Abstract
In this study, optimization of spray drying for double emulsions containing pectin-whey protein concentrate (WPC) was investigated for nano-encapsulation of folic acid. Five independent variables including pectin and WPC content, dispersed phase content, pH, surfactant type of Span, and polyglycerol polyricinoleate (PGPR) were considered along with encapsulation efficiency (EE) as the main response. The experiment design was performed by a Taguchi approach. Final double emulsions were formulations containing an internal nano/micro-emulsion composed of a water in oil (W/O) system with folic acid present in the water phase, re-emulsified within an aqueous phase of pectin-WPC complexes. The average of folic acid EE was approximately 88.3 % in a range of 82.3 to 95.0 %. The main effect analysis with a Taguchi technique revealed that the dispersed phase content of double emulsions was the most important factor affecting EE (36 %) and surfactant had the minimum influence (5 %). Also, it was revealed that the most important interaction between independent variables in terms of EE was between WPC and dispersed phase content while pectin-pH had the lowest interaction. Finally, optimum conditions were determined as 1.0 % pectin, 4.0 % WPC, and 15 % dispersed phase, pH = 6, with a PGPR nano-emulsion which resulted in 99.0 % EE of folic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fortification of food formulations with vitamins is usually made by incorporating them directly as a solution or powder into final products, but these bioactive components are susceptible to breakdown: easy release, rapidly eliminated from the body, have little absorption in the gastrointestinal tract, and are influenced by storage factors such as temperature, pH, light, oxygen, enzymes, proteins, and metallic ions (Donsì et al. 2011; Sessa et al. 2014; McClements 2015). Therefore, many researchers have investigated delivery systems to protect the vitamins from these detrimental effects (Katouzian and Jafari 2016). The water in oil (W/O) micro-emulsion as a delivery system can improve the antioxidative effects, bioavailability, and solubility of hydrophilic bioactive vitamins (Gutiérrez et al. 2008; Talegaonkar et al. 2008). On the other hand, emulsification of W/O micro-emulsions within an aqueous phase containing hydrophilic emulsifiers could lead to production of water in oil in water (W/O/W) double emulsions. As a result, the hydrophilic bioactive compounds are entrapped in the inner aqueous phase of W/O/W emulsions which have shown greater stability in gastrointestinal tract (GIT) fluid, high stability with controlled release in food formulations, and enhanced bioavailability (Benichou et al. 2004; Hemar et al. 2010; Dickinson 2011). However, thermodynamic instability and diffusion of hydrosoluble vitamins through the oil layers limits the usage of W/O/W multiple emulsions in food systems (Garti 1997; Garti and Bisperink 1998; Garti and Aserin 2013).
Research over recent decades has shown that low or inadequate folate concentrations may contribute to some malfunctions and disorders (Lamers 2011; Iyer and Tomar 2009). In many countries, particularly the developing populations, people suffer from folate deficiency. Cereal flours have been a primary candidate for fortification as they are consumed by most of the people (Rader et al. 2000; Gujska et al. 2009; Berry et al. 2010). Fortification with folic acid (FA) has some possible limitations since added vitamin may be lost during processing and handling due to its high sensitivity to heat, oxidation, pH, and other environmental conditions (Bakhshi et al. 2013; Perez et al. 2015). Due to its properties as a vitamin and medicinal compound, FA must be protected against environmental conditions and should have a controlled release (Assadpour et al. 2016). There are several ways to encapsulate and protect hydrophilic bioactive components such as FA including liposomes, multiple emulsions, solid fat particles, biopolymer complexes, niosomes, and biologically derived systems (Ezhilarasi et al. 2013; Esfanjani et al. 2015; Mohammadi et al. 2016; da Silva et al. 2016).
High stability of W/O/W multiple emulsions could be achieved by the using primary W/O emulsions with smaller droplets and selecting appropriate hydrophilic emulsifiers in outer aqueous phase (Sapei et al. 2012; Schuch et al. 2013). In the last couple of years, whey proteins have been widely investigated as hydrophilic emulsifiers for W1/O/W2 emulsions and encapsulating hydrophilic bioactive compounds within the inner aqueous phase (W1) (Benichou et al. 2007; Esfanjani et al. 2015; Pérez-Masiá et al. 2015; Teng et al. 2015). Also, it has been shown that whey proteins can be used as an excellent functional agent in food formulations. Esfanjani et al. (2015) showed pectin combined with whey protein concentrate (WPC) could in W/O/W multiple emulsions create an elastic and aggregated layer around oil droplets. Murugesan and Orsat (2012) explained that a spray drying process could help to improve the stability of encapsulated nutraceuticals and bioactive components against processing. Also, it has been proved that functional properties (like antioxidant activity, solubility, gelation, and foaming behavior) of whey proteins would improve when combined with pectin (Kovacova et al. 2009; Lutz et al. 2009a; Teng et al. 2015).
In the present work, encapsulation efficiency of folic acid was monitored as a function of pectin and WPC content, dispersed phase content, and pH of external aqueous phase along with surfactant type, and the optimum conditions to make double emulsions with the highest encapsulation efficiency was determined by Taguchi’s robust design methodology. Taguchi is a process-product optimization method based on different steps of planning, conducting, and evaluating results of matrix experiments to determine the best level of control factors on characteristic properties and hence optimal conditions for any complex process (Mosavian and Hassani 2010; Milić et al. 2014). It has been used in many areas of manufacturing since the 1960s, with many discrete product engineering and manufacturing companies using it to great effect. This technique is an alternative to standard full factorial designs. Since it reduces the number of experiments, it is easier to use, faster, and at the same time, accurate and reliable, saving time and cost (Jose et al. 2012; Sonam et al. 2014). A Taguchi method can determine the experimental conditions having the best effect on the desired characteristics (optimal conditions).
Although encapsulated powders have been produced for coating of different bioactive compounds (Rajabi et al. 2015; Khazaei et al. 2014), studies about the usage of nano-carrier complex biopolymers with controllable release of folic acid from W/O/W multiple emulsions have not been performed. In the present study, our main goal was to produce a new nano-carrier for folic acid through W1/O/W2 multiple emulsions stabilized by functional biopolymers (a complex of pectin/WPC in W2 phase). Since there are various parameters affecting biopolymer complex formation and emulsification such as the ratio of applied biopolymers, pH of medium, concentration of individual biopolymers, bioactive content, volume fraction of dispersed phase, and surfactant type, it is necessary to select the most important factors and to gain a full knowledge of interaction between these factors and, finally, to optimize this process. A Taguchi approach could be an appropriate technique for these goals which designs minimum experimental treatments with a maximum number of independent variables. With the aim of improving encapsulation efficiency of folic acid in W/O/W emulsion formulations by applying complexes of WPC/pectin in the outer phase, we applied for the first time a Taguchi approach.
Materials and Methods
Folic acid (purity >97 %, molecular weight 441.4) and Span 80 (sorbitan monooleate) were purchased from Sigma-Aldrich Co. (St. Louis, MO), and Merck Chemicals Co. (Germany), respectively. Polyglycerol polyricinoleate (PGPR, E476) was kindly donated by Palsgaard, Denmark. Canola oil was purchased from a local market. Double-distilled water was used for preparing all emulsions. Whey protein concentrate (80 % protein) and maltodextrin were obtained from Arla (Denmark) and Qinhuangdao Lihua Starch Co., Ltd. (DE 16-20, China), respectively. Citrus pectin with a degree of methyl esterification of 71.1 % and galacturonic acid >65 % was purchased from Sigma-Aldrich Co. (St. Louis, MO). All other chemicals used in this study were of analytical grade.
Micro/Nano-emulsion Production
W/O micro-emulsions were prepared by spontaneous emulsification according to our previously used procedures for making W/O emulsions (Assadpour et al. 2016). Briefly, aqueous phase was prepared by mixing FA solution and Span 80 using a magnetic stirrer (IKA, Germany) at 1000 rpm and then added dropwise to oil phase while magnetically stirring. For nano-emulsions, the same procedure was applied except replacing the magnetic stirrer with a rotor-stator homogenizer (Ultra-Turrax, IKA, Germany, at 10,000 rpm). The formulation was composed of 76:12:12 % of canola oil, surfactant (Span or PGPR), and folic acid solution (with a concentration of 3 mg FA/mL in phosphate buffer at pH = 9), respectively.
Biopolymer Solution Preparation
Pectin powder was dissolved in boiling deionized water to prepare 100 g pectin solutions with concentrations of 0.5, 1.0, 1.5, and 2.0 % w/w. At the same time, different aqueous solutions of whey protein concentrate were prepared by dispersing required amounts of WPC powder (as shown in Table 1) into deionized water to obtain 100 g solutions containing 0.02 % sodium azide as an antimicrobial agent. Solutions were gently stirred for at least 30 min on a magnetic stirrer. Then, pectin and WPC solutions were mixed together and stored overnight at room temperature for complete hydration of biopolymers. For increasing dry matter of the feed into a spray dryer, 30 g maltodextrin solution (Brix = 50) was added into mixed biopolymer solutions and their pH was adjusted to predetermined pH values (Table 1) using HCl and NaOH (0.1 M).
Preparation of W/O/W Double Emulsions
W/O/W emulsions were prepared in two steps, according to Esfanjani et al. (2015), briefly:
-
(a)
First, primary W/O nano-emulsions were produced by the formulation procedure described in the “Micro/Nano-emulsion Production” section.
-
(b)
W/O/W double emulsions were prepared by gradually adding W/O nano-emulsions into the outer aqueous phase of mixed biopolymer solutions (WPC/pectin/maltodextrin) while blending by a homogenizer (Heidolph Silent Crusher, Germany) at 12,000 rpm for 5 min at 10 °C, and then these coarse emulsions were further emulsified using the mentioned homogenizer (15,000 rpm for 8 min at 10 °C). The composition of W/O/W multiple emulsions is given in Table 2.
Droplet Size Measurement
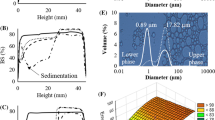
Emulsions were analyzed firstly through microscopic pictures taken from a Zeiss optical microscope (Germany). The ocular (eyepiece) lens had a ×10 magnification, and we used objective lens of ×100 magnification. The pictures were analyzed by ImageJ software (Hosseini et al. 2015). For droplet size, samples were analyzed simultaneously using a dynamic light scattering method (Zetasizer Nano ZS, Malvern Instrument, Malvern, UK). To avoid multiple scattering, all samples were diluted using 0.1 % SDS. The size analysis of treatment no. 1 is shown in Fig. 1.
Spray Drying of Double Emulsions
The in-fed double emulsions were transformed into encapsulated powders in a lab spray dryer (model SP1500, Fanyuan Instrument Co., Shanghai, China) equipped with a pressure air-atomizing nozzle at 2.5 bar air pressure, inlet air temperature of 180 ± 5 °C, and outlet air temperature of 90 ± 5 °C with a feed flow rate of 450 mL/h. These conditions were obtained as an optimum range after running some initial experiments. The dried powder was collected and stored in dark, air-tight bottle containers at 4 °C until further analysis.
Encapsulation Efficiency of Folic Acid
For determination of entrapment efficiency of different formulations, it was necessary to analyze encapsulated folic acid powders in terms of total content and surface content of folic acid in final powders. For surface content, 0.5 g of each sample was dispersed in 20 mL hexane, vortexed for 2 min, and filtered using Whatman filter paper no. 41. After adding 10 mL ethanol, the folic acid content was determined spectrophotometrically at 282.5 nm (Matias et al. 2014). For total content, 0.1 g of powders was dissolved in 7 mL hexane and 3 mL phosphate buffer (pH = 9.5). These solutions were ultrasonicated for 5 min and centrifuged at 3500 rpm for 20 min. The supernatant was withdrawn carefully without disturbing the cake left at the bottom of tubes, diluted sufficiently, and analyzed for estimation of total folic acid available in nano-encapsulated powders. The encapsulation efficiency of folic acid nano-encapsulated within powders was calculated using the following formula (Jafari et al. 2007; Pourashouri et al. 2014):
Statistical Analysis
The experiments (Table 2) were designed by the Taguchi approach in Minitab software (version 16). The collected data were analyzed by Qualitek software (version 4, Nutek, USA). On the basis of preliminary trials, the Taguchi orthogonal array design (L16) factors (independent and dependent) were selected (Table 1), generated designs were prepared, and their responses were evaluated (Table 2) for statistical optimization of formulations by investigating effects of major factors on responses with a minimum number of formulation trials. All factors with optimum conditions were determined by estimating the experimental conditions having a minimum variability using orthogonal array analysis of variance (ANOVA) tables. The responses were analyzed to determine the significance of each factor plotted against each level (main effects plot which reveals the effect of each factor on responses). It also revealed the best optimum level of each factor which should be used to prepare an optimized formulation.
Results and Discussion
Determination of Factors and Their Levels
In order to select important factors and their working ranges having major influence on encapsulation efficiency of folic acid, preliminary trials were conducted. The experimental range of pectin, WPC, dispersed phase volume fraction (DPVF), and pH were found as 0.5–2.0 % w/w, 4.0–10.0 % w/w, 10.0–30.0 % v/v, and 6 to 9, respectively, as shown in Table 1. Also, two surfactants of Span and PGPR were used to prepare the internal phase of double emulsions in two different forms of micro- and nano-emulsions as the fifth independent factor. In order to prepare an optimized formulation, one factor at a time has to be changed which increases the number of trials to be performed, resulting in wastage of a large number of chemicals and is also a very time-consuming process. Therefore, to minimize the number of trials and to study the effect of factors in all possible combinations, a Taguchi orthogonal array design was applied on experimental ranges of factors to formulate an optimized encapsulation of folic acid with the highest efficiency.
Main Effect of Independent Variables on Folic Acid Encapsulation Efficiency
Although the design trails produced good encapsulation efficiency in the range of 82.30 to 95.00 (Table 2), the selection of the best formulation was difficult. Therefore, the response (Y) obtained for each trial was analyzed based on independent factors. It also helps to establish the significance of each factor and their optimum levels for optimized formulation. The results for responses were interpreted by considering the “highest is the best” for better accuracy of measured data. The highest and lowest encapsulation efficiency was obtained for treatment no. 5 (95.00 %) and no. 9 (82.30 %), respectively. The main effects plot was drawn for each factor at different levels by taking levels as x-coordinates and encapsulation efficiency as y-coordinates (Fig. 2). The plots could show the effects of individual factors on encapsulation efficiency of folic acid by different double emulsion formulations.
A prerequisite for the formation of electrostatic complexes is naturally the existence of opposing charges on the reacting species. Conventionally, this is achieved by the utilization of an anionic polysaccharide (e.g., pectin) in combination with a protein (whey proteins in our case) bearing positive charge below its isoelectric point (Murillo-Martínez et al. 2011; Salminen and Weiss 2014). For polysaccharides such as pectin, the negative charge most commonly arises out of the presence of carboxyl moieties, and for proteins, the positive charge normally is achievable below their isoelectric point (Bouyer et al. 2012; Evans et al. 2013; Hosseini et al. 2015).
Our results (Fig. 2) showed that the dispersed phase content of the inner W/O micro-emulsion and biopolymer concentration in the outer aqueous phase had a significant effect on the encapsulation efficiency of folic acid in W/O/W multiple emulsions. As it can be seen in Fig. 2, the highest encapsulation efficiency of folic acid was obtained when the concentration of pectin and WPC was 1.0 and 4.0 % w/w, respectively. In other words, a weight ratio of 1.0:4.0 for pectin/WPC resulted in the best encapsulation efficiency. This finding shows indirectly that double emulsions prepared by 1.0 % pectin and 4.0 % WPC have a good stability and low release of folic acid from internal nano-emulsions into outer aqueous phase, which results in the highest encapsulation efficiency. At lower or higher ratios of pectin/WPC, the formation of the complex between these two oppositely charged biopolymers is not strong enough to protect the internally encapsulated folic acid and this bioactive ingredient migrates out and releases to the external aqueous phase of the double emulsions, thus lowering the encapsulation efficiency. Lutz et al. (2009a) working on double emulsion prepared with whey protein isolate (WPI)-pectin found that a weight ratio of 4:0.5 (WPI/pectin) came with the smallest droplet size and best stability of produced emulsions. The difference between their optimum ratio and our observation could be due to the difference in purity of used whey protein (WPC vs. WPI) and applied pectin (low methoxyl vs. modified pectin) and physicochemical/rheological variations among samples.
Considering DPVF, in general, lower contents of the dispersed phase produced double emulsions with higher encapsulation efficiency in agreement with the results of Schuch et al. (2013). They found that for high internal phase concentrations, inner droplets coalesce with each other. The so-formed bigger inner droplets seem to increase the overall release rate and lower the encapsulation efficiency (Schuch et al. 2013). In this study, the dispersed phase itself was a W/O nano- (or micro-)emulsion composed of surfactant (Span or PGPR), oil, and water containing folic acid. It can be concluded that increasing DPVF results in an increase in the dispersed phase volume of double emulsions which could result in bigger dispersed droplets with lower emulsion stability (Jafari et al. 2008). Thus, folic acid molecules within the internal phase (dispersed droplets) could easily release and exit into the external aqueous phase of final double emulsions without any protection from the complex biopolymer layer of pectin-WPC and led to lower encapsulation efficiency.
Interestingly, by an increase of pH from 6 to 9, encapsulation efficiency of folic acid within produced double emulsions was decreasing continuously (Fig. 2). pH strongly affects both the net charge of biopolymers and the interactions between them. Moreover, the net surface charge (zeta-potential) of the emulsion droplets affects the emulsion stability (Lutz et al. 2009a, b). In our preliminary tests, it was found that at pH level of 4, which is the isoelectric point of the mixture solution of both pectin-WPC biopolymers, the two species are almost neutralized and complex coacervation occurs, forming relatively large precipitating aggregates (flocs). Because the absorption of the large flocculated aggregates on the oil/water interface was poor and incomplete, the repulsion between the droplets was also insufficient (Kovacova et al. 2009; Salminen and Weiss 2014; Teng et al. 2015). Consequently, relatively large droplets were obtained. Below the isoelectric point of the whey proteins, at pH 2, the proteins are mostly positively charged, while the net charges of the pectin are negative and the zeta-potential is positive. The complex aggregates forming at this pH were smaller than those formed at pH 4, but larger than those obtained at pH 6.
At pH 6, the interactions could occur between several positively charged groups on the WPC backbone, in spite of being negatively charged overall and totally negative pectin. Those interactions are the driving force for the formation of these complexes. These are limited strength attraction interactions not sufficient to cause phase separation but rather forming small molecular aggregates. On the other hand, the aggregates at pH 2 are based on very strong charge interactions and precipitate out of the solution. The complex aggregates at pH 2 are very large and have very limited molecular absorbing capability onto the interface of the droplets and thus cannot serve as a good emulsifier. Hence, only the blend of WPC and pectin at pH 6 acted as a good emulsifying agent and formed emulsions with small droplets, similar to the results of Lutz et al. (2009a, b). Furthermore, the negatively charged complexes, at pH 6, added some beneficial repulsive forces between the droplets and improved the emulsion stability. At pH 8, the amino moieties on the WPC were not protonated and the carboxylic groups were fully ionized. So, the total net surface charge is negative.
When considering the influence of surfactant system on the encapsulation efficiency of folic acid through double emulsions of pectin-WPC, it was found that micro-emulsions (droplet size of less than 100 nm) of both Span and PGPR had a higher efficiency which could be due to thermodynamic stability of these emulsions compared with their nano-emulsion (100–1000 nm) counterparts which are only kinetically stable. Also, PGPR was generally acting better probably due to its branching anchors and stronger protection of the oil-water interface from coalescence (Mehrnia et al. 2016).
Determination of the Most Important Factors and Their Interactions
Estimated model coefficients for means of encapsulation efficiency and ANOVA for independent factors are given in Tables 3 and 4, respectively.
As it is shown in Table 3 by the constant value, the average of encapsulation efficiency for all treatments is equal to 88.29 %. When the influence of independent variables on the encapsulation efficiency of folic acid is positive, the plus sign precedes their corresponding coefficients in the model and, when it is negative, a minus sign comes before their coefficient which is consistent with the results of Fig. 2. By looking at Table 4, it can be concluded that the order of independent factors affecting the encapsulation efficiency of folic acid through double emulsions is (with DPVF having the highest influence of 36 %)
Also, as shown in Fig. 3, the highest interaction between two independent variables was for WPC-surfactant with a severity index of about 67 % and the lowest for pectin-pH with an index of less than 5 %.
Optimization Results
By analysis of the results through a Taguchi approach, it was found that the optimum conditions to have the highest encapsulation efficiency were pectin content of 1.0 %, WPC content of 4 %, DPVF of 15 %, pH = 6, and applying a PGPR-based micro-emulsion of folic acid as the internal phase of double emulsions (Table 5). These conditions theoretically result in an encapsulation efficiency of 99 % which was compatible to our experimentally obtained result (98.3 %).
Conclusion
In this study, we evaluated a Taguchi approach for encapsulation optimization of folic acid through W/O/W multiple emulsions. The independent variables were pectin and WPC content (as a complex double-layer biopolymer), dispersed phase volume fraction, pH, and surfactant system. It was found that the most affecting parameter on the encapsulation efficiency of folic acid was the volume of dispersed phase (36 %) and the surfactant system had the lowest influence (5 %). Also, there was a strong interaction between WPC and surfactant and then dispersed phase content and pH. We observed that a pH equal to 6 resulted in the highest encapsulation efficiency as it leads to formation of strong complexes between pectin and WPC which, in turn, produce double emulsions with a good stability and smaller emulsion droplet sizes. Finally, the optimum conditions to have the highest encapsulation efficiency for folic acid through double emulsions were determined as pectin = 1.0 %, WPC = 4.0 %, DPVF = 15 %, pH = 6, and applying PGPR micro-emulsions as the internal phase.
References
Assadpour, E., Maghsoudlou, Y., Jafari, S.-M., Ghorbani, M., & Aalami, M. (2016). Optimization of folic acid nano-emulsification and encapsulation by maltodextrin-whey protein double emulsions. International Journal of Biological Macromolecules, 86, 197–207.
Bakhshi, P. K., Nangrejo, M. R., Stride, E., & Edirisinghe, M. (2013). Application of electrohydrodynamic technology for folic acid encapsulation. Food and Bioprocess Technology, 6(7), 1837–1846.
Benichou, A., Aserin, A., et al. (2004). Double emulsions stabilized with hybrids of natural polymers for entrapment and slow release of active matters. Advances in Colloid and Interface Science, 108, 29–41.
Benichou, A., Aserin, A., et al. (2007). W/O/W double emulsions stabilized with WPI–polysaccharide complexes. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 294(1–3), 20–32.
Berry, R. J., Bailey, L., et al. (2010). Fortification of flour with folic acid. Food & Nutrition Bulletin, 31(1), 22S–35S.
Bouyer, E., Mekhloufi, G., et al. (2012). Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: alternatives to synthetic surfactants in the pharmaceutical field? International Journal of Pharmaceutics, 436(1–2), 359–378.
da Silva, M. M., Nora, L., Cantillano, R. F. F., Paese, K., Guterres, S. S., Pohlmann, A. R., & Rios, A. d. O. (2016). The production, characterization, and the stability of carotenoids loaded in lipid-core nanocapsules. Food and Bioprocess Technology, 9(7), 1148–1158.
Dickinson, E. (2011). Double emulsions stabilized by food biopolymers. Food Biophysics, 6(1), 1–11.
Donsì, F., Sessa, M., et al. (2011). Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Procedia Food Science, 1, 1666–1671.
Esfanjani, A. F., Jafari, S. M., et al. (2015). Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. Journal of Food Engineering, 165, 149–155.
Evans, M., Ratcliffe, I., et al. (2013). Emulsion stabilisation using polysaccharide–protein complexes. Current Opinion in Colloid & Interface Science, 18(4), 272–282.
Ezhilarasi, P. N., Karthik, P., Chhanwal, N., & Anandharamakrishnan, C. (2013). Nanoencapsulation techniques for food bioactive components: a review. Food and Bioprocess Technology, 6(3), 628–647.
Garti, N. (1997). Progress in stabilization and transport phenomena of double emulsions in food applications. LWT-Food Science and Technology, 30(3), 222–235.
Garti, N. and A. Aserin (2013). Double emulsions. Encyclopedia of colloid and interface science. T. Tadros, Springer: Berlin Heidelberg: 303–337.
Garti, N., & Bisperink, C. (1998). Double emulsions: progress and applications. Current Opinion in Colloid & Interface Science, 3(6), 657–667.
Gujska, E., Michalak, J., et al. (2009). Folates stability in two types of rye breads during processing and frozen storage. Plant Foods for Human Nutrition, 64(2), 129–134.
Gutiérrez, J. M., González, C., et al. (2008). Nano-emulsions: new applications and optimization of their preparation. Current Opinion in Colloid & Interface Science, 13(4), 245–251.
Hemar, Y., Cheng, L., et al. (2010). Encapsulation of resveratrol using water-in-oil-in-water double emulsions. Food Biophysics, 5(2), 120–127.
Hosseini, S. M. H., Emam-Djomeh, Z., et al. (2015a). Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocolloids, 50, 16–26.
Hosseini, A., Jafari, S. M., et al. (2015b). Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydrate Polymers, 126, 1–8.
Iyer, R., & Tomar, S. K. (2009). Folate: a functional food constituent. Journal of Food Science, 74(9), R114–R122.
Jafari, S. M., He, Y., & Bhandari, B. (2007). Role of powder particle size on the encapsulation efficiency of oils during spray drying. Drying Technology, 25(6), 1081–1089.
Jafari, S. M., Assadpoor, E., et al. (2008). Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocolloids, 22(7), 1191–1202.
Jose, S., Fangueiro, J. F., et al. (2012). Cross-linked chitosan microspheres for oral delivery of insulin: Taguchi design and in vivo testing. Colloids and Surfaces B: Biointerfaces, 92, 175–179.
Katouzian, I., & Jafari, S. M. (2016). Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends in Food Science & Technology, 53, 34–48.
Kovacova, R., Synytsya, A., et al. (2009). Characterisation of whey proteins–pectin interaction in relation to emulsifying properties of whey proteins. Czech Journal of Food Science, 27, 54–58.
Lamers, Y. (2011). Folate recommendations for pregnancy, lactation, and infancy. Annals of Nutrition & Metabolism, 59(1), 32–37.
Lutz, R., Aserin, A., et al. (2009a). Double emulsions stabilized by a charged complex of modified pectin and whey protein isolate. Colloids and Surfaces B: Biointerfaces, 72(1), 121–127.
Lutz, R., Aserin, A., et al. (2009b). Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids and Surfaces B: Biointerfaces, 74(1), 178–185.
Mahdavee Khazaei, K., Jafari, S. M., Ghorbani, M., & Kakhki, A. H. (2014). Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydrate Polymers, 105, 57–62.
Matias, R., Ribeiro, P. R. S., et al. (2014). A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Analytical Methods, 6(9), 3065–3071.
McClements, D. J. (2015). Encapsulation, protection, and release of hydrophilic active components: potential and limitations of colloidal delivery systems. Advances in Colloid and Interface Science, 219, 27–53.
Mehrnia, M. A., Jafari S. M., et al. (2016). Crocin loaded nano-emulsions: factors affecting emulsion properties in spontaneous emulsification. International Journal of Biological Macromolecules, 84, 261–267.
Milić, J., Petrinić, I., et al. (2014). Ultrafiltration of oil-in-water emulsion by using ceramic membrane: Taguchi experimental design approach. Central European Journal of Chemistry, 12(2), 242–249.
Mohammadi, A., Jafari, S. M., et al. (2016). Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chemistry, 190, 513–519.
Mosavian, M. T. H., & Hassani, A. (2010). Making oil-in-water emulsions by ultrasound and stability evaluation using Taguchi method. Journal of Dispersion Science and Technology, 31(3), 293–298.
Murillo-Martínez, M. M., Pedroza-Islas, R., et al. (2011). Designing W1/O/W2 double emulsions stabilized by protein–polysaccharide complexes for producing edible films: rheological, mechanical and water vapour properties. Food Hydrocolloids, 25(4), 577–585.
Murugesan, R., & Orsat, V. (2012). Spray drying for the production of nutraceutical ingredients—a review. Food and Bioprocess Technology, 5(1), 3–14.
Pérez-Masiá, R., López-Nicolás, R., et al. (2015). Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chemistry, 168, 124–133.
Pourashouri, P., Shabanpour, B., Razavi, S. H., Jafari, S. M., Shabani, A., & Aubourg, S. P. (2014). Impact of wall materials on physicochemical properties of microencapsulated fish oil by spray drying. Food and Bioprocess Technology, 7(8), 2354–2365.
Rader, J. I., Weaver, C. M., et al. (2000). Total folate in enriched cereal-grain products in the United States following fortification. Food Chemistry, 70(3), 275–289.
Rajabi, H., Ghorbani, M., Jafari, S. M., Sadeghi, A. R., & Rajabzadeh, G. (2015). Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocolloids, 51, 327–337.
Salminen, H., & Weiss, J. (2014). Effect of pectin type on association and pH stability of whey protein—pectin complexes. Food Biophysics, 9(1), 29–38.
Sapei, L., Naqvi, M. A., et al. (2012). Stability and release properties of double emulsions for food applications. Food Hydrocolloids, 27(2), 316–323.
Schuch, A., J. Wrenger, et al. (2013). “Production of W/O/W double emulsions. Part II: influence of emulsification device on release of water by coalescence.” Colloids and Surfaces A: Physicochemical and Engineering Aspects(0).
Sessa, M., Balestrieri, M. L., et al. (2014). Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chemistry, 147, 42–50.
Sonam, H. C., et al. (2014). Taguchi design for optimization and development of antibacterial drug-loaded PLGA nanoparticles. International Journal of Biological Macromolecules, 64, 99–105.
Talegaonkar, S., Azeem, A., et al. (2008). Microemulsions: a novel approach to enhanced drug delivery. Recent Patents on Drug Delivery & Formulation, 2(3), 238–257.
Teng, Z., Xu, R., et al. (2015). Beta-lactoglobulin-based encapsulating systems as emerging bioavailability enhancers for nutraceuticals: a review. RSC Advances, 5(44), 35138–35154.
Acknowledgments
It is necessary to appreciate the Iran Nanotechnology Initiative Council (INIC) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assadpour, E., Maghsoudlou, Y., Jafari, SM. et al. Evaluation of Folic Acid Nano-encapsulation by Double Emulsions. Food Bioprocess Technol 9, 2024–2032 (2016). https://doi.org/10.1007/s11947-016-1786-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1786-y