Abstract
Pomegranate seed oil (PSO) is rich in bioactive compounds and is susceptible to oxidation. This research sought to encapsulate PSO in conventional and Pickering emulsions using whey protein isolate (WPI) microgels, WPI in its natural form, gum Arabic (GA), and WPI combinations with GA, maltodextrin (MD), and modified starch (Capsul®) as aqueous phase/emulsifier followed by spray drying. Emulsions with 1.39–2.55 μm droplet size, low viscosity (1.47–3.96 mPa s), and final interfacial tensions of 4.21–9.97 mN m−1 were obtained. All formulations were stable with the Turbiscan stability index between 4.57% and 12.95% at 24 h. Emulsions resulted in particles with encapsulation efficiency and yield of 56.28–73.83% and 28.07–93.99%, respectively. PSO powders had small particle sizes (9.86–22.60 μm), high glass transition temperature (103.24–121.62 °C), and oxidative stability index (OSI) of 2.71 h and in the range of 4.11–21.23 h for non-encapsulated and encapsulated PSO, respectively. All formulations promoted the oil oxidative protection when compared with the non-encapsulated one. Treatments presented feasible values of Aw, moisture, solubility, and hygroscopicity for handling and storage of the powders. WPI, WPI:Capsul®, and Pickering treatments promoted greater protection of the encapsulated oil; however, the combination of WPI with modified starch was considered the best wall material, allowing protection of PSO and future applications in the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Punicic acid, known as conjugated linolenic acid, is from a family of octatrienoic acid positional and geometric isomers that regulate lipid metabolism and exert anticancer properties. Pomegranate (Punica granatum L.) seed oil, a by-product of the juice industry (Elfalleh et al. 2011), is an important source of this fatty acid (FA) and also contains linoleic acid (omega-6 FA), phospholipids, tocopherols, and triterpenoids in its composition (Siano et al. 2016). Due to the presence of double bonds, unsaturated FAs are susceptible to oxidation when in contact with light, oxygen, moisture, and high temperature (Siano et al. 2016), which hinders the application of oils rich in these FAs in food products. Encapsulation represents an alternative to minimize these problems.
The encapsulation process involves the protection or immobilization of one or more compounds (core or internal phase) by one or more materials (wall material or external phase) in order to avoid contact with light, heat, moisture, and oxygen; moreover, it masks unpleasant flavors and odors and promotes the controlled release of the core (Shahidi and Han 1993). Microencapsulation based on emulsification is used extensively in the food and pharmaceutical industries (Jiang et al. 2019). In oil-in-water (O/W) emulsions, the two immiscible liquids are combined to produce a system in which small oil droplets are dispersed within a continuous aqueous phase. In general, most emulsions are thermodynamically unstable, tending to destabilize easily through different mechanisms such as phase separation, creaming, coalescence, and flocculation (Guzey et al. 2004).
In order to prepare emulsions with the stability required for their application in food products, surface-active agents of amphiphilic nature (i.e., emulsifiers) are added, which decreases the surface tension at the O/W or water/air interface (McClements 2011). Alternatively, there is an increasing focus on the utilization of nano/solid particles for the stabilization of emulsions (Calabrese et al. 2018). Pickering emulsions—emulsions stabilized by solid particles—exhibit enhanced resistance to coalescence, making them attractive systems for applications in food products (Costa et al. 2018a, 2018b). Whey protein isolate (WPI) is an example of a suitable food-grade ingredient used for Pickering emulsion formation (Ravanfar et al. 2018). The main whey protein in WPI is β-lactoglobulin, whose native globular state is denatured upon heat treatment. WPI nanoparticles (microgels) can be formed by heat treatment and pH changes. Non-covalent and covalent interactions between free thiol groups and disulfide bonds can occur, resulting in aggregation of denatured proteins (Bryant and McClements 2000; Donato et al. 2009).
In addition to the emulsification technique, spray drying is a fast and continuous method, and one of the most used techniques for encapsulation of food ingredients (Bucurescu et al. 2018). It consists of the atomization of a solution, dispersion, or emulsion containing the active compound (material to be encapsulated) and the carrier (encapsulating material, usually a biopolymer) into a chamber in which the material comes into contact with hot air and is dried instantaneously, producing microparticles (Gharsallaoui et al. 2007).
The combination of spray drying technique with Pickering emulsions using WPI microgels for the encapsulation of food ingredients is still little studied, and no research on the encapsulation of pomegranate seed oil by the combination of these techniques has been found in the literature. Moreover, the comparison of this process with the use of different carrier agents is also of great importance for studying the properties of the particles’ structure, which is directly related to the protection and adequate release of the bioactive compound and have also been little evaluated. Studies on the influence of different types of wall material during the spray drying process are still needed.
This work aimed to study the encapsulation of pomegranate seed oil in a Pickering O/W emulsion using WPI microgels and in traditional systems utilizing WPI in its natural form, gum Arabic (GA), modified starch (Capsul®), maltodextrin (MD), and their combinations (WPI:GA, WPI:MD, and WPI:Capsul®) as the aqueous phase/emulsifier, followed by a spray drying process. Apart from the use of a milk-based protein (WPI), the materials (GA and MD) are of low cost and wide availability, and Capsul® is an excellent emulsifier. It is known that particles obtained by atomization using traditional carrier materials present high water solubility (Aghbashlo et al. 2013; Jiang et al. 2019; Samborska et al. 2019). However, the combination of a Pickering emulsion with spray drying technique allows the possibility of different food applications (hydrophilic and hydrophobic food matrices) since these particles have low water solubility. The process proposed here should be explored to obtain innovative vehicles that will afford health benefits to consumers, protect the encapsulated compound, and allow the possibility of controlled release.
Material and Methods
Materials
As a source of polyunsaturated FAs, pomegranate seed oil (De Wit Specialty Oils, De Waal, Texel, Netherlands) was used. Whey protein isolate (WPI 894-Inst) and gum Arabic were obtained from Fonterra (Auckland, New Zealand) and Nexira (São Paulo, Brazil), respectively. Maltodextrin DE 10 (MOR-REX 1910) and modified starch (Capsul®) were obtained from Ingredion Brazil (São Paulo, Brazil).
Methods
WPI Microgel Preparation
The WPI microgels were produced according to the methods used by Ravanfar et al. (2018) and Zamani et al. (2018) with some modifications. Aqueous solution of WPI (8.0% w/v) was prepared and heated at 50 °C for 45 min under constant magnetic stirring to ensure homogenization. The solution was then cooled to room temperature and the pH adjusted to 5.9 with HCl (0.2 N). The WPI solution was re-heated at 82 °C for 45 min, followed by rapid cooling in an ice bath, to stop aggregation.
The ζ-potential and particle size of the microgels were analyzed using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Before analysis, the samples were diluted to 0.1% using deionized water at room temperature. Triplicate analyses were conducted. Microgels were further investigated for their morphology using a transmission electron microscope (Jeol 1200 EX II; Tokyo, Japan) equipped with a Gatan 791 multiscan camera (Gatan, Pleasanton, CA, USA) for image acquisition. A concentrated pellet of WPI microgels was prepared, and a drop of this pellet was placed under a formvar/carbon-coated nickel grid. The sample was then transferred to 50 μL drops of filtered saturated uranyl, allowed to dry for 5 min, and washed four times in pure water.
Emulsion Preparation
The O/W emulsions were prepared using pomegranate seed oil as the oil phase (disperse phase) and different proteins and polysaccharides in the aqueous phase (continuous phase). The continuous phase was prepared with 8% (w/w) solids and was composed of WPI, GA, and combinations of WPI and GA (WPI:GA), WPI and MD (WPI:MD), WPI and modified starch (WPI:Capsul®), or WPI microgels (Pickering emulsion). The proportion of oil phase : aqueous phase (solids basis) was 1 : 4, and the homogenization conditions were 18,000 rpm for 10 min, using an Ultra-Turrax IKA T18 (Campinas, Brazil), resulting in six treatments (Table 1).
Emulsion Characterization
Optical and Fluorescence Microscopy
Emulsions were visualized by optical and fluorescence microscopy techniques, using a Zeiss microscope (Axio Scope A1, Oberkochen, Germany) with × 40 and × 100 magnification lenses. To obtain fluorescence micrographs, the samples were added to a mixture (3 : 1) of the fluorescent dyes Nile Red (0.10 g L−1 in polyethylene glycol) and Fast Green (0.01 g L−1 in water), as described by Drapala et al. (2017). Nile Red labels the oil, and Fast Green allows detection of the protein.
Droplet Size and Droplet Size Distribution
The droplet size and droplet size distribution (polydispersity) of the emulsions were examined by light scattering using a Mastersizer 2000 (Malvern Instruments, Malvern, UK) and distilled water as the dispersant medium. The mean droplet size was expressed as the surface-weighted D[3,2] properties.
Rheology
Rheological measurements of the emulsions were achieved using an AR 1500ex rheometer (TA Instruments, New Castle, USA) equipped with double concentric cylinder geometry with an inner cylinder (outer radius of 17.53 mm and inner radius of 16.02 mm) and an outside cup (outer radius of 18.45 mm and inner radius of 15.10 mm). The gap was 500 μm, and the shear rate varied from 0.1 to 300 s−1. Three flow curves (up-down-up cycles) were acquired, and the third flow curve data were used to obtain the apparent viscosity at shear rate of 50 1/s, as well as the viscosity–shear rate plots. The experiments were carried out at 25 °C, in duplicate.
Emulsion Stability by Laser Scanning Turbidimetry
The stability of the emulsions was assessed using an optical scanning instrument (Turbiscan ASG, Formulation, Toulouse, France). The samples were placed in cylindrical glass tubes, and the backscattered light intensity was measured at 25 °C, at 0, 1, 2, 3, 4, 5, 6, and 24 h after emulsion preparation to ascertain the Turbiscan stability index (TSI). Analyses were carried out in duplicate.
Interfacial Tension
The interfacial tension (mN m−1) at the O/W interface of commercial canola oil (Liza, Cargill, Brazil) with aqueous solutions of WPI, GA, WPI:GA, WPI:MD, WPI:Capsul®, and WPI microgels at 8% (w/v) was measured by the pendant droplet method using a Track-S tensiometer (Teclis, Marseille, France). Canola oil was used since the dark coloration of pomegranate seed oil did not allow a contrast with the aqueous phase. A comparison of the different continuous phases of the O/W emulsions was conducted. The analyses were done in triplicate, at 25 °C for 2000 s.
Spray Drying
The process of spray drying was performed in a Büchi Mini Spray Dryer B-290 (Flawil, Switzerland), with a nozzle atomization system with 0.7 mm diameter nozzle, feed flow rate of 10 mL min−1, drying air flow rate of 35 m3 h−1, compressor air pressure of 0.4–0.6 MPa, and inlet and outlet air temperature of 150 ± 1.0 and 80.0 ± 1.0 °C, respectively.
Particle Characterization
Encapsulation Yield and Efficiency
The oil EE was determined as a function of the mass difference of the oil contained in the microparticles and the mass of oil present on the surface (Eq. 1). The oil EY was determined as a function of the mass difference of the oil contained in the microparticles and the total amount used in the encapsulation process (Eq. 2).
where Ototal = mass of total oil determined in the particle; Osurface = mass of free oil determined on the particle surface; and Oinitial = the initial oil mass used in the process prior to drying.
For the determination of oil mass on the particle surface, the methodology adopted by Premi and Sharma (2017) was used, with modifications. A mass of 1.5 g of particles was added to 15 mL of hexane in glass tubes and stirred gently for 2 min. The mixture was filtered on filter paper with grammage of 80 g m−2. The sample was washed again with 15 mL of hexane, and the filtrate was kept in an oven (60 °C) until complete evaporation of the solvent.
The determination of the oil mass contained in the microparticles was done according to the method of Bligh and Dyer (1959). In order to rupture the particles, 2.5 g of particles was added to 10 mL of 5% (w/v) KCl solution and vortexed for 1 min. Then, 10 mL of chloroform and 20 mL of methanol were added, and the mixture was stirred for 30 min with the aid of an AP22 Phoenix Luferco tube stirrer (Araraquara, Brazil); 10 mL of chloroform and 5 mL of 3% (w/v) Na2SO4 solution were added to the mixture and kept under stirring for 2 min. Samples were allowed to stand for 24 h for phase separation. The chloroform phase was filtered twice on filter paper with grammage of 80 g m−2, and 5 mL of this filtrate was kept in an oven (60 °C) until complete evaporation of the solvent.
Scanning Electron Microscopy
Microparticles were analyzed by SEM using a Leo 440i EDS 6070 scanning electron microscope with X-ray dispersive energy detector (LEO Electron Microscopy, Oxford/Cambridge, England). The samples were subjected to metallic coating using an EMITECH K450 sputter COATER (Kent, UK), with an Au thickness estimated at 200 Å.
Moisture, Water Solubility, Water Activity, and Hygroscopicity
Particle moisture was determined using a Moc63u moisture-determining scale (Shimadzu, Kyoto, Japan). Water activity (Aw) was determined using AQUALAB equipment (Decagon Devices, Pullman, USA). Determination of particles’ water solubility was done by the gravimetric method, according to Eastman and Moore (1984), cited by Cano-Chauca et al. (2005). The method consists of the addition of 0.50 g of sample to an Erlenmeyer flask containing 50 mL of distilled water. The mixture was homogenized at 100 rpm for 30 min at room temperature. The solution was then centrifuged at 1400 g for 5 min. An aliquot of 25 mL of the supernatant was transferred to a beaker of known mass and kept in an oven at 105 °C until complete evaporation of water.
To determine particles’ hygroscopicity, the methodology used by Cai and Corke (2000) was adopted, with some modifications. A mass of 0.25 g of sample was conditioned for 1 week in a desiccator containing saturated NaCl solution, which imparts to the medium a relative humidity of 75%. Hygroscopicity was determined by the mass of water absorbed per 100 g of sample after 7 days of storage.
Particle Size and Particle Size Distribution
To obtain the particle size and particle size distribution of the atomized treatments, a Mastersizer S laser diffraction particle analyzer (Malvern Instruments, Malvern, UK) was used with ethanol as the dispersion medium. The mean particle size was expressed as D[4,3] by volume distribution.
Thermogravimetric Analysis
The TGA curves were obtained using a TGA-50 M thermogravimetric analyzer from Shimadzu (Kyoto, Japan), with a heating rate of 10 °C min−1. The sample (10 mg) was packed in a pre-tared alumina sample carrier (70 μL). The oven atmosphere was saturated with nitrogen at a flow rate of 50 mL min−1. The analysis temperature range was from 25 to 600 °C.
Differential Scanning Calorimetry
For determination of the glass transition temperature, the atomized samples were submitted to DSC analysis with the aid of a Mettler Toledo DSC1 differential scanning calorimeter (Schwerzenbach, Switzerland). Cooling controlled by a mechanical cooler was used, operating with nitrogen gas, and using helium as purge gas. Samples were conditioned on aluminum standard (40 μL) container. The heating range was from 25 to 200 °C, at 10 °C min−1, and the sample was maintained at 200 °C for 3 min. The samples were then cooled to 25 °C and kept at 25 °C for 3 min. Finally, the particles were subjected to a new heating up to 200 °C, at a rate of 10 °C min−1. The value of the glass transition temperature was obtained from the second heating curve.
Loose bulk, Tap Bulk, and Apparent Particle Density
The loose bulk density (ρa) was determined according to Jinapong et al. (2008). ρa was calculated from the relation of mass (g) and volume (mL) of a known quantity (about 2 g) of the sample put into a graduated cylinder. The tap bulk density (ρc) was determined according to Ferrari et al. (2012), from the relation mentioned to loose bulk density; however, the system was subjected to repeated surface strikes until the difference in volume observed was insignificant. The apparent particle density (ρp) was determined by pycnometry with the sample dispersed in toluene (Ferrari et al. 2012).
Porosity
Porosity (ε) was determined according to Amin et al. (2014), and calculated from Eq. 3:
where ρc = tap bulk density and ρp = apparent particle density.
Accelerated Oxidation by the Rancimat Method
Accelerated oxidation tests were performed with Rancimat equipment (model 873, Metrohm, Herisau, Switzerland) according to Wang et al. (2014). Samples (1.5 g of powder and 4 g of oil) were submitted to heating with an air flow rate of 20 L h−1 at 90 °C. The sample induction time, measured in hours, was used as an oxidative stability index (OSI).
Statistical Analysis
Data were analyzed statistically using the statistical program SAS (Statistical Analysis System), version 9.2, by analysis of variance ANOVA and Tukey’s test, at the 5% level.
Results and Discussion
Microgels
The morphological characteristics of WPI nanoparticles are associated with the heating conditions and pH values, resulting in spherical or elongated particles. WPI microgels obtained with 8% (w/v) protein at pH 5.9 presented a particle size of 214.72 ± 4.06 nm, polydispersity of 0.213 ± 0.04, monomodal behavior, and zeta potential of − 44.47 ± 1.65 mV. From the transmission electron micrographs, microgels (Fig. 1) displayed rounded particles (highlighted in the figure). In addition, the micrograph corroborated the particle size data corresponding to WPI microgel, and the smaller the particle size of WPI nanoparticles, the smaller the droplet size of emulsions obtained with these particles, resulting in stable systems, which are presented in the next sections.
Emulsion Characterization
Optical and Fluorescence Microscopy, Droplet Size, and Droplet Size Distribution
The morphology of all formulations was depicted using optical and fluorescence microscopy and is presented in Fig. 2. In the case of the Pickering emulsion (Fig. 2a), the Nile Red-stained oil droplets (highlighted red) were surrounded by the Fast Green-stained WPI microgels (highlighted green), proving the formation of this type of system. This emulsion had a slightly green continuous aqueous phase. However, the strongest color predominated at the O/W interface. For the conventional emulsion with WPI (Fig. 2b), the whole continuous phase appeared intensely green, illustrating that the protein was not only present at the O/W interface. Similar structures were evidenced for the other conventional emulsions. This implies that the use of WPI microgels, WPI in its natural form, or other biopolymers (GA, MD, and Capsul®), and their combination does not influence the morphology of the emulsion droplets.
The average droplet size for all emulsions was within the range of 1.39–2.55 μm, and polydispersity (Span) was between 1.30 and 3.39 (Table 1). Different biopolymers and their combination influenced the droplet size: formulations WPI, WPI:MD, and WPI:Capsul® presented the smallest droplet size. Moreover, it is possible to relate the emulsion droplet size to the EE of particles (“EY and EE”) since WPI, WPI:MD, and WPI:Capsul® emulsions presented a smaller droplet size and higher EE (Table 4).
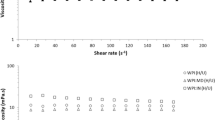
Rheology
Rheological tests enable characterization of the flow behavior of a material when submitted to a certain shear rate. This behavior can be observed in Fig. 3, and the apparent viscosities (ƞ) at shear rate of 50 1/s are presented in Table 1.
Apparent viscosity was in the range of 1.47–3.96 mPa.s and was significantly different among the formulations due to the differences in the composition of the continuous aqueous phase. The Pickering emulsion had twice the viscosity of the WPI emulsion, even though it contained the same type and concentration of protein in the aqueous phase. This discrepancy is explained by the change in the structure of β-lactoglobulin during the preparation of microgels. Comparing the formulations with only one polymer in the aqueous phase, the ƞ decreased in the order of the GA emulsion, followed by the Pickering emulsion, and then the WPI emulsion. Among the emulsions with two polymers, WPI:GA emulsion had the highest viscosity, due to the presence of GA. Despite these differences, all formulations presented good apparent viscosity values for the spray drying process since they flowed easily and resulted in low energy expenditure.
Liu et al. (2019) noted a different behavior when studying the stability, rheology, and β-carotene accessibility of high internal phase emulsion gels using WPI and glucono-δ-lactone (GDL) in their formulations. The WPI concentrations studied varied from 2.5% to 10% w/v, and the apparent viscosity increased with increasing WPI concentration and GDL addition.
Kinetic Stability: Laser Scanning Turbidimetry
The kinetic stability of the emulsions was characterized by laser scanning turbidimetry measurements. The stability was studied based on the TSI (Table 2). The higher the TSI, the more unstable the sample (and the lower the % stability). Comparing Pickering and WPI emulsions, the conventional emulsion was more stable (stability of 87.05% and 94.33% for Pickering and WPI emulsions, respectively) at 24 h because, although both emulsions had WPI in their composition, the β-lactoglobulin structure was modified in only one of them. Regardless of this difference between Pickering and WPI emulsions, both presented excellent conditions for drying and application in food products. At 24 h, the stability values of GA (88.41%) and WPI:GA emulsions (88.12%) were similar to that of the Pickering emulsion, but the best behavior was seen for the WPI:Capsul® combination, which reached a stability value of 95.43%. These stability results support those attained for interfacial tension (“Interfacial Tension”) that indicate that WPI and WPI:Capsul® emulsions gave the lowest values (4.21 and 5.04 mN m−1, respectively).
Zheng et al. (2018), who prepared and characterized water-in-oil emulsions of isoprothiolane, analyzed the laser scanning turbidimetry for 4 h and found TSI values of the most stable emulsion samples in the range of 1–10%. In comparison, the TSI values of the pomegranate O/W emulsions were better, since similar values were obtained at 24 h. Furthermore, the findings indicate that the materials and technique proposed here (the use of WPI microgels, WPI in its natural form, GA, and the combinations WPI:GA, WPI:MD, and WPI:Capsul® as emulsifiers/aqueous phases, and homogenization at 18,000 rpm/10 min) is suitable for producing stable emulsions.
Interfacial Tension
Table 3 gives the initial and final interfacial tensions of each system. A statistical difference occurred among the aqueous phases, and the interfacial tension of all the samples decreased over 2000 s, with final values in the range of 4.21–9.97 mN m−1. The systems containing WPI, WPI:GA, and WPI:Capsul® solutions showed the lowest interfacial tension, meaning that emulsions with these aqueous phases were the most stable. This finding can be explained since the function of an emulsifier is to reduce interfacial tension, resulting in stabilization of the system. In other words, the lower the interfacial tension between the oil and aqueous phases, the more stable the emulsion. These results correlate with the TSI data (Table 2), mainly for WPI and WPI:Capsul® emulsions.
Particle Characterization
The six emulsions formulations were atomized and the particles evaluated as follows:
EY and EE
The EY and EE for pomegranate seed oil particles are presented in Table 4. Values in the range of 56.28–73.83% and 28.07–93.99% were obtained for EE and EY, respectively, and they were significantly influenced by the type of carrier agent.
Regarding EY, Pickering and WPI:Capsul® treatments presented higher values (93.99% and 94.18%, respectively), showing that these materials allowed less loss of oil during the process. This can be explained due to the formation of a structure with fewer pores (Pickering compared with the other treatments) (“Accelerated Oxidation by the Rancimat Method” and Table 5) and thicker walls (WPI:Capsul® treatment) (“Moisture, Water Solubility, Water Activity (Aw), and Hygroscopicity” and Fig. 4). Particles with gum Arabic as wall material presented lower EY values (28.07%), showing that GA is not indicated to obtain high retention values of this oil during the atomization process. In relation to the EE, WPI:MD, and WPI:Capsul® treatments presented the highest values (73.65% and 73.83%, respectively). The use of WPI microgels and WPI combined with maltodextrin and Capsul® as wall materials allowed the acquisition of high EY and EE values. These data also suggest that smaller emulsion droplet size promotes better encapsulation of pomegranate seed oil since the WPI, WPI:MD, and WPI:Capsul® emulsions presented the smallest droplet size (“Optical and Fluorescence Microscopy, Droplet Size, and Droplet Size Distribution”) and higher EE.
It was expected that the most stable emulsions in a period of 24 h also presented higher EE and EY values for the particles. However, as the atomization was done in a short time, a period in which there was no emulsion destabilization, there was no influence of emulsion stability on the amount of oil encapsulated in particles.
Fioramonti et al. (2019) studied the encapsulation of flaxseed oil by spray drying using milk whey protein–alginate double layer emulsions and obtained EE values in the range of 30.69–59.88%. In other words, the materials used for encapsulation of pomegranate seed oil allowed the acquisition of higher EE values than those obtained for flaxseed oil. Aghbashlo et al. (2013) observed similar EE values for fish oil encapsulated by spray drying using different agent carriers (skim milk powder, whey protein concentrate and isolate, and sodium caseinate). However, even with similar EE, the particles obtained by Aghbashlo et al. (2013) can be applied in only few types of food product since they are water-soluble.
SEM
The micrographs obtained by SEM are presented in Fig. 4. All the treatments presented round particles, characteristic of samples obtained by spray drying. Pickering and WPI treatments resulted in particles with a smooth surface, however, with the presence of pores for the WPI particles (indicated in red). This difference can be explained by alteration of the protein structure during the preparation of WPI microgels (β-lactoglobulin agglomerates formation) to obtain Pickering emulsions. This result corroborates those for porosity (“Accelerated Oxidation by the Rancimat Method” and Table 5), which shows that Pickering particles present fewer pores than other formulations. GA, WPI:GA, and WPI:MD treatments showed shrunken structures due to the evaporation of water during the atomization process. The WPI:Capsul® formulation presented this shrinkage characteristic; however, it was less accentuated since these particles resulted in thicker structures, as observed in Fig. 4. This characteristic can be explained by the combination of WPI protein and modified starch, forming a more rigid material, confirmed by TGA (“DSC”; Fig. 6).
Belscak-Cvitanovic et al. (2015) studied the encapsulation of green tea bioactive compounds by spray drying using different natural biopolymers (inulin, whey proteins, pectin, oligofructose, modified starch, alginate, carrageenan, pea proteins, acacia gum, guar gum, locust bean, and xanthan gums) and observed that the use of these alternative materials resulted in pronounced shape distortion and cracked (broken) particles, which did not happen for the pomegranate seed oil particles obtained in this research.
Moisture, Water Solubility, Water Activity (Aw), and Hygroscopicity
The moisture (dry base), water solubility, Aw, and hygroscopicity values are presented in Table 4. Values in the ranges 0.139–0.211, 3.77–5.65%, 17.62–91.90%, and 2.61–3.60 g water 100 g−1 powder were obtained for Aw, moisture, solubility, and hygroscopicity, respectively. These Aw and moisture values are considered good for powders obtained by spray drying and ensure their microbiological stability. With respect to water solubility, there was no significant difference among formulations, with the exception of the Pickering treatment, which presented an average value of 17.62%. This lower water solubility is explained by the formation of solid nanoparticles (formation of β-lactoglobulin agglomerates) during preparation of WPI microgels. These nanoparticles are obtained during adjustment of the WPI solution pH, closer to the isoelectric point (IP) of the protein, which results in agglomerates. Producing particles with low water solubility (Pickering treatment) is considered an advantage since they can be applied in several types of food product, which is a limitation for particles obtained by spray drying as they solubilize or release the active ingredient when in contact with water. Thus, one of the differences between the WPI and Pickering treatments, even with the same carrier material, is the possibility of different food applications since they have different solubilities. All formulations presented similar hygroscopicity values; in other words, different wall materials or their combination did not influence the amount of water adsorbed from the environment. All treatments presented feasible values of Aw, moisture, solubility, and hygroscopicity for handling and storage of the powders.
Hoyos-Leyva et al. (2019) studied the characterization of almond oil particles using spherical taro starch aggregates obtained by spray drying, and observed moisture values of 3.47% and 2.79% when adding 10% and 20% oil to the particles, respectively. Fioramonti et al. (2019) encapsulated flaxseed oil by spray drying using milk whey protein–alginate as wall materials and obtained Aw values in the range of 0.27–0.45. The values obtained by these authors corroborate those obtained for pomegranate oil particles due to the similarity of carrier agents. Hygroscopicity values in the range of 12.06–15.40 g of adsorbed moisture 100 g−1 of sample were obtained for spray dried jussara extract microparticles when maltodextrins with different dextrose equivalent values and gum Arabic were used as wall materials (Carvalho et al. 2016).
Particle Size and Particle Size Distribution
The particle size and polydispersibility (Span) values are presented in Table 4, and particle size distribution is presented in Fig. 5. Values in the range of 9.86–22.60 μm and 2.21–4.45 were obtained for particle size and Span, respectively. Different wall materials and their combination influenced particle size; Pickering treatment presented particles twice as big (15.76 μm) as for WPI treatment (9.86 μm). This difference can be explained due to WPI agglomerates formed during WPI microgel preparation. WPI:MD treatment presented the biggest particle size (22.60 μm); one hypothesis for this is that because maltodextrin does not present emulsifying property, the structure was not enough stable to obtain smaller particles, and then they agglomerated. Moreover, there was no significant difference between GA and WPI:Capsul® treatments. Regarding Span values, there was no difference among formulations, with the exception of WPI:MD treatment. However, even with this difference, all treatments presented feasible particle size since they were below 100 μm, so would not be perceived by consumers if applied to food products. Regarding particle size distribution, all formulations presented unimodal behavior (Fig. 5a, b), which justifies the low Span values (Table 4).
TGA
The thermogravimetric behavior of the particles when subjected to heating in the range of 25 to 600 °C is shown in Fig. 6. The thermogravimetric curve is composed of three steps: (1) loss of mass observed in the range of 25–100 °C, attributed to the loss of water; (2) loss of mass in the range of 100–300 °C, attributed to the evaporation of surface-bound oil; and (3) loss of mass in the range of 300–500 °C, attributed to the degradation of wall materials. The WPI:Capsul® treatment presented a more rigid structure since higher temperatures were required for the loss of mass. In other words, the increase in decomposition temperature observed for the particles with combination of WPI and modified starch suggested a protective layer for pomegranate seed oil. The WPI and Pickering treatments had slightly weaker structures than for the WPI:Capsul® treatment; however, they were stronger than WPI:GA, GA, and WPI:MD. This result corroborates the micrographs obtained by SEM, which showed particles with thicker walls (WPI:Capsul®) and smooth and compact surfaces (Pickering and WPI). These results are also in agreement with those obtained in the study of accelerated oxidation by the Rancimat method (Fig. 8), which showed that Pickering, WPI, and WPI:Capsul® treatments promoted greater oil protection compared with other formulations.
The TGA behavior obtained by Loughrill et al. (2019), who encapsulated docosahexaenoic acid (DHA) by spray drying using hydroxy propyl methyl cellulose acetate succinate as carrier, confirms the three-step curve obtained for pomegranate seed particles.
DSC
The temperature at which a material changes from a glassy solid to an amorphous rubber is called the glass transition temperature (Tg), and DSC provides this information (Bhandari and Howes 1999). Our objective was to study the different biopolymers and their influence on increase or decrease of Tg since it is related to the molecular weight of carrier agents (Ross 1995).
The glass transition temperature (Tg) and DSC curves are presented in Fig. 7. Values in the range of 103.24–121.62 °C were obtained for Tg, showing that the formulation WPI:Capsul® presented the highest value of Tg. Lower Tg values were observed for GA, WPI:GA, and WPI:MD formulations, confirming the efficiency of the use of WPI microgels (Pickering treatment), WPI in its natural form, and its combination with Capsul® for the enhancement of powders’ physicochemical properties since these biopolymers offered better stability of pomegranate oil particles due to an increase of the glass transition temperature.
Loose Bulk, Tap Bulk, and Apparent Particle Densities, and Porosity
Values in the range of 0.206–0.287, 0.258–0.358, and 1.158–1.421 g mL−1 and 70.64–81.70% were obtained for loose bulk, tap bulk, and apparent particle densities and porosity, respectively (Table 5). Particle density and porosity are directly related to the oxidative stability of encapsulated oil since particles with high porosity present empty spaces, which contribute to oxygen diffusion and oxidation reactions. Pickering, WPI, GA, and WPI:Capsul® treatments presented lower porosity values when compared with WPI:GA and WPI:MD formulations, confirming the best oil protection of Pickering, WPI, and WPI:Capsul® treatments.
Powders obtained by spray drying are characterized by the presence of pores depending on the wall materials (Table 5 and “SEM”). Moreover, apparent particle density includes the volume of all closed pores, which explains the difference observed among loose bulk, tap bulk, and apparent pomegranate seed oil particle densities. In addition, different types of bulk density can be distinguished (loose or tap) depending on the treatment to which the powders are subjected and is also related to particle density, the amount of interstitial air, and flowability. Furthermore, the study of bulk density is important for samples in powder form since it allows the improvement of storage, processing, and packaging by the food industry (Webb 2001; Santomaso et al. 2003; Barbosa-Cánovas and Juliano 2005; Domian et al. 2017).
Accelerated Oxidation by the Rancimat Method
OSI values and the behavior of each formulation are presented in Fig. 8a, b. OSI values of 2.71 h and in the range of 4.11–21.23 h were obtained for non-encapsulated and encapsulated pomegranate seed oil, respectively. All formulations promoted oil oxidative protection when compared with the non-encapsulated oil. Comparing the treatments with only one wall material (Pickering, WPI, and GA), the WPI formulation increased the oxidative stability of the oil 10 times, confirming the efficiency of the protein in the protection of the encapsulated material. When WPI microgels (Pickering treatment) were used, there was an increase in the oil oxidative protection of three times. In other words, the use of β-lactoglobulin agglomerates as wall material also promoted the protection of the oil; however, the protection was greater when the protein was used in its natural form. One hypothesis for this is that oxidation of the protein occurs more easily in microgels since it is denatured and is then more susceptible to degradation, affecting negatively its action as wall material.
When comparing treatments with the combination of two wall materials (WPI:GA, WPI:MD, and WPI:Capsul®), the WPI:Capsul® treatment promoted greater protection of the oil. Generally, WPI, WPI:Capsul®, and Pickering treatments promoted greater protection of encapsulated oil when compared with free oil and other formulations, confirming the behavior observed by TGA (“DSC”) and DSC (“Loose Bulk, Tap Bulk, and Apparent Particle Densities, and Porosity”). These results suggest that the TSI of the emulsions is related to oxidation of the particles since the emulsions with WPI and WPI:Capsul® materials presented the best stability index and particles with these materials also promoted the best oil protection. Carvalho et al. (2014) obtained similar OSI values for green coffee oil encapsulated by spray drying using corn syrup and modified starch Hi-Cap 100 as wall materials.
Conclusions
Pomegranate seed oil was successfully encapsulated by emulsification, obtaining stable Pickering and conventional emulsions. Pickering emulsions did not exhibit better stability than conventional emulsions; both emulsions demonstrated feasible characteristics for stabilization of O/W systems. WPI microgels, WPI in its natural form, GA, and the combinations WPI:GA, WPI:MD, and WPI:Capsul® can be considered as excellent materials for the continuous aqueous phase since the emulsions presented small droplet size, low viscosity, and good stability, facilitating the drying of these systems and their possible application in food products. The spray dried emulsions showed powders with physicochemical properties feasible for their handling and storage, high values of EE and EY, and oil oxidative protection. WPI, WPI:Capsul®, and Pickering were considered the best treatments since they showed higher EE, thermostability, glass transition temperature, and oil oxidative protection.
References
Aghbashlo, M., Mobli, H., Madadlou, A., & Rafice, S. (2013). Influence of wall material and inlet drying air temperature on the microencapsulation of fish oil by spray drying. Food and Bioprocess Technology, 6, 1561–1569.
Amin, M. C. I. M., Abadi, A. G., & Katas, H. (2014). Purification, characterization and comparative studies of spray-dried bacterial cellulose microparticles. Carbohydrate Polymers, 99, 180–189.
Barbosa-Cánovas, G. V., & Juliano, P. (2005). Physical and chemical properties of food powders. In C. Onwulata (Ed.), Encapsulated and powdered foods (pp. 39–71). Taylor & Francis Group, Boca Raton: CRC Press LLC.
Belscak-Cvitanovic, A., Levic, S., Kalusevic, A., Spolkaric, I., Dordevic, V., Komes, D., Mrsic, G., & Nedovic, V. (2015). Efficiency assessment of natural biopolymers as encapsulants of green tea (Camellia sinensis L.) bioactive comounds by spray drying. Food and Bioprocess Technology, 8, 2444–2460.
Bhandari, B. R., & Howes, T. (1999). Implication of glass transition for the drying and stability of dried foods. Journal of Food Engineering, 40, 71–79.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Bryant, C. M., & McClements, D. J. (2000). Optimizing preparation conditions for heat-denatured whey protein solutions to be used as cold-gelling ingredients. Journal of Food Science, 65, 259–263.
Bucurescu, A., Blaga, A. C., Estevinho, B. N., & Rocha, F. (2018). Microencapsulation of curcumin by a spray-drying technique using gum arabic as encapsulating agent and release studies. Food and Bioprocess Technology, 11, 1705–1806.
Cai, Y. Z., & Corke, H. (2000). Production and properties of spray-dried Amaranthus Betacyanin pigments. Journal of Food Science, 65(6), 1248–1252.
Calabrese, V., Courtenay, J. C., Edler, K. J., & Scott, J. L. (2018). Pickering emulsions stabilized by naturally derived or biodegradable particles. Current Opinion in Green and Sustainable Chemistry, 12, 83–90.
Cano-Chauca, M., Stringheta, P. C., Ramos, A. M., & Cal-Vidal, J. (2005). Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innovative Food Science and Emerging Technologies, 5(4), 420–428.
Carvalho, A. G. S., Silva, V. M., & Hubinger, M. D. (2014). Microencapsulation by spray drying of emulsified green coffee oil with two-layered membranes. Food Research International, 61, 236–245.
Carvalho, A. G. S., Machado, M. T. C., Silva, V. M., Sartoratto, A., Rodrigues, R. A. F., & Hubinger, M. D. (2016). Physical properties and morphology of spray dried microparticles containing anthocyanins of Jussara (Euterpe edulis Martius) extract. Powder Technology, 294, 421–428.
Costa, A. L. R., Gomes, A., & Cunha, R. L. (2018a). One-step ultrasound producing O/W emulsions stabilized by chitosan particles. Food Research International, 107, 717–725.
Costa, A. L. R., Gomes, A., Tibolla, H., Menegalli, F. C., & Cunha, R. L. (2018b). Cellulose nanofibers from banana peels as a Pickering emulsifier: High-energy emulsification processes. Carbohydrate Polymers, 194, 122–131.
Domian, E., Brynda-Kopytowska, A., & Marzec, A. (2017). Functional properties and oxidative stability of flaxseed oil microencapsulated by spray drying using legume proteins in combination with soluble fiber or trehalose. Food and Bioprocess Technology, 1–13.
Donato, L., Schmitt, C., Bovetto, L., & Rouvet, M. (2009). Mechanism of formation of stable heat-induced β-lactoglobulin microgels. International Dairy Journal, 19, 295–306.
Drapala, K. P., Auty, M. A. E., Mulvihill, D. M., & O’Mahony, J. A. (2017). Influence of emulsifier type on the spray-drying properties of model infant formula emulsions. Food Hydrocolloids, 69, 56–66.
Eastman, J.E.; Moore, C.O. Cold Water Soluble Granular Starch for Gelled Food Composition. U.S. Patent 4465702, 14 ago. 1984.
Elfalleh, W., Ying, M., Nasri, N., Sheng-Hua, H., Guasmi, F., & Ferchichi, A. (2011). Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds. International Journal of Food Sciences and Nutrition, 62(3), 200–206.
Ferrari, C. C., Germer, S. P. M., Alvim, I. D., Vissotto, F. Z., & Aguirre, J. M. A. (2012). Influence of carrier agents on the physicochemical properties of blackberry powder produced by spray drying. International Journal of Food Science and Technology, 47(6), 1237–1245.
Fioramonti, S. A., Stepanic, E. M., Tibaldo, A. M., Pavón, Y. L., & Santiago, L. G. (2019). Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Research International, 119, 931–940.
Gharsallaoui, A., Roudaut, G., Chambin, O., Voilley, A., & Saurel, R. (2007). Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Research International, 40(9), 1107–1121.
Guzey, D., Kim, H. J., & McClements, D. J. (2004). Factors influencing the production of o/w emulsions stabilized by β-lactoglobulin-pectin membranes. Food Hydrocolloids, 18, 967–975.
Hoyos-Leyva, J. D., Bello-Perez, L. A., Agama-Acevedo, J. E., Alvarez-Ramirez, J., & Jaramillo-Echeverry, L. M. (2019). Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT- Food Science and Technology, 101, 526–533.
Jiang, S., Yildiz, G., Ding, J., Andrade, J., Rababahb, T., Almajwalc, A., Abulmeatyc, M. M., & Feng, H. (2019). Pea protein nanoemulsion and nanocomplex as carriers for protection of cholecalciferol (vitamin D3). Food and Bioprocess Technology, 12, 1031–1040.
Jinapong, N., Suphantharika, M., & Jamnong, P. (2008). Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. Journal of Food Engineering, 84(2), 194–205.
Liu, W., Gao, H., McClements, D. J., Zhou, L., & Zou, L. (2019). Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocolloids, 88, 210–217.
Loughrill, E., Thompson, S., Owusu-Ware, S., Snowden, M. J., Douroumis, D., & Zand, N. (2019). Controlled release of microencapsulated docosahexaenoic acid (DHA) by spray-drying processing. Food Chemistry, 286, 368–375.
McClements, D. J. (2011). Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter, 7, 2297–2316.
Premi, M., & Sharma, H. K. (2017). Effect of different combinations of maltodextrin, gum arabic and whey protein concentrate on the encapsulation behaviour and oxidative stability of spray dried drumstick (Moringa oleifera) oil. International Journal of Biological Macromolecules, 105(Pt 1), 1232–1240.
Ravanfar, R., Comunian, T. A., Dando, R., & Abbaspourrad, A. (2018). Optimization of microcapsules shell structure to preserve labile compounds: A comparison between microfluidics and conventional homogenization method. Food Chemistry, 241, 460–467.
Ross, Y. H. (1995). Phase transitions in foods. San Diego: Academic Press.
Samborska, K., Jedlinska, A., Wiktor, A., Derewiaka, D., Wolosiak, R., Matwijczuk, A., Jamróz, W., Skwarczynska-Maj, K., Kielczewski, D., Blazowski, L., Tulodziecki, M., & Witrowa-Rajchert, D. (2019). The effect of low-temperature spray drying with dehumidified air on phenolic compounds, antioxidant activity, and aroma compounds of rapeseed honey powders. Food and Bioprocess Technology, 12, 919–932.
Santomaso, A., Lazzaro, P., & Canu, P. (2003). Powder flowability and density ratios: The impact of granules packing. Chemical Engineering Science, 58, 2857–2874.
Shahidi, F., & Han, X. Q. (1993). Encapsulation of food ingredients. Critical Reviews in Food Science and Nutrition, 33, 501–547.
Siano, F., Addeo, F., Volpe, M. G., Paolucci, M., & Picariello, G. (2016). Oxidative stability of pomegranate (Punica granatum L.) seed oil to simulated gastric conditions and thermal stress. Journal of Agricultural and Food Chemistry, 64, 8369–8378.
Wang, B., Adhikari, B., & Barrow, C. J. (2014). Optimisation of the microencapsulation of tuna oil in gelatin-sodium hexametaphosphate using complex coacervation. Food Chemistry, 158, 358–365.
Webb, P. A. (2001). Volume and density determinations for particle technologists. Micromeritics instruments Corp. 2/16/01 (www.micromeritics.com).
Zamani, S., Malchione, N., Selig, M. J., & Abbaspourrad, A. (2018). Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels. Food & Function, 9(2), 982–990.
Zheng, L., Cao, C., Li, R.-Y., Cao, L.-D., Zhou, Z.-L., Li, M., & Huang, Q.-L. (2018). Preparation and characterization of water-in-oil emulsions of isoprothiolane. Colloids and Surfaces, A: Physicochemical and Engineering Aspects, 537, 399–410.
Acknowledgments
The authors thank FAPESP (Fundação de Auxílio à Pesquisa do Estado de São Paulo) for scholarship conceded to Talita A. Comunian (Process 2018/01710-5) and to Ana Gabriela da Silva Anthero (Process 2018/02132-5), CNPq for scholarship conceded to Eveling Oliveira Bezerra (Process 118279/2018-01), De Wit Specialty Oils for the pomegranate oil donation, Fonterra for WPI donation, Ingredion for maltodextrin and modified starch donation and Centro de Microscopia Eletrônica (Universidade Federal de São Paulo, Escola Paulista de Medicina - UNIFESP) for assistance with transmission electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1 Pomegranate oil was successfully encapsulated by emulsification followed by spray drying;
2 Emulsions were 87-95 % stable at 24 h after preparation;
3 Particles with whey protein (WPI) as wall material promoted the highest oil oxidative protection;
4 The combination of WPI and Capsul® resulted in particles with higher thermostability;
5 Pickering particles presented lower water solubility due to protein denaturation.
Rights and permissions
About this article
Cite this article
Comunian, T.A., da Silva Anthero, A.G., Bezerra, E.O. et al. Encapsulation of Pomegranate Seed Oil by Emulsification Followed by Spray Drying: Evaluation of Different Biopolymers and Their Effect on Particle Properties. Food Bioprocess Technol 13, 53–66 (2020). https://doi.org/10.1007/s11947-019-02380-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02380-1