, TO:β-CD ratio) exposed at different RH during 3 weeks at 20 °C was determined (Fig. 6). When RH increased, the amount of TOL released from the complex increased. Gradually at each RH, ≈15% of TOL is displaced, and almost 76% is released at the highest RH, according to the results obtained by GC-FID. Mourtzinos et al. (2008) obtained 30% of TOL released after 10 h exposure in water from the spray- or freeze-dried powders with β-CD [33]; however, in that study the antifungal activity of the released volatiles was not measured. Oil–cyclodextrin interactions are mainly hydrophobic–hydrophilic. In this context, hydrophobicity is intimately related to hydration. If hydrophobic interactions play a major role in a binding process, the latter must be accompanied by substantial release of water molecules from the hydration shell of nonpolar groups of the cyclodextrin [32]. On the other hand, when water availability increases around the capsules, water starts to interact with the polar groups of the β-CD unbalancing the equilibrium and oil constituents are displaced. Therefore, all of these results suggest the application of the oil in systems where it can be released passively and controlled.
The generated release system of TO capsules could be useful in the active packaging area. Headspace artifacts were the first antimicrobial active packaging commercialized in the market, in the form of sachets that are enclosed in the interior of the package or attached to it. Headspace artifacts with direct antimicrobial activity include antimicrobial volatile compounds such as sulfur dioxide [34], ethanol [34, 35], organic acids, and EOs [1, 36–38]. The effectiveness of headspace artifacts depends on the permeability of the sachet material to water vapor, the release of the volatile compound absorbed or encapsulated, the diffusion through the polymer, their vapor partial pressure, and the way in which the antimicrobial interacts with the microorganism. It has been hypothesized that internal moisture in packed food can be the driving force that releases the antimicrobial compound from the complex.
Antifungal activity
The antifungal potential of free TO and their capsules (8:92, TO:β-CD ratio) was determined. To establish this antifungal potential, a mold related to plant food spoilage, A. alternata, was selected. The antifungal activity was determined according to the agar dilution (Fig. 7) and headspace methods (Fig. 8). In the case of the agar dilution method, free TO showed a concentration-dependent behavior, with a complete mold growth inhibition at the highest concentration. On the other hand, β-CD by itself increased the mycelial growth of A. Alternata. The reason for this increment was probably because β-CD acts as a carbohydrate source for the fungus. This could explain the behavior of capsules, which showed that as the amount of capsules (β-CD) increased, the mold mycelial growth also increased. A 55.6% inhibition of the fungus was achieved with 0.005 g/mL of capsule, compared with the control.
By the headspace antifungal method, free TO also showed a concentration-dependent behavior, with a complete inhibition at 0.28 g of TO; 0.02 g of TO were used since they are equivalent to the amount of oil contained in 0.12 g/mL of capsules. Therefore, this concentration was used to compare results between complexed and free TO. The release of volatiles to the headspace can achieve 25% inhibition of the fungus in presence of TO capsules during 21 days, compared to the highest free TO concentration. Similar results were obtained when the free TO was used (0.05 g). Results showed that with this method, TO volatiles were released from capsules to the headspace and were not directly in contact with the culture medium, for this reason while the amount of capsules increased the mycelial growth was reduced. These results are totally different to those obtained with the agar dilution method, and reveals that the way in which cyclodextrins are used will directly modify their behavior against mold growth.
Sokmen et al. (2004) demonstrated the capacity of TO at 10 μL to inhibit the growth of molds such as Alternaria spp. [17]. In other studies, Viuda-Martos et al. (2007) showed that 2 μL/18 mL culture medium is enough to reduce the growth of A. niger [18]. This antifungal capacity of TO has also been demonstrated on fungi such as Aspergillus and Fusarium [39–41]. The antifungal activity of TO is mainly attributable to its major components, although the possibility of other phenomena, such as synergy or antagonism with minor components has to be taken into count [42]. Some studies have shown that specific phenolic compounds and terpenes can control the growth rate and spore germination time of spoilage fungi [43, 44]. The possible modes of actions and mechanisms have not been completely elucidated. However, some studies have reported the effect of phenolic compounds on enzyme activities that cause protein denaturation and microbial cell permeability [45]. On the other hand, the antimicrobial activity of TOL could result from damage to enzymatic cell systems, including those associated with energy production and synthesis of structural compounds, as well as from disturbing genetic material functionality [17, 45–47].
Conclusion
From the reported results, it can be concluded that the encapsulation of antimicrobial volatile compounds from TO in β-CD could be an alternative to solve the problem of volatility, providing a molecular complex with antifungal activity that can be released to the atmosphere of interest passively and controlled using RH. Additionally, thyme oil is from natural origin, being the main reason for their suitability, which consumers find comforting and beneficial for the environment. The optimization of the use of natural antimicrobials as food preservatives will facilitate the transition through a more clean and sustainable environment.
Abbreviations
- TO:
-
Thyme essential oil
- β-CD:
-
β-cyclodextrin
- TOL:
-
Thymol
- EOs:
-
Essential oils
- RH:
-
Relative humidity
- IR:
-
Infrared
- 1H NMR:
-
Proton nuclear magnetic resonance
- GC-MS:
-
Gas chromatography coupled to mass spectrometry detector
- GC-FID:
-
Gas chromatography coupled to flame ionization detector
- HPLC:
-
High performance liquid chromatography
References
Ayala-Zavala, J.F., del-Toro-Sanchez, L., Alvarez-Parrilla, E., Gonzalez-Aguilar, G.A.: High relative humidity in-package of fresh-cut fruits and vegetables: advantage or disadvantage considering microbiological problems and antimicrobial delivering systems? J. Food Sci. 73, R41–R47 (2008)
Carraminana, J.J., Rota, C., Burillo, J., Herrera, A.: Antibacterial efficiency of Spanish Satureja montana essential oil against Listeria monocytogenes among natural flora in minced pork. J. Food Prot. 71, 502–508 (2008)
Wang, C.: The use of essential oils as natural preservatives for berry fruits. HortScience 41, 1042–1043 (2006)
Ravi, R., Prakash, M., Bhat, K.K.: Aroma characterization of coriander (Coriandrum sativum L.) oil samples. Eur. Food Res. Technol. 225, 367–374 (2007)
Zeller, A., Rychlik, M.: Impact of estragole and other odorants on the flavour of anise and tarragon. Flavour Fragr. J. 22, 105–113 (2007)
Mukherjee, M., Datta, A.K.: The basils—a review. Plant Arch. 7, 473–483 (2007)
Senhaji, O., Faid, M., Kalalou, I.: Inactivation of Escherichia coli O157 : H7 by essential oil from Cinnamomum zeylanicum. Brazilian J. Infect. Dis. 11, 234–236 (2007)
Jugl-Chizzola, M., Ungerhofer, E., Gabler, C., Hagmuller, W., Chizzola, R., Zitterl-Eglseer, K., Franz, C.: Testing of the palatability of Thymus vulgaris L. and Origanum vulgare L. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berliner Und Munchener Tierarztliche Wochenschrift. 119, 238–243 (2006)
Arcila-Lozano, C.C., Loarca-Pina, G., Lecona-Uribe, S., de Mejia, E.G.: Oregano: properties, composition and biological activity. Arch. Latinoam. Nutr. 54, 100–111 (2004)
Novak, I., Zambori-Nemeth, E., Horvath, H., Seregely, Z., Kaffka, K.: Study of essential oil components in different Origanum species by GC and sensory analysis. Acta Alimentaria 32, 141–150 (2003)
Edris, A.E., Farrag, E.S.: Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Nahrung Food 47, 117–121 (2003)
Ayala-Zavala, J.F., del-Toro-Sánchez, L., Alvarez-Parrilla, E., Soto-Valdez, H., Martín-Belloso, O., Ruiz-Cruz, S., González-Aguilar, G.A.: Natural antimicrobial agents incorporated in active packaging to preserve the quality of fresh fruits and vegetables. Stewart Postharvest Rev. 4, 1–9 (2008)
Ayala-Zavala, J.F., Oms-Oliu, G., Odriozola-Serrano, I., Gonzalez-Aguilar, G.A., Alvarez-Parrilla, E., Martin-Belloso, O.: Bio-preservation of fresh-cut tomatoes using natural antimicrobials. Eur. Food Res. Technol. 226, 1047–1055 (2008)
Ayala-Zavala, J.F., Soto-Valdez, H., Gonzalaz-Leon, A., Alvarez-Parrilla, E., Martin-Belloso, O., Gonzalez-Aguilar, G.A.: Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in beta-cyclodextrin. J. Incl. Phen. Macrocycl. Chem. 60, 359–368 (2008)
Fisher, K., Phillips, C.: Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 19, 156–164 (2008)
Ayala-Zavala, J.F., González-Aguilar, G.A., del Toro-Sánchez, L.: Enhancing safety and aroma appealing of fresh-cut fruits and vegetables using the antimicrobial and aromatic power of essential oils. J. Food Sci. 74, R84–R91 (2009)
Sokmen, A., Gulluce, M., Akpulat, H.A., Daferera, D., Tepe, B., Polissiou, M., Sokmen, M., Sahin, F.: The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 15, 627–634 (2004)
Viuda-Martos, M., Ruiz-Navajas, Y., Fernandez-Lopez, J., Perez-Alvarez, J.A.: Antifungal activities of thyme, clove and oregano essential oils. J. Food Saf. 27, 91–101 (2007)
Hudaib, M., Aburjai, T.: Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour Fragr. J. 22, 322–327 (2007)
Martinez, S., Madrid, J., Hernandez, F., Megias, M.D., Sotomayor, J.A., Jordan, M.J.: Effect of thyme essential oils (Thymus hyemalis and Thymus zygis) and monensin on in vitro ruminal degradation and volatile fatty acid production. J. Agric. Food Chem. 54, 6598–6602 (2006)
Aydin, S., Basaran, A.A., Basaran, N.: The effects of thyme volatiles on the induction of DNA damage by the heterocyclic amine IQ and mitomycin C. Mutat. Res. Genet. Toxicol. Environ. Mutag. 581, 43–53 (2005)
Tripathi, P., Dubey, N.K.: Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 32, 235–245 (2003)
Baranauskiene, R., Venskutonis, P.R., Dewettinck, K., Verhe, R.: Properties of oregano (Origanum vulgare L.), citronella (Cymbopogon nardus G.) and marjoram (Majorana hortensis L.) flavors encapsulated into milk protein-based matrices. Food Res. Int. 39, 413–425 (2006)
Guillen, M.D., Manzanos, M.J.: Composition of the extract in dichloromethane of the aerial parts of a Spanish wild growing plant Thymus vulgaris L. Flavour Fragr. J. 13, 259–262 (1998)
Torras, J., Grau, M.D., Lopez, J.F., de Las Heras, F.X.C.: Analysis of essential oils from chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 87, 2327–2333 (2007)
McGimpsey, J.A., Douglas, M.H., Van Klink, J.W., Beauregard, D.A., Perry, N.B.: Seasonal variation in essential oil yield and composition from naturalized Thymus vulgaris L. in New Zealand. Flavour Fragr. J. 9, 347–352 (1994)
Piccaglia, R., Marotti, M.: Composition of the essential oil of an italian Thymus vulgaris L. ecotype. Flavour Fragr. J. 6, 241–244 (1991)
Moon, T.W., Lee, J.W., Jhee, K.H., Khang, K.W., Jeong, H.S., Yang, S.A., Kim, H.J.: Supramolecular encapsulation of pulegone from oriental herb, Schizonepeta tenuifolia Briquet by beta- and gamma-cyclodextrins. Bull. Korean Chem. Soc. 29, 1579–1582 (2008)
Locci, E., Lai, S.M., Piras, A., Marongiu, B., Lai, A.: C-13-CPMAS and H-1-NMR study of the inclusion complexes of beta-cyclodextrin with carvacrol, thymol, and eugenol prepared in supercritical carbon dioxide. Chem. Biodivers. 1, 1354–1366 (2004)
Pose-Vilarnovo, B., Perdomo-Lopez, I., Echezarreta-Lopez, M., Schroth-Pardo, P., Estrada, E., Torres-Labandeira, J.J.: Improvement of water solubility of sulfamethizole through its complexation with beta- and hydroxypropyl-beta-cyclodextrin—Characterization of the interaction in solution and in solid state. Eur. J. Pharm. Sci. 13, 325–331 (2001)
Fernandes, C.M., Carvalho, R.A., da Costa, S.P., Veiga, F.J.B.: Multimodal molecular encapsulation of nicardipine hydrochloride by beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin and triacetyl-beta-cyclodextrin in solution. Structural studies by H-1 NMR and ROESY experiments. Eur. J. Pharm. Sci. 18, 285–296 (2003)
Taulier, N., Chalikian, T.V.: Hydrophobic hydration in cyclodextrin complexation. J. Phys. Chem. B. 110, 12222–12224 (2006)
Mourtzinos, I., Kalogeropoulos, N., Papadakis, S.E., Konstantinou, K., Karathanos, V.T.: Encapsulation of nutraceutical monoterpenes in beta-cyclodextrin and modified starch. J. Food Sci. 73, S89–S94 (2008)
Ozdemir, M., Floros, J.D.: Active food packaging technologies. Crit. Rev. Food Sci. Nutr. 44, 185–193 (2004)
Vermeiren, L., Devlieghere, F., Debevere, J.: Effectiveness of some recent antimicrobial packaging concepts. Food Addit. Contam. 19, 163–171 (2002)
Cha, D.S., Chinnan, M.S.: Biopolymer-based antimicrobial packaging: a review. Crit. Rev. Food Sci. Nutr. 44, 223–237 (2004)
Suppakul, P., Miltz, J., Sonneveld, K., Bigger, S.W.: Active packaging technologies with an emphasis on antimicrobial packaging and its applications. J. Food Sci. 68, 408–420 (2003)
Tunc, S., Chollet, E., Chalier, P., Preziosi-Belloy, L., Gontard, N.: Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Int. J. Food Microbiol. 113, 263–270 (2007)
Montes-Belmont, R., Carvajal, M.: Control of Aspergillus flavus in maize with plant essential oils and their components. J. Food Prot. 61, 616–619 (1998)
Basilico, M.Z., Basilico, J.C.: Inhibitory effect of some spice essentials oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin production. Lett. Appl. Microbiol. 29, 238–241 (1999)
Inouye, S., Tsuruoka, T., Watanabe, M., Takeo, K., Akao, M., Nishiyama, Y., Yamaguchi, H.: Inhibitory effect of essential oils on apical growth of Aspergillus fumigatus by vapour contact. Mycoses 43, 17–23 (2000)
Daferera, D.J., Ziogas, B.N., Polissiou, M.G.: The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp and Clavibacter michiganensis subsp michiganensis. Crop Protect. 22, 39–44 (2003)
Aggarwal, K.K., Khanuja, S.P.S., Ahmad, A., Kumar, T.R.S., Gupta, V.K., Kumar, S.: Antimicrobial activity profiles of the two enantiomers of limonene and carvone isolated from the oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 17, 59–63 (2002)
Sharma, N., Tripathi, A.: Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 163, 337–344 (2008)
Burt, S.: Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94, 223–253 (2004)
Edris, A.E.: Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res. 21, 308–323 (2007)
Gutierrez, J., Barry-Ryan, C., Bourke, R.: The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 124, 91–97 (2008)
Acknowledgements
Special appreciation to the Mexican Council of Science and Technology (CONACYT project 6457) for financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Toro-Sánchez, C.L., Ayala-Zavala, J.F., Machi, L. et al. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J Incl Phenom Macrocycl Chem 67, 431–441 (2010). https://doi.org/10.1007/s10847-009-9726-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9726-3