Abstract
The present study was aimed to evaluate the different proportions of carrier materials: Moringa oleifera gum (MG), maltodextrin (MD), and inlet temperature on the powder characteristics of encapsulated tender coconut water (TCW) by spray drying. The characterization of microparticle was studied as encapsulation efficiency, antioxidant activity, total phenolic content, moisture content, water activity, solubility, particle morphology, and encapsulation yield. The investigation was conducted using an experimental design of 22 mixes with five replicates at the center point. The encapsulation efficiency was affected by all tested variables and reached significantly (p < 0.05) higher value (94.86%) when higher MG concentration, and lower MD concentration, and inlet temperature were applied. The DPPH (53.66%) and ABTS radical scavenging activity (54.92%) was observed to be higher at the highest MG concentration (1.5%). The obtained powder retained a higher amount of phenol content (21.82 mg GAE/g) at increasing MG concentration, with decreasing MD concentration, and inlet temperature. X-ray diffraction (XRD) analysis revealed that TCW powder encapsulated with 1.5% MG exhibited higher crystalline nature as compared to microparticle encapsulated with 0.5 and 1% MG. The optimum wall material composition and inlet temperature were determined as follows: MD 30%, MG 1.5%, and 120 °C inlet temperature. Hence, our results suggest that the application of this technology could increase the use of TCW in various industrial applications and imply MG as a potent candidate for microencapsulation of food materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coconut (Cocos nucifera L.) is one of the major tropical crops which are considered as a principal source of food, water, isotonic, energy, fuel, animal feed, and shelter (Chauhan et al. 2014). In certain parts of the world, tender coconut water (TCW) is regarded as the hydrating drink of choice (Kalman et al. 2012) and is used as an oral rehydration to replace the loss of fluid in patients suffering from severe diarrhea and vomiting. It has also been successfully used intravenously (Campbell-Falck et al. 2000). Mantena et al. (2003) reported that the coconut water has antioxidant properties, which may help in neutralizing reactive oxygen species production resulting from long-duration exercise. Naturally, TCW contains a high amount of potassium, iron, sodium, chloride, and carbohydrates (Kalman et al. 2012). The sugars comprise of glucose, fructose, and sucrose and sugar alcohols, mainly sorbitol (Chauhan et al. 2014).
In spite of a number of well-known uses for coconut water, the market is not sufficiently large enough. Due to high demand in the market for consumption, a variety of products made out of TCW has been coming up. Recently, few researchers have formulated coconut water beverage from mature nuts and also developed drinks from tender coconut water by addition of various fruit juices (Chauhan et al. 2008). Further studies on coconut water need to be carried out, and its qualities must be known to attain improved market demand. To achieve this, new options such as the possibility of storing TCW should be considered. Microencapsulation technique is becoming popular as an alternative method to store perishable juices in the form of powder with increasing stability for a long time. Among the available microencapsulation techniques, the most commonly used in commercialization is the spray-drying process as it has lower water-retaining properties (Desai and Park 2005). Maltodextrins (MD) and gum arabic are some of the polysaccharide gums derived from plants that are generally used as carrier material for the microencapsulation of vitamins, minerals, colorants as well as fat and oils (Lolodi 2011). However, the significant shortcomings of using MD as an encapsulating agent are their virtual lack of emulsifying capacity and low retention of volatile compounds (Yousefi et al. 2015). Immature coconut water contains high amount of volatile compounds like ketones, aldehydes, acids, esters, lactones, alcohols, etc. (Prades et al. 2012). Therefore, it is desirable to use MD in combination with a surface active biopolymer, such as esterified modified starches, gum arabic, or milk proteins (Jafari et al. 2008). Gum arabic is costly, and its irregular availability has motivated research for alternative encapsulation matrices (Madene et al. 2006; Cano-Higuita et al. 2015).
Carbohydrates like Moringa oleifera gum (MG) as carrier materials could suitably be used to produce more stable emulsions and to improve the powder yield (Zbicinski and Marciniak 2008; Raja et al. 2016). Exudate gums have been valuable items of international trade in the food, pharmaceutical, and other industries for thousands of years (Whistler 1993; Verbeken et al. 2003). It is suggested that during the encapsulation process of fruit juices using natural gum as wall material, hydrogen bonding and dipole-dipole interactions occur between natural gums and phenol compounds, mainly due to the presence of free hydroxyl groups in the phenolic compounds present in the juice and also in gum (Rutz et al. 2013). Food manufacturers continually explore new plant gums for the commercial introduction to provide broader purposes. MG is obtained from the tree of the Moringaceae family, which is an arabinogalactan, composed of 1,6-, 1,3-, and 1,3,6-linked β-Galp units (Raja et al. 2016). The therapeutic potential of Moringa oleifera plant and gum includes antiinflammatory, antifungal, antibacterial, antidyslipidemic, antihyperglycemic, and antioxidant effects (Mbikay 2012; Gopalakrishnan et al. 2016). MG exudate is primarily white in color, during the exposure; the color becomes reddish brown to brownish black. It is highly viscous in nature as it swells in contact with water, whereas, MG is sparingly soluble in water (Panda et al. 2006). Singhal et al. (2012) reported the use of MG as a potential carrier of curcumin for colon-specific drug delivery. Previously, MG has been used as an emulsifying agent (Panda 2014). Owing to the easy availability of the plant, the exudate from the stem of the tree Moringa oleifera was used for microencapsulation of TCW.
The primary objective of this study was to optimize the microencapsulation of TCW using response surface methodology (RSM) and to investigate the effect of the spray-drying process at various temperature and wall material concentration (MG/MD) on encapsulation efficiency. Microencapsulation was done to minimize nutritional loss and maximize antioxidants, phenols, and other nutritive compounds.
Materials and Methods
Materials
Tender coconut (Cocos nucifera) was purchased from a local farm (Kattankulathur, Chennai, India), and dried Moringa oleifera gum was obtained from Annai Aravindh Herbals (Chennai, India). Maltodextrin was procured from Himedia, Mumbai, India. All other chemicals were purchased from SRL, Mumbai, India, and used without further purification.
Physicochemical Analyses of TCW Water
Physicochemical analyses were performed in triplicate. Total soluble solids (TSS) were measured in a refractometer (Erma, Tokyo, Japan), according to the method 932.12 (AOAC 1997). The pH value was determined using a digital pH meter (Susima, MP-1PLUS) according to the method 981.12 (AOAC 1997). Titratable total acidity (TTA) was measured by titration with NaOH (0.1 N) using phenolphthalein as indicator, according to the method 942.15 (AOAC 1997).
Preparation of Carrier Formulations
The first step in microencapsulation is to prepare a fine and stable formulation of the core material (TCW) in the wall material solution. The core material was collected by cut opening the tender coconut after washing with distilled water. TCW was filtered through muslin cloth, and a constant volume of the freshly collected core material (100 mL) was used throughout the study. The wall material preparation was based on the experimental design conditions of response surface methodology (RSM) (Table 1). The dry MG was ground in a mortar and pestle and passed through a mesh sieve (250-μm aperture) to obtain a fine powder. MG for each formulation was dissolved in warm distilled water (50 °C) under constant stirring for 2–3 h (Akhavan Mahdavi et al. 2016). Gum was sparingly soluble in water and swelled in contact with it producing a highly viscous solution. The supernatant of the gum solution was separated and collected from the residue by centrifugation (REMI, R-8C, Mumbai, India) at 5000 rpm for 10 min. For each formulation, MD was dissolved separately in warm water (50 °C), and the clear solution was obtained by passing through a muslin cloth (Akhavan Mahdavi et al. 2016; Panda et al. 2006). The wall materials were combined with core material under ambient temperature, and the resulting solution was blended in a shear homogenizer (Indo-french Industries Engineers, Mumbai, Model type SPM-9) at 3000 rpm for 5 min until complete dispersion of the components. The final solution was slightly reddish brown in color, which might be due to the presence of natural pigment in MG. The color of the solution increased with increasing concentration of MG from 0.5 to 1.5%.
Microencapsulation of TCW by Spray Drying
The stable formulation was spray dried in a tall type spray dryer (S.M Scientech, Kolkata, India) with 1000-mm cylindrical length, 300-mm cylindrical diameter, and 0.7-mm diameter nozzle. The experimental setup was conducted at a feed flow rate of 0.4 kg/h, and for the inlet air temperatures 100, 120, and 140 °C, the respective outlet air temperatures were 90, 95, and 97 °C. The pressure of compressed air flow of the spray was adjusted to 350 kPa. The microencapsulated TCW powders were packed in an airtight polypropylene bag (Knilam Packaging (Pty) Ltd., South Africa) and stored in a desiccator to avoid moisture absorption until further analysis.
Experimental Design
RSM was employed to investigate the variation of encapsulation efficiency with respect to operating parameter including MD and MG concentration, and inlet temperature. The effect of inlet temperatures and wall material concentration on encapsulation efficiency in terms of total phenol content, solubility, water activity, encapsulation yield, crystallinity, antioxidant activity, particle morphology, and color characteristics of the microencapsulated powder was determined. The composition of the three variables was designed by central composite design (CCD) approach using 22 mixes with three factors and three levels. The variables and their ranges are inlet temperature range (X 1) from 100 to 140 °C, the proportion of MD (X 2) from 10 to 50%, and concentration of MG (X 3) from 0.5–1.5% as the coating materials (Table 1). By adopting Y 1, Y 2, Y 3, and Y 4 as the response values, 18 experiments were designed, in which 13 were factorial experiments, and the remaining five runs were zero-point tests. Zero-point tests were performed five times to estimate the errors derived from CCD. The experiments were performed in triplicate for different combinations of the process parameters using statistical designs (Table 1).
A second-order polynomial quadratic equation was used to express the response variables as a function of the independent variables as follows:
Where Y represents the response variables; b o is a constant; b 1, b 2, and b 3 are the linear coefficients; b 12, b 13, and b 23 are the interaction coefficients; and b 11, b 22, and b 33 are quadratic coefficients, respectively. X 1, X 2, and X 3 are the levels of the independent variables.
Encapsulation Efficiency
The encapsulation efficiency (EE) was calculated by following the method of Aceval Arriola et al. (2016); the percentage of EE was determined as Eq. (2):
The analysis was conducted in triplicate.
DPPH Radical Scavenging Activity
DPPH method was performed to analyze the antioxidant (stability of 1, 1-diphenyl-2-picrylhydrazyl radical) activity of microencapsulated powder according to Kuskoski et al. (2005) with slight modification. Initially, the spray-dried powder (50 mg), MD, and MG were added separately to 2.95 mL of methanol-water (60:40) solution, and the mixture was magnetically stirred (REMI, Mumbai, India) for 5 min. Following which the solution was centrifuged (REMI, R-8C, Mumbai, India) at 6500 rpm for 15 min and to the resulting supernatant 2 mL of the DPPH (0.1 μmol/L methanolic solution) was added. Similarly, filtered TCW (0.05 mL) was added separately to the 2.95 mL of methanol (60%) and 2 mL of the 0.1 μmol/L of DPPH solution (Castro-Muñoz et al. 2015; Da Fonseca et al. 2009). The absorbance of samples was recorded at 517 nm (UV-Vis spectrophotometer, Jasco V-730ST, Japan) after incubating for 30 min in the dark. Milli-Q water was used instead of the sample for control. The experiment was carried out in triplicate. The antioxidant activity was calculated using the following Eq. (3).
ABTS Radical Scavenging Activity
The ABTS·+ radical scavenging activity was assayed according to the method described by Nakagawa et al. (2016) with some modifications. The working solution of ABTS·+ was prepared by mixing stock solutions of 7.4 mM ABTS·+ with 2.6 mM potassium persulphate solution in equal quantities and allowing them to react for 12–16 h at room temperature in the dark. One milliliter of ABTS·+ solution was then diluted with methanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm after equilibration at 30 °C. Exactly 10 μL of TCW, spray-dried powders, and wall materials were reacted with 1 mL of the ABTS·+ reagent separately and incubated at 30 °C for 6 min after vortexing. The absorbance of the mix was measured at 734 nm. Similarly, 10 μL of Milli-Q water was used instead of the sample for the control. The experiment was carried out in triplicate. Radical scavenging activity was calculated using Eq. (3).
Determination of Total Phenol Content
Total phenolic content was analyzed according to the Liu et al. (2008) method with slight modification. Microencapsulated powder (250 mg) was mixed with 60% acetone and stirred for 30 min vigorously. The resulting supernatant (60 μL), Folin-Ciocalteu reagent (300 μL), and 20% sodium carbonate (750 μL) were added to 4.75 mL of water. The resulting mixture was incubated for 45 min, and the absorbance was measured at 760 nm (UV-Vis spectrophotometer, Jasco V-730ST, Japan). The total phenol content was calculated from the calibration curve (y = 0.0039 × + 0.045; R 2 = 0.9928), and the results were expressed as milligrams of gallic acid equivalent per gram (mg GAE/g) of encapsulated TCW powder. Simultaneously, the total phenol content of TCW, MG, and MD was also determined. The analysis was carried out in triplicate.
Microencapsulation Yield
The encapsulation yield of spray-dried powder was calculated according to Eq. (4):
Where YE is the yield (g/100 g), W 2 is the mass (g) of the solids of powder collected after spray drying, and W 1 is the weight (g) of the non-solvent mass (wall and core material) in the feed (Xue et al. 2013). The triplicate analysis was performed.
Color Measurements
The color measurements (L*, a*, and b* color parameters of the CIE scale) of encapsulated powders, MG and MD, were carried out using ColorQuest XE, Hunter colorimeter (USA). To obtain the L*, a*, and b* values, 15 g of wall material and microencapsulated powder were transferred separately to a cuvette for equipment reading. The experiment was performed in triplicate. The hue angle (h°) and chroma (C) values were calculated using the Eqs. (5) and (6), respectively (Mapari et al. 2006):
Moisture Content and Water Activity
Moisture content was determined by following the method of AOAC (2000), 5 g of the sample (encapsulated powder) was dried in a hot air oven (Hitech Equipments, Chennai, India) at 105 °C for 3 h. Moisture loss was calculated as the percentage on a dry basis by weighing and comparing the initial weight with the final weight after drying. The measurement of the water activity was conducted at 27 ± 1 °C using a water activity meter (Aqualab model 0301962, USA). All the analysis was done in triplicate.
Solubility
The microencapsulated TCW powder (2.5 g) was dispersed in 25 mL of distilled water at 30 °C. The suspension was mixed thoroughly by vortexing (REMI, CM 101, Cyclo mixer, Mumbai, India) followed by centrifugation (REMI, R-8C, Mumbai, India) at 4000 rpm for 5 min. The resulting supernatant was then kept in a petri dish, weighed as initial weight, and allowed to dry in an oven at 100 °C for 5 h. The final weight of the petri dish containing the resulting solids was taken after drying, and the solubility (%) was calculated based on the weight difference (Shittu and Lawal 2007). Analyses were done in triplicate.
Microstructure
The microstructure, surface morphology of microencapsulated powder was investigated using a Field Emission-Scanning Electron Microscopy (FEI Quanta FEG 200 HR-SEM) after mounting the specimens onto SEM stubs by using double-sided tape and spluttering the specimen with gold. Scanning electron microscope was operated at 20 kV at magnifications of 5000× and 15,000×.
X-ray Diffraction
XRD pattern of TCW microparticles was performed on a Bruker D8 Advance X-ray diffractometer (Bruker AXS GmbH, Germany) with Cu anode and Lynx eye detector. Crystal monochromator with filter radiation of Cu-Kα1 (λ = 1.5406 Å) at 40 kV and 30 mA. Samples were analyzed in Bragg’s angles 2θ of 10 to 100° with a step of 0.02° (1.2°/min).
Statistical Analysis
For the experimental design and test, The Design Expert 10v, USA, was used. All experiments were performed in independent triplicates, and suitability of optimized model was done using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) for windows. One-way ANOVA followed by Duncan’s multiple range tests was applied to analyze significant differences (p < 0.05).
Results and Discussion
Physicochemical Analyses
Physicochemical properties of fresh TCW samples are depicted in Table 2. Physicochemical properties, such as pH, TTA, and TSS, play a significant role in taste, color, and microbial stability of juice (Cheong et al. 2012). The pH, TSS, and TTA value of the TCW were 4.5, 6.5 °Bx, and 0.09 g citric acid/100 mL, respectively, which are close to the values reported by Uphade et al. (2008) and Costa et al. (2015). Previous studies on fruit quality have found good relationships between TSS levels or TSS/TTA ratios and consumer acceptability of fruits (Niu et al. 2008; Marsh et al. 2004). The TSS and TSS/TTA ratios are generally taken as an essential characteristic of juice quality. The TSS/TTA ratio of TCW was around 72.2 which is near to the values of 61.72 and 74.8 reported by Costa et al. (2015) and Terdwongworakula et al. (2009), respectively.
Encapsulation Efficiency
In this study, EE was expressed as the amount of successful entrapment of phenol content in the powder, and the value was between 38.26–94.86%. Table 1 represents the effects of inlet temperature (X 1), MD concentration (X 2), and MG concentration (X 3) on each response variable (Y 1, Y 2, Y 3, and Y 4) of the encapsulated TCW powder. Inlet temperature (X 1) produced maximum effect on EE followed by MD concentration (X 2), interactive effect (X 12), and MG concentration (X 3) (Table 1). The model showed high significant (p < 0.001) value with the experimental data, and analysis of variance (ANOVA) showed a significant (p < 0.05) linear (X 1, X 2, and X 3), and its interactive (X 12) effect on EE (Table 3). The determination coefficient (R 2) value was 0.975. The analysis showed a lack of fit (p = 0.2803) of the model (Table 3). The regression equation for the response EE (Y 1) is given as follows:
The type of encapsulating agent and core to coating ratio is the most important variable for the total phenol encapsulation (Robert et al. 2010; Sun-Waterhouse et al. 2013), and it was noted that higher MG concentration and lower MD concentration significantly (p < 0.05) improved the EE. The ability of MG to protect the EE could be due to the presence of hydrophilic groups such as hydroxyl groups in the MG polysaccharide structure which would form interactions with hydrophilic groups of some phenolic components in the juice (TCW) via hydrogen bonds (Yousefi et al. 2015). Also unlike MD, MG has intrinsic phenol content (Table 2) which could also have contributed to the phenol content of encapsulated powder (Khare et al. 1997). The highest EE was observed in the powder produced with the lowest MD (10%). This could be explained by the fact that the lower total solid (MD: 10%) content in liquid feed used for the production of the powder in comparison to the much higher total solid (MD: 50%) in liquid feed probably enables faster and better drying conditions, and by that better protection of bioactive compounds like phenol, what results in higher EE (Vladic et al. 2016). The interaction effect between the inlet temperature (X 1) and the MD (X 2) concentration was found to be positive on the EE, i.e. decrease in MD and inlet temperature increased the EE (Table 3). Besides, the increase in inlet air temperature significantly (p < 0.001) decreased the EE. The variation in efficiency could be due to the susceptibility of some phenol acids to destruction during the application of heat in the spray-drying process (Changchub and Maisuthisakul 2011). The maximum EE (94.86%) was obtained for wall material concentration: 10% MD, 1.5% MG at 100 °C inlet temperature. Figure 1 represents the 3D contour plots of the predicted model for encapsulation efficiency. As observed in Fig. 1, there was a negative non-significant (p > 0.05) interaction between MG (X 3) and MD (X 2) concentration, and between MG (X 3) and inlet temperature (X 1). At lower MD concentration (10%) with increasing MG concentration (0.5–1.5%), a rapid increase in EE was observed (Fig. 1); however, as the MD concentration increased (30%), EE gradually decreased, also there was no major change in EE on further addition of MD (50%). Similarly, with decreasing inlet temperature (140–100 °C) with increasing MG concentration (0.5–1.5%), EE increased gradually. During spray drying, atomization of the feed solution results into very fine mist-like droplets with increased surface area. The increase in surface area means more exposure to heat. Further, there may be cases when due to atomization, some part of the wall material could get removed from the core material even after homogenization. Such partially covered encapsulates are easily affected by heat (Saikia et al. 2015). The quadratic effect of variables was not found significant (p > 0.05) for all the responses (Table 3).
Antioxidant Activity and Total Phenol Content
As shown in Table 2, the total phenol content and radical scavenging activity of TCW was relatively low when compared to MG, whereas, MD did not contain phenol content and antioxidant activity. The linear effect of MG concentration (X 3) showed a significant (p < 0.005) positive effect on the DPPH and ABTS·+ radical scavenging activity (Y 2 and Y 3) of the spray-dried powder (Table 3). The possible reason of this could be the high antioxidant activity of MG (Table 2) which could be attributed to their polyphenol compound leucoanthocyanin which might have increased the antioxidant activity of the encapsulated powder (Khare et al. 1997; Park et al. 2006). This result is in agreement with the previous findings of Jariene et al. (2015), where leucoanthocyanin present in potatoes had good free radical scavenging activity. Earlier, Raja et al. (2016) reported that a polysaccharide isolated from MG showed high DPPH radical scavenging activity in a dose-dependent manner. The phenol compounds in TCW such as catechin and epicatechin contain a high number of hydroxyls, and this chemical feature could be attributed to the antioxidant activity of the TCW (Chang and Wu 2011). The regression equation for the response DPPH radical scavenging activity (Y 2) and ABTS radical scavenging activity (Y 3) is given as follows:
The interactive effect of inlet temperature (X 1) and MD (X 2) concentration showed a negative non-significant (p > 0.05) effect (Table 3) on radical scavenging activity, whereas, interactive effect of MG (X 3) with MD (X 2) and inlet temperature (X1) were positive (Figs. 2 and 3). When MG concentration was increased at various inlet temperatures and MD concentration, a higher radical scavenging activity was observed. At lower temperature (100 °C) with the rise in MD concentration (10–50%), there was a decrease in antioxidant activity. However, increasing the inlet temperature from 100 to 120 °C, an increase in radical scavenging activity was observed, further increase in temperature (120–140 °C) reduced the antioxidant activity. Similar results were reported by Mishra et al. (2014), where an increase in MD and inlet temperature decreased the phenol content in spray-dried amla powder. Even though, MD and inlet temperature had no significant effect (p > 0.05) on the antioxidant activity, the lowest antioxidant activity (DPPH: 14.50% and ABTS: 16.80%) was observed in the powder obtained at the highest inlet temperature and MD concentration which correlates with the result of phenol content (Table 1). The optimum condition for antioxidant activity was inlet temperature: 120 °C, MG: 1.5%, and MD: 30%. However, the optimum condition for phenol and antioxidant activity was not the same. This could be because the antioxidant activities have been not only associated with phenol content (Da Fonseca et al. 2009) but also with acids (Loki and Rajamohan 2003), aromatic compounds (Agnaniet et al. 2005), aldehydes (Yi and Kim 1982), and esters (Narain et al. 2007). The quadratic effect of variables was not found significant (p > 0.05) for all the responses. Figures 2 and 3 show that all three tested variables had a negative quadratic effect on the DPPH and ABTS·+ radical scavenging activity. When the MD concentration was increased from 10 to 30%, a maximum DPPH (53.66%) and ABTS (54.92%) radical scavenging activity was attained; however, when the MD concentration was increased furthermore (30–50%), the antioxidant activity gradually decreased. This result is concomitant with the reports suggested by Kha et al. (2010), where an increase in MD concentration decreased the antioxidant activity of the spray-dried gac fruit aril powder. The statistical analysis showed a lack of fit p = 0.1181 and p = 0.0778 of the model for DPPH radical scavenging activity and ABTS·+ radical scavenging activity, respectively, and the R 2 value was 0.8007 and 0.7971, respectively (Table 3).
Various factors showed a significant (p < 0.05) effect on phenol content; these are linear (X 1, X 2, and X 3) and interactive (X 12) effects (Table 3). The phenol concentration demonstrated a significant (p < 0.005) decrease when the level of coating material (MD), and inlet temperature was increased from 10 to 50% and 100 to 140 °C, respectively (Table 3). The phenol content value was between 8.8 and 21.82 mg GAE/g (Table 1) for the microparticles, and the optimum condition for the retention of total phenol content was MG (1.5%), MD (10%), and inlet temperature (100 °C). Even though TCW contains natural phenol compounds like catechin and epicatechin (Chang and Wu 2011), the phenol content was much low when compared to MG (Table 2). The high phenol content of MG could be attributed to the presence of polyphenols like leucoanthocyanin. MG concentration increased the encapsulation efficiency of total phenol content; this is a substantial effect as the encapsulation with MG not only efficiently protected the total phenol content of the TCW but also may have provided additional phenol content to the encapsulated powder (Khare et al. 1997). The above observation is evident in the result, as increasing MG concentration significantly (p < 0.05) increased the total phenol content of the powder (Fig. 4). The observed trend is in agreement with the results demonstrated by other researchers (Yousefi et al. 2015; Rutz et al. 2013). Microencapsulation processes using the spray-drying method have proven to be a useful technology for protecting compounds like phenols for long periods and release them under digestive conditions.
The regression equation for the response total phenol content (Y 4) is
The statistical analysis illustrates a lack of fit of the model (p = 0.2803) for total phenol content; the R 2 value was 0.97 (Table 3). The linear, interactions, and quadratic effect on phenol content showed similar trends as EE, showing a significant (p < 0.05) positive interaction effect between inlet temperature (X 1) and MD concentration (X 2) and negative interaction between MG (X 3) and MD (X 2), MG (X 3), and inlet temperature (X1) (Fig. 4). Carbohydrates, like maltodextrin, gum arabic, and other plant gums are among key wall materials, which are used as encapsulating agents who keep the core material intact during the process (Mapari et al. 2006; Jafari et al. 2008). This material has the ability to form a coating for the core, encapsulating aromas and flavors (Pourashouri et al. 2014). Therefore, this process is suitable for increasing total phenol stability during long-term storage while preserving their biological activity (Mahdavee Khazaei et al. 2014). However, it was noted in our study that wall material (MD) did not contain phenolic content; hence, a decrease in phenol content of microparticle was observed as a result of increasing concentration of MD in the core: coating mixture.
Microencapsulation Yield
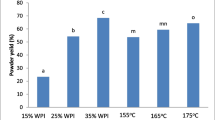
Powder yield is affected by the wall material concentration, diminishing from 37.90 to 8.98 g/100 g when the wall material is in low proportions (Table 4). The addition of further amounts of MD (50%) and MG (1.5%) enhanced the powder yield. According to Bhandari et al. (1997), more than 50% recovery in the cyclone is a criterion for efficient drying. One of the approaches to improve the yield and diminish the stickiness during spray drying is to increase the wall material concentration (Tontul and Topuz 2017). However, in our study, when the MD concentration was increased, encapsulation efficiency and radical scavenging activity were decreased. Similarly, the color of the powder was negatively influenced by the addition of the higher amount of MG. Thus, the conditions (wall material and temperature) were optimized to maximize the retention of all the tested responses. A previous study on spray drying of Morinda citrifolia L. using κ-carrageenan and maltodextrin as an encapsulating agent resulted in low powder yield of 5.15–48.13% (Krishnaiah et al. 2012). In the same way, Obon et al. (2009) reported that spray drying of purple cactus pear using gelatin and maltodextrin mixtures as carrier material lead to very low product yield (<10%). The increase in wall material concentration results in a soar in solid content leading to a surge in the feed viscosity. Enhancing the feed viscosity up to an optimum point will suppress the internal circulations and oscillations of droplets, and prevents the colliding of wet droplets onto the drying surface, therefore improving the encapsulation yield (Rajabi et al. 2015). However, increasing the viscosity beyond that optimum level causes a decrease in the yield, due to the difficulties in droplet formation (Jafari et al. 2008). The increase in total solid content reduces the droplet size which in turn reduces the size of the particle, leading to increasing the powder yield (Rajabi et al. 2015). A higher level of moisture content in larger droplet generates more interparticle cohesion that increases the product stuck on the wall of the drying chamber, thereby reducing the yield (Tontul and Topuz 2017). The total solid content was increased by the addition of MG (0.5, 1, and 1.5%) and MD (10, 30, and 50%) which may be the amount of water to be evaporated decreased, leading to a lesser moisture content and greater yield. Moreover, Ameri and Maa (2006) suggested that increasing the total solid content of the feed solution could increase the yield of the powder. Sticky particles were obtained when the inlet temperature and MD/MG concentration was lower, and non-sticky fine powders were obtained when the wall material ratio and the temperature were higher. This finding is in agreement with the results of Obon et al. (2009) who obtained powder food colorant by spray drying of O. stricta fruit juices with glucose syrup, which suggested that increase in the concentration of drying aid decreased the stickiness of the final product. The encapsulation yield reached their maxima (37.90 g/100 g) at 140 °C. The increase in drying temperature usually results in a faster drying rate, and higher powder productivity which is achieved by greater efficiency of heat and mass transfer processes occurs in the case of higher inlet temperatures (Leon-Martinez et al. 2010; Tonon et al. 2008). Hence, low inlet temperature (100 °C) caused the formation of encapsulated powders with high moisture content. Generally, in a spray-drying system, the temperature of air leaving the drying chamber (exhaust) controls residual moisture content (Leon-Martinez et al. 2010). The variations in moisture content were mentioned in Table 4, where lower inlet temperature (100 °C) and wall material concentration (MD: 10%; MG: 0.5%) gave the lowest yield of powder with higher moisture content. Also, at higher inlet temperatures, there is a greater temperature gradient between the atomized feed and drying air, resulting in a greater driving force for water evaporation, thus producing powders with lower moisture content (Tonon et al. 2008).
Color Measurements
The L* value of MD is towards lightness (Table 4), and the addition of maltodextrin favorably influenced the L* value of the particle by increasing the value from 52.15 to 96.66 (Table 4). Conversely, the addition of MG had a negative effect on microcapsules due to natural pigment (reddish brown), causing the L* value to decrease. Similar results were demonstrated by Chauhan et al. (2008) where the reduction of L* value of the TCW beverage was observed on the addition of pomegranate juice, giving a darker shade to the beverage. Hue angle is attributed to the perceived color, and hue angle 0° represents the red color, and 90° represents the yellow color (Tolvaj and Nemeth 2008). With increasing MG concentration, and decreasing MD concentration and inlet temperature, the change towards redness was greater, decreasing the hue angle from 84.37 to 51.79°. The chroma (C) measurement is indicative of the vividness of color, and the C value decreased with increasing MD concentration and inlet temperature and decreasing MG concentration. The possible reason for the lightness of powder at high temperature may be due to the destruction of some inherent pigments present in the Moringa gum on the application of high heat during spray drying. This result is concurrent with the previous study carried out by Saikia et al. (2015), where microencapsulated powder of phenol extract from Averrhoa carambola pomace by freeze and spray drying was lighter in color with increasing inlet temperature. The optimal condition for color value is MD: 50%; MG: 0.5%; and 140 °C.
Water Activity, Moisture Content, and Solubility
The water activity (a w), solubility, and moisture content of the encapsulated powder are depicted in Table 4. As shown in Table 4, higher inlet temperature led to decrease in the water activity. The decrease in water activity might have occurred due to the greater evaporation of water at higher temperature, therefore reducing the capture of water molecules by the sample. The long shelf life of the dried product is closely linked to the moisture content and water activity (Caliskan and Dirim 2013). The microencapsulated powder had a water activity (a w) between 0.21–0.39 and decreased with increasing wall material concentration. Hence, the use of MD and MG was useful for the reduction of stickiness in the spray dryer. Also, the higher wall material to core material concentration showed lesser moisture content. These results could be explained by the fact that additional concentrations of drying aid led to a rise in feed solids and lower water available for evaporation, leading to lower moisture content (Landim 2008). The outcome was similar to the findings reported by Quek et al. (2007) where an increasing percentage of maltodextrin exhibited decreasing moisture content of watermelon powder. In our study, higher inlet temperature caused lower moisture content since greater heat transfer rate provides great driving force for moisture evaporation (Silva et al. 2014). The solubility of the microparticles ranged from 76.68 to 95.83%, which is similar to the value of 67.71–99.71% reported by Castro-Muñoz et al. (2015) for mango juice powders. The greater solubility of the powder is owing to the properties of the water-soluble encapsulants that were used for drying, because the mixes with the highest solubilities correspond to those produced with the highest amount of the encapsulant.
Scanning Electron Microscopy
SEM micrographs of encapsulated TCW powder at different MG concentrations are shown in Fig. 5. The particles were spherical in shape, irregular, and dents were visible. Coating material composition and core/coating ratio may be responsible for dented characteristics of the spray-dried microcapsules. Outer structures were affected by the coating material which was composed of different composition. These structural irregularities might have occurred because of the possible removal of water content during the spray-drying process. However, there were no cracks or fissures in the particles. The ideal structure in the microencapsulation process is a uniform and smooth surface, slightly spherical shape with minimum cracks and collapses on its walls (Tolun et al. 2016). In this case, the coating material mixture with higher MG level gave the best results (Fig. 5c, d). The microencapsulated particles with low MG level had more rough surfaces. The particles with rough surfaces are more sensitive to oxidation reaction compared to those of smooth surfaces because of their larger surface areas. The microparticle with 1.5% MG exhibited more rigid structures (Fig. 5c, d) compared to the particle with 1% MG level (Fig. 5a, b). The rigid structure indicates that the increase of MG level led to the formation of structures more resistant to the spray-drying processes, probably due to the higher viscoelastic capacity of Moringa gum. Alamilla-Beltran et al. (2005) reported that increase in temperature leads to increasing rate of evaporation, resulting in smoother and defined surfaces, also lower air inlet temperatures resulted in irregularly shaped microparticles. The surface structure feature is dependent mainly on the surfaces dried at a low speed, as determined by the low operating temperatures (Tonon et al. 2009).
X-ray Diffraction
XRD is an important tool to study the crystal lattice arrangement, and it gives very useful information about the sample crystallinity. XRD analysis of the encapsulated TCW powder with different MG concentration at 120 °C with 30% MD is presented in Fig. 6a–c. All the samples had an amorphous structure with a minimum of crystallinity. Powders containing amorphous components hydrate quickly because of the low energy levels of bonds between molecules, when compared to the crystalline state. On the other hand, products with crystalline components tend to dissolves slowly, as the dissolution of crystals occurs only on the outer surface, exposed to the solvent (Marabi et al. 2007). Usually, amorphous samples tend to be very hygroscopic and absorb water during storage. This characteristic prejudices the storage of the samples since the water absorption implicates in weight gain and nutrient degradation, microstructure collapse, and potential microbiological instability (Borrmann et al. 2013). The main diffraction peak at 2θ = 22.5° in Fig. 6c (MG 1.5%, MD 30%, and 120 °C), appeared to be sharper and have a larger magnitude than the peak produced by other encapsulated samples, which denotes that an increase in MG concentration might have slightly increased the degree of crystallinity of encapsulated sample.
Optimization
Optimum spray-drying conditions depend on inlet temperature, wall material concentration of the solution, and drying without expansion and cracks of final particles (Bimbenet et al. 2002). Taking into account that total phenol content was chemically unstable in the presence of moisture content, the optimization procedure of multiple responses was applied by reducing the maximum value of the inlet temperature (120 °C) to maximize the EE% and to preserve the quality of encapsulated powder. The optimized response suggested that a combination of X 1: 120 °C, X 2: 30%, and X 3: 1.5% would lead to EE of 56.65%. At this optimized condition, the predicted values for encapsulation efficiency were 54.85%, close to the experimental values of 56.65%, indicating the suitability of the model to be used in optimizing the spray-drying process for the production of quality TCW powder (Table 5). The results showed that no significant differences were found between the estimated value of the model and the experimentally observed value for EE which suggested a good fit of the model to experimental data.
Conclusion
The effect of different proportions of MD and MG, and inlet temperature on physicochemical properties of TCW powder, was evaluated. Maltodextrin concentration (10–50%), Moringa oleifera gum concentration (0.5–1.5%), and inlet air temperature (100–140 °C) significantly (p < 0.05) affected moisture content, water activity, solubility, color attributes, antioxidant activity, and total phenol content of the encapsulated powder. The spray-dried powder demonstrated good water solubility (76.68–95.83%) which is essential for reconstitution. The TCW powder dried at 100 °C, and MD concentration of 10% and MG concentration 1.5% showed better retention of total phenol content (21.82 mg GAE/g), and DPPH (28.80%) and ABTS (31.41%) free radical scavenging activity. However, due to the high stickiness of the powder, lower yield, higher moisture content, and water activity, it cannot be recommended as the optimum product. TCW dried at 120 °C, 30% MD, and 1.5% MG concentration was effective to produce the powder with less moisture content, water activity, potent total phenol content, radical scavenging activity, and acceptable color. Hence, this study suggests that MG offers promising possibilities as matrices for microencapsulation of TCW, which might increase the application of TCW in food formulations. Moreover, MG provides an effective microencapsulation system which could be used for powder production with better retention of the bioactive compounds in food industries.
References
Aceval Arriola, N. D., De Medeiros, P. M., Prudencio, E. S., Olivera Müller, C. M., & De Mello Castanho Amboni, R. D. (2016). Encapsulation of aqueous leaf extract of Stevia rebaudiana Bertoni with sodium alginate and its impact on phenolic content. Food Bioscience, 13, 32–40.
Agnaniet, H., Bikanga, R., Bessiere, J. M., & Menut, C. (2005). Aromatic plants of tropical central Africa. Part XLVI. Essential oil constituents of Cassia alata (L.) from Gabon. Journal of Essential Oil Research, 17(4), 410–412.
Akhavan Mahdavi, S., Jafari, S. M., Assadpoor, E., & Dehnad, D. (2016). Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. International Journal of Biological Macromolecules, 85, 379–385.
Alamilla-Beltran, L., Chanona-Pere, J. J., Jimenez-Aparicio, A. R., & Gutierrez-Lopez, G. F. (2005). Description of morphological changes of particles along spray drying. Journal of Food Engineering, 67(1–2), 179–184.
Ameri, M., & Maa, Y.-F. (2006). Spray drying of biopharmaceuticals: stability and process considerations. Drying Technology, 24(6), 763–768.
AOAC. (1997). Official methods of analysis of AOAC International. 16th edn. Gaithersburg.
AOAC. (2000). Official methods of analysis of AOAC International. 17th ed., Arlington.
Bhandari, B. R., Datta, N., Crooks, R., Howes, T., & Rigby, S. (1997). A semi-empirical approach to optimise the quantity of drying aids required to spray dry sugar rich foods. Drying Technology, 15(10), 2509–2525.
Bimbenet, J. J., Bonazzi, C., & Dumoulin, E. (2002). Drying of foodstuffs. Proceeding of the 13th International Drying Symposium IDS, Beijing, China, pp. 64–80.
Borrmann, D., Pierucci, A. P. T. R., Leite, S. G. F., & Leao, M. H. M. D. R. (2013). Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatised starch using spray-drying. Food and Bioproducts Processing, 91(1), 23–27.
Caliskan, G., & Dirim, S. N. (2013). The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food Bioproducts and Processing, 91(4), 539–548.
Campbell-Falck, D., Thomas, T., Falck, T. M., Tutuo, N., & Clem, K. (2000). The intravenous use of coconut water. American Journal of Emergency Medicine, 18(1), 108–111.
Cano-Higuita, D. M., Velez, H. A. V., & Telis, V. R. N. (2015). Microencapsulation of Turmeric oleoresin in binary and ternary blends of gum arabic, maltodextrin and modified starch. Science and Agrotechnology, 39, 173–182.
Castro-Muñoz, R., Barragan-Huerta, B. E., & Yañez-Fernandez, J. (2015). Use of gelatin-maltodextrin composite as an encapsulation support for clarified juice from purple cactus pear (Opuntia stricta). LWT- Food Science and Technology, 62(1), 242–248.
Chang, C.-L., & Wu, R.-T. (2011). Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC–MS. Food Chemistry, 126(2), 710–717.
Changchub, L., & Maisuthisakul, P. (2011). Thermal stability of phenolic extract and encapsulation from mango seed kernel. Agricultural Science Journal, 42(2), 397–400.
Chauhan, O. P., Asha, S., Raju, P. S., & Bawa, A. S. (2008). Development of pomegranate juice blended tender coconut water beverage. Cord, 2, 46–60.
Chauhan, O. P., Archana, B. S., Singh, A., Raju, P. S., & Bawa, A. S. (2014). A refreshing beverage from mature coconut water blended with lemon juice. Journal of Food Science and Technology, 51(11), 3355–3361.
Cheong, M. W., Zhu, D., Sng, J., Liu, S. Q., Zhou, W., Curran, P., & Yu, B. (2012). Characterization of calamansi (Citrus microcarpa). Part II: volatiles, physicochemical properties and non-volatiles in the juice. Food Chemistry, 134(2), 696–703.
Costa, H. B., Souza, L. M., Soprani, L. C., Oliveira, B. G., Ogawa, E. M., Korres, A. M. N., Ventura, J. A., & Romao, W. (2015). Monitoring the physicochemical degradation of coconut water using ESI-FT-ICR MS. Food Chemistry, 174, 139–146.
Da Fonseca, A. M., Bizerra, A. M. C., De Souza, J. S. N., Monte, F. J. Q., De Oliveira, M. D. C. F., De Mattos, M. C., et al. (2009). Constituents and antioxidant activity of two varieties of coconut water (Cocos nucifera L.) Brazilian Journal of Pharmacognosy, 19(1 B), 193–198.
Desai, K. G. H., & Park, H. J. (2005). Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. Journal of Microencapsulation, 22(2), 179–192.
Gopalakrishnan, L., Doriya, K., & Kumar, D. S. (2016). Moringa oleifera: a review on nutritive importance and its medicinal application. Food Science and Human Wellness, 5(2), 49–56.
Jafari, S. M., Assadpoor, E., He, Y., & Bhandari, B. (2008). Encapsulation efficiency of food flavours and oils during spray drying. Drying Technology, 26(7), 816–835.
Jariene, E., Vaitkeviciene, N., Danilcenko, H., Gajewski, M., Chupakhina, G., Fedurajev, P., et al. (2015). Influence of biodynamic preparations on the quality indices and antioxidant compounds content in the tubers of coloured potatoes (Solanum tuberosum L.) Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 43(2), 392–397.
Kalman, D. S., Feldman, S., Krieger, D. R., & Bloomer, R. J. (2012). Comparison of coconut water and a carbohydrate-electrolyte sport drink on measures of hydration and physical performance in exercise-trained men. Journal of the International Society of Sports Nutrition, 9, 2–10.
Kha, T. C., Nguyen, M. H., & Roach, P. D. (2010). Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. Journal of Food Engineering, 98(3), 385–392.
Khare, G. C., Singh, V., & Gupta, P. C. (1997). A new leucoanthocyanin from Moringa oleifera gum. Journal of Indian Chemical Society, 74(3), 247–248.
Krishnaiah, D., Sarbatly, R., & Nithyanandam, R. (2012). Microencapsulation of Morinda citrifolia L. extract by spray-drying. Chemical Engineering Research and Design, 90(5), 622–632.
Kuskoski, E. M., Asuero, A. G., Troncoso, A. M., Mancini-Filho, J., & Fett, R. (2005). Application of several chemical methods to determine antioxidant activity in fruit pulps. Ciencia y Tecnología de Alimentos, Campinas, 25(4), 726–732.
Landim, E. M. C. (2008). Obtaining, characterization and evaluation of the stability of microencapsulated pigmentosnaturais, Dissertation (Food Technology), Brazil, Federal University of Ceará, Fortaleza, p. 89.
Leon-Martinez, F. M., Mendez-Lagunas, L. L., & Rodriguez-Ramirez, J. (2010). Spray drying of nopal mucilage (Opuntia ficus-indica): effects on powder properties and characterization. Carbohydrate Polymers, 81(4), 864–870.
Liu, X., Cui, C., Zhao, M., Wang, J., Luo, W., Yang, B., & Jiang, Y. (2008). Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chemistry, 109(4), 909–915.
Loki, A. L., & Rajamohan, T. (2003). Hepatoprotective and antioxidant effect of tender coconut water on carbon tetrachloride induced liver injury in rats. Indian Journal of Biochemistry and Biophysics, 40(5), 354–357.
Lolodi, O. (2011). Microencapsulation of colistin sodium methanesulfonate in gum arabic and maltodextrin by spray drying. Trends in Applied Sciences Research, 6, 877–889.
Madene, A., Jacquot, M., Scher, J., & Desobry, S. (2006). Flavour encapsulation and controlled release—a review. International Journal of Food Science and Technology, 41(1), 1–21.
Mahdavee Khazaei, K., Jafari, S. M., Ghorbani, M., & Hemmati Kakhki, A. (2014). Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydrate Polymers, 105(1), 57–62.
Mantena, S. K., Jagadish, Badduri, S. R., Siripurapu, K. B., & Unnikrishnan, M. K. (2003). In vitro evaluation of antioxidant properties of Cocos nucifera Linn. water. Nahrung, 47(2), 126–131.
Mapari, S. A., Meyer, A. S., & Thrane, U. (2006). Colorimetric characterization for comparative analysis of fungal pigments and natural food colorants. Journal of Agricultural Food Chemistry, 54(19), 7027–7035.
Marabi, A., Mayor, G., Raemy, A., Bauwens, I., Claude, J., Burbidge, A. S., et al. (2007). Solution calorimetry: a novel perspective into the dissolution process of food powders. Food Research International, 40(10), 1286–1298.
Marsh, K., Attanayake, S., Walker, S., Gunson, A., Bolding, H., & MacRae, E. (2004). Acidity and taste in kiwifruit. Postharvest Biology and Technology, 32(2), 159–168.
Mbikay, M. (2012). Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Frontiers in Pharmacology, 3, 1–12.
Mishra, P., Mishra, S., & Mahanta, C. L. (2014). Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food and Bioproducts Processing, 92(3), 252–258.
Nakagawa, K., Ritcharoen, W., Sri-Uam, P., Pavasant, P., & Adachi, S. (2016). Antioxidant properties of convective-air-dried Spirulina maxima: evaluation of phycocyanin retention by a simple mathematical model of air-drying. Food and Bioproducts Processing, 100, 292–302.
Narain, N., Galvão, M. S., & Madruga, M. S. (2007). Volatile compounds captured through purge and trap technique in caja-umbu (Spondias sp.) fruits during maturation. Food Chemistry, 102(3), 726–731.
Niu, L., Wu, J., Liao, X., Chen, F., Wang, Z., Zhao, G., et al. (2008). Physicochemical characteristics of orange juice samples from seven cultivars. Agricultural Sciences in China, 7(1), 41–47.
Obon, J. M., Castellar, M. R., Alacid, M., & Fernandez-Lopez, J. A. (2009). Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. Journal of Food Engineering, 90(4), 471–479.
Panda, D. S. (2014). Studies on gum of Moringa oleifera for its emulsifying properties. Journal of Pharmacy and Bioallied Sciences, 6(2), 92–96.
Panda, D., Si, S., Swain, S., Kanungo, S. K., & Gupta, R. (2006). Preparation and evaluation of gels from gum of Moringa oleifera. Indian Journal of Pharmaceutical Sciences, 68(6), 777–780.
Park, Y. S., Jung, S. T., Kang, S. G., Delgado-Licon, E., Leticia Martinez Ayala, A., Tapia, M. S., et al. (2006). Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. LWT-Food Science and Technology, 39(7), 748–755.
Pourashouri, P., Shabanpour, B., Razavi, S. H., Jafari, S. M., Shabani, A., & Aubourg, S. P. (2014). Impact of wall materials on physicochemical properties of microencapsulated fish oil by spray drying. Food and Bioprocess Technology, 7(8), 2354–2365.
Prades, A., Ablan, R., Dornier, M., Pain, J., & Boulanger, R. (2012). Characterisation of the volatile profile of coconut water from five varieties using an optimised HS-SPME-GC analysis. Journal of the Science of Food and Agriculture, 92(12), 2471–2478.
Quek, S. Y., Chok, N. K., & Swedlund, P. (2007). The physicochemical properties of spray-dried watermelon powder. Chemical Engineering and Processing: Process Intensification, 46(5), 386–392.
Raja, W., Bera, K., & Ray, B. (2016). Polysaccharides from Moringa oleifera gum: structural elements, interaction with blactoglobulin and antioxidative activity. RSC Advances, 6(79), 75699–75706.
Rajabi, H., Ghorbani, M., Jafari, S. M., Sadeghi, A., & Rajabzadeh, Q. (2015). Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocolloids, 51, 327–337.
Robert, P., Gorena, T., Romero, N., Sepulveda, E., Chavez, J., & Saenz, C. (2010). Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. International Journal of Food Science and Technology, 45(7), 1386–1394.
Rutz, J. K., Zambiazi, R. C., Borges, C. D., Krumreich, F. D., Da Luz, S. R., Hartwig, N., & Da Rosa, C. G. (2013). Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydrate Polymers, 98(2), 1256–1265.
Saikia, S., Mahnot, N. K., & Mahanta, C. L. (2015). Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chemistry, 171, 144–152.
Shittu, T. A., & Lawal, M. O. (2007). Factor affecting instant properties of powdered cocoa beverages. Food Chemistry, 100(1), 91–98.
Silva, P. T. D., Fries, L. L. M., Menezes, C. R. D., Holkem, A. T., Schwan, C. L., Wigmann, E. F., et al. (2014). Microencapsulation: concepts, mechanisms, methods and some applications in food technology. Cienca Rural, 44(7), 1304–1311.
Singhal, A. K., Jarald, E. E., Showkat, A., & Daud, A. (2012). In vitro evaluation of Moringa oleifera gum for colon-specific drug delivery. International Journal of Pharmaceutical Investigation, 2(1), 48–51.
Sun-Waterhouse, D., Wadhwa, S. S., & Waterhouse, G. I. N. (2013). Spray drying microencapsulation of polyphenol bioactives: a comparative study using different natural fibre polymers as encapsulants. Food and Bioprocess Technology, 6(9), 2376–2388.
Terdwongworakula, A., Chaiyapong, S., Jarimopas, B., & Mccklangsacn, W. (2009). Physical properties of fresh young Thai coconut for maturity sorting. Biosystems Engineering, 103(2), 208–216.
Tolun, A., Altintas, Z., & Artik, N. (2016). Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: development and characterization. Journal of Biotechnology, 239, 23–33.
Tolvaj, L., & Nemeth, K. (2008). Correlation between hue-angle and colour lightness of steamed black locust wood. Acta Silvatica Et Lignaria Hungarica, 4, 55–59.
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2008). Influence of process conditions on the physicochemical properties of acai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering, 88, 411–418.
Tonon, R. V., Brabet, C., Pallet, D., Brat, P., & Hubinger, M. D. (2009). Physicochemical and morphological characterisation of acai (Euterpe oleraceae Mart.) powder produced with different carrier agents. International Journal of Food Science and Technology, 44(10), 1950–1958.
Tontul, I., & Topuz, A. (2017). Spray-drying of fruit and vegetable juices: effect of drying conditions on the product yield and physical properties. Trends in Food Science and Technology, 63, 91–102.
Uphade, B. K., Shelke, S. S., & Thorat, D. G. (2008). Studies on some physico-chemical characteristics of coconut water near sugar and chemical factory, Kopergaon (M. S.) International Journal of Chemical Sciences, 6(4), 2052–2054.
Verbeken, D., Dierckx, S., & Dewettinck, K. (2003). Exudate gums: occurrence, production, and applications. Applied Microbiology and Biotechnology, 63(1), 10–21.
Vladic, J., Ambrus, R., Szabó-Révész, P., Vasic, A., Cvejina, A., Pavlic, B., & Vidovic, S. (2016). Recycling of filter tea industry by-products: production of A. millefolium powder using spray drying technique. Industrial Crops and Products, 80, 197–206.
Whistler, R. L. (1993). Exudate gums, in industrial gums: polysaccharides and their derivatives. In R. L. Whistler & J. N. Bemiller (Eds.), (pp. 318–337). San Diego: Academic Press.
Xue, F., Li, C., Liu, Y., Zhu, X., Pan, S., & Wang, L. (2013). Encapsulation of tomato oleoresin with zein prepared from corn gluten meal. Journal of Food Engineering, 119(3), 439–445.
Yi, B. H., & Kim, D. H. (1982). Antioxidant activity of maltol, kojic acid, levulinic acid, furfural, 5-hydroxymethylfurfural, and pyrazine. Korean Journal of Food Science and Technology, 14(3), 265–270.
Yousefi, S., Emam-Djomeh, Z., Mousavi, M., Kobarfard, F., & Zbicinski, I. (2015). Developing spray-dried powders containing anthocyanins of black raspberry juice encapsulated based on fenugreek gum. Advanced Powder Technology, 26(2), 462–469.
Zbicinski, I., & Marciniak, E. (2008). Microencapsulation in spray drying—an overview, 16th International Drying Symposium (IDS 2008), Hyderabad, India, November A, pp. 9–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nambiar, R.B., Sellamuthu, P.S. & Perumal, A.B. Microencapsulation of Tender Coconut Water by Spray Drying: Effect of Moringa oleifera Gum, Maltodextrin Concentrations, and Inlet Temperature on Powder Qualities. Food Bioprocess Technol 10, 1668–1684 (2017). https://doi.org/10.1007/s11947-017-1934-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1934-z