Abstract
The purpose of this study was to investigate the influence of pectin type on complex formation between whey protein isolate (WPI) and high methoxy pectins with varying degrees of esterification (DE), and their pH stability. The biopolymer particles with protein-to-polysaccharide mass ratio set to 2:1 were formed at pH 3–7 by heating at 85 °C for 20 min. The particle size, electrical charge, turbidity and microstructure of the biopolymer complexes were evaluated. The optimal conditions for forming WPI-pectin complexes were at the initial pH of 4.5–4.75, just below the isoelectric point of the WPI, where complex formation occurs. At this pH range, the smallest biopolymer complexes (d = 225–300 nm) could be created. Pectins with 50, 55, 62 and 70 % DE formed relatively small and monomodal complexes with WPI, except for pectin with 71 % DE, which showed major aggregation. The pH stability against aggregation was best with the biopolymer complexes assembled from pectins with 50 % DE (stable at pH 3.5–6.0) and with 62 % DE (stable at pH 3.0–6.0). The results suggest that pectins with varying DE can be used to form small particles and therefore can offer new possibilities in designing novel hierarchical structures and delivery systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fabrication of biopolymer particles can be used to create novel structures and delivery systems for encapsulation, protection and delivery of bioactive or functional ingredients such as lipids, flavors, peptides, proteins, enzymes, dietary fiber, minerals and vitamins as well as drugs [1, 2]. Biopolymer particles may also be used as functional ingredients to enhance the stability, structure, texture, and viscoelastic properties of food products. For example, spherical biopolymer particles can mimic the optical and rheological (textural) properties of lipid droplets, and can therefore be used to replace fat in certain products [3].
Protein-polyelectrolyte interactions have been extensively reviewed [4–10]. Biopolymer particles can be constructed from proteins and anionic polysaccharides through associative interactions [4, 5]. These mainly electrostatically driven interactions begin at pH > pI, when the charged regions of proteins start binding to the negatively charged polysaccharides to form primary soluble complexes at the first critical pH (pHc) [5, 9, 11]. At the second critical pH (pHϕ1), the soluble protein-polysaccharide complexes begin to aggregate into insoluble complexes due to the charge neutralization. This will eventually lead to extensive aggregation and phase separation [5, 9]. When pH is further decreased to the third critical pH (pHϕ2), the complexes begin to dissociate to soluble complexes, or even to non-interacting individual protein and polysaccharide molecules [4–6]. Biopolymer interactions can be controlled by solutions conditions (pH, ionic strength), environmental conditions (temperature, shearing), and by choosing the type of biopolymers (molecular weight, charge density, flexibility, hydrophobicity) [7, 8].
Research on complex formation/coacervation in food grade materials has mainly focused on investigating complexation of milk proteins such as mixed whey protein-gum arabic [11], whey protein-pectin [2, 12], β-lactoglobulin-acacia gum [13], β-lactoglobulin-chitosan [14, 15], β-lactoglobulin-carrageenan [16], casein-pectin [17], serum albumin-pectin [18], and β-lactoglobulin-pectin solutions [16, 19–27]. In these studies, the major focus has been on β-lactoglobulin-pectin complexes formed from a variety of pectin types from different sources (sugar beet, citrus): (i) low-methoxy (LM) pectins with 28–32 % degree of esterification (DE) [16, 20, 24–26], (ii) amidated pectins with 30 % DE [12], and (iii) high-methoxyl (HM) pectins with 54–57 % DE [19, 22, 23] or 70–73 % DE [16, 20, 24, 26]. The ratio of methylesterified galacturonic acid groups to total galacturonic acid groups is termed as the degree of esterification (DE). Thus, HM pectins have ≥50 % DE, whereas LM have <50 % DE. On the other hand, only a few published articles have studied the biopolymer complexation of whey protein isolate (WPI), and even then with a focus on either LM [2] or amidated LM pectins [12].
Consequently, there are no systematic studies on the physical characterization of biopolymer complexes made with more readily available and low cost WPI and varying esterification of HM pectins. Whey proteins are widely used as emulsifiers in food industry to form and stabilize emulsion based-food products due to their amphiphilic and surface active properties [28]. Pectins are mainly used as gelling and thickening agents, but also as emulsifiers and stabilizers in food, pharmaceutical and cosmetic formulations [29]. The functionality of pectins can be attributed to their highly complex polymeric structure consisting of multiple polygalacturonic acid groups. A certain amount of their carboxyl groups are esterified with methanol [30]. We hypothesized that the DE of HM pectins will influence the properties and stability of the formed biopolymer complexes. Therefore, the purpose of this study was to investigate the association behavior of biopolymer complexes between WPI and various HM pectins, and test their stability against pH changes. In this study, biopolymer complexes between WPI and HM-pectins formed upon heat treatment likely because heating above the denaturation temperature of the protein increased their hydrophobicity thereby increasing attractive hydrophobic interactions. In addition, neighboring proteins may have undergone chemical crosslinking induced by the thermal treatment. The so formed biopolymer complexes may be smaller and more compact and exhibit better pH, salt and temperature stabilities than those formed at ambient temperatures [9, 10, 22, 23, 31].
Materials and Methods
Materials
Food grade whey protein isolate (WPI) (DSE 9273, dry matter 99.0 %, protein 93.9 %, lactose <0.5 %, fat 0.2 %, moisture 5.2 %, ash 1.5 %) was donated by Fonterra GmbH (Hamburg, Germany). WPI was obtained by ion exchange and ultra-filtration. WPI was mainly composed of a mixture of β-lactoglobulin A (46.1 %), β-lactoglobulin B (43.9 %), and α-lactalbumin (9.7 %). The β-lactoglobulin A and B forms differ in positions 64 and 188 where aspartic acid and valine of β-lactoglobulin A are replaced by glycine and alanine in β-lactoglobulin B [32]. The pI of β-lactoglobulin is 5.1–5.2 [29, 33], whereas the pI of α-lactalbumin is 4.1 [11, 34]. The mineral content was: Na 0.48 %, S 0.24 %, Ca 0.07 %, K 0.05 %, P 0.03 %, Fe 0.0003 %, Al 0.00001 %, and Se 0.0000006 %. Apple pectins (Pectin Classic AU606, 50 % DE; Pectin Classic AU301-LV, 62 % DE; and Pectin Classic AU202, 70 % DE), citrus pectin (Pectin Classic CU-L, 71 % DE) and sugar beet pectin (Betapec RU 301, 55 % DE) were donated from Herbstreith & Fox KG (Neuenbürg/Württ, Germany) (Table 1). Sugar beet pectin contained 0.75 ± 0.02 % ferulic acid. Sodium acetate anhydrous was obtained from Merck (Darmstadt, Germany) and glacial acetic acid was from Carl Roth GmbH + Co. KG (Karlsruhe, Germany). Sodium azide was obtained from Sigma-Aldrich (Steinheim, Germany). Distilled, deionized water was used throughout the study.
Biopolymer Solution Preparation
Powdered WPI and different pectins were dissolved in 10 mM sodium acetate (pH 7.0) containing sodium azide (0.02 %) as an antimicrobial agent, and stirred at ambient temperature for at least 4–8 h. Protein and pectin solutions were initially adjusted to pH 7.0 using 1.0 and 0.1 N sodium hydroxide solutions before being mixed together. After mixing, the final protein concentrations in the solutions were 0.5 %, while the polysaccharide concentrations were 0.25 % (w/w). Individual and mixed biopolymer solutions were adjusted to pH values below 7.0 using 1.0 N, 0.5 N, and/or 0.01 N hydrochloric acid solutions.
Biopolymer Complex Formation
Protein-pectin solutions were adjusted to a specific pH (3.5–7.0) and stirred for 30 min. Then the mixed solutions were heat treated (85 °C, 20 min) and cooled at room temperature for 2 h. The resulting suspensions were evaluated for their turbidity, appearance, particle size and charge. The pH stability measurements of the biopolymer complexes were conducted only to selected samples that indicated formation of relatively small biopolymer particles with narrow particle distributions.
pH Stability of Biopolymer Complexes
The pH stability of selected biopolymer complexes formed by heating to subsequent changes in pH (3.0–6.0) was examined by adjusting the pH using 1.0 N, 0.5 N and/or 0.01 N hydrochloric acid or sodium hydroxide solutions.
Turbidity Measurements
The turbidity of biopolymer solutions before and after heat treatment was analyzed with a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA). The samples (300 μL) were placed in a 96-well microplate and the absorbance was measured at 630 nm at ambient temperature. Sodium acetate buffer at appropriate pH was used as a blank reference.
Visual Observation
Samples (10 mL) were placed in test tubes and photographic images were taken using a digital camera (PowerShot SX200 IS, Canon, Tokyo, Japan).
Microstructure
Microstructures of samples were assessed by light microscopy. Microscopic images were taken using an AXIO Scope.A1 Light Microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) equipped with a Canon Powershot G10 Digital camera (Canon, Tokyo, Japan). An A-Plan objective with a magnification of 40×, numerical aperture of 0.65 with phase contrast of Ph2 (Carl Zeiss, MicroImaging GmbH, Jena, Germany) without immersion was used. The settings of the Canon digital camera were as follows: Mode Dial AV, White Balance (WB) Tungsten, Flash off, Digital zoom 1.7×, Exposure compensation +1, and Aperture >F5.6 with manual focus. For microscopic imaging, representative images were chosen from among at least four similar images.
Particle Size Analysis
The particle sizes were determined using a dynamic light scattering device (Nano-ZS, Malvern Instruments, Worcestershire, UK). Biopolymer complex samples were diluted 1:100 using an appropriate buffer solution (at the same pH as the sample) to avoid multiple scattering effects. The instrument reports the mean particle diameter (z-average) and the polydispersity index (PDI) ranging from 0 (monodisperse) to 1.0 (very broad distribution). The Nanosizer ZS operates by detecting back-scattered laser-light (θ = 173°) and comparing the coherence of scattering patterns as a function of time. Decay of coherence is converted to apparent particle sizes and distributions through the software, which relies on Mie theory calculations.
ζ-Potential Measurements
Individual biopolymer samples were diluted to a droplet concentration of approximately 1:10, whereas mixed biopolymer solutions were diluted to 1:100 using an appropriate buffer solution (at the same pH as the sample), and placed into the measurement chamber of the particle electrophoresis instrument (Zetasizer Nanoseries Nano-ZS, Malvern Instruments, Worcestershire, UK). The electrical charge (ζ-potential) was determined by measuring the direction and velocity that the droplets moved in the electric field applied. The Smoluchowsky mathematical model was used by the software to convert the electrophoretic mobility measurements into ζ-potential values.
Statistical Analysis
All measurements were performed on three samples. Means and standard deviations were calculated using Excel (Microsoft, Redmond, WA, USA).
Results and Discussion
Characterization of Individual Biopolymers
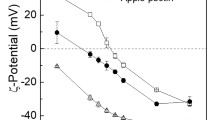
Initially, the impact of pH on the electrical characteristics, and turbidity of WPI and different pectin types used in this study were evaluated. The influence of pH (3.0–7.0) on the ζ-potential (Fig. 1) and turbidity (Fig. 2) of 0.5 wt% WPI and 0.25 wt% pectins were measured at ambient temperature and after heat treatment (85 °C, 20 min).
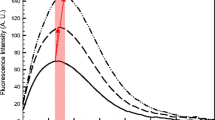
Influence of pH on turbidity in individual and mixed whey protein isolate (WPI, 0.5 %)–pectin (0.25 %) solutions in 10 mM sodium acetate before and after heat treatment (85 °C, 20 min): (a) apple pectin DE 50 %, (b) beet pectin DE 55 %, (c) apple pectin DE 62 %, (d) apple pectin DE 70 %, and (e) citrus pectin DE 71 %. Each figure is accompanied with a visual observation of the corresponding WPI-pectin solutions after heat treatment at pH 3.0–6.5
The ζ-potential of WPI solution changed from positive to negative as the pH was increased from pH 3.5 to 7.0 with a zero charge around pH 4.7–4.9 (Fig. 1) This is because the electrical charge of WPI molecules goes from positive to negative as the pH moves from below to above its pI. The measured pI of WPI was in an agreement with previous studies (pI ≈ 5) [8, 10]. The turbidity of the WPI solution at ambient temperature was low across the entire pH range most likely due to low concentration (Fig. 2). Nevertheless, whey proteins generally form aggregates close to their pI due to loss of electrostatic repulsion of protein molecules. Indeed, when the WPI concentration was increased to 1 wt%, white sediment was visually observed at the bottom of the test tubes at pH 4.0–5.25 (data not shown). After heat treatment, WPI showed a greatly increased turbidity at pH 4.0–6.0 (Fig. 2), which can be attributed to extensive protein aggregation associated with the increased hydrophobic attraction between protein molecules when they are heated above their thermal denaturation temperature [35]. This is because at ≥60 °C, β-lactoglobulin dissociates from a lipocalin dimer (36.2 kDa) to a monomer (18.4 kDa), thus exposing its thiol groups and interior hydrophobic residues, enabling thiol/disulfide exchange reactions [36]. Moreover, the electrical charge of WPI changed between pH 4.0–6.0 upon heating (Fig. 1b). At pH 5–6, the ζ-potential of heated WPI was more negative than the unheated protein, whereas at pH 4–4.75 the electrical charge was more positive than the unheated protein (Fig. 1). This corroborates that the unfolding of the protein molecule upon heating exposes charged groups from the core. Similar variations in ζ-potential have also been reported between unheated and heated (85 °C for 15 min) β-lactoglobulin, and this phenomenon was suggested to occur due to a redistribution of the counterions upon heat aggregation and rearrangements of the protein structure [37].
The various pectins showed overall negative charges across the entire pH range before (Fig.1a) and after heat treatment (Fig. 1b), which was attributed to their low pKa values (≈ 3.5–4.1) [38]. Pectins contain a substantial amount of various functional carboxyl, methyl, and acetyl groups attached to either linear homogalacturonan (HG) or branched rhamnogalacturonan (RG) or xylogalacturonan backbones that are more or less protonated depending on pH. Linear HG are made of linked α-(1 → 4) galacturonic acid residues. Branched RG-I are formed by a backbone built by repeating disaccharide galacturonic acid and rhamnose with neutral sugar residues and polymeric side chains linked to the rhamnose residues. RG-II has a backbone similar to HG with very complex side chains. Xylogaracturonans consist of a linear chain as HG, which is partially substituted with xylose residues, and/or xylan chains that can be further substituted with sugar residues [30]. Linear HG comprise a majority (55–70 %) of the pectin and can contain more than 80 % of galacturonic acid residues [30, 39]. It should be noted that aside from the plant source, the characteristics of pectin are also highly dependent on the extraction conditions. The citrus pectin used in this study contained the highest amount of galacturonic acid (87 %) followed by apple pectin (83–84 %) and sugar beet pectin (65 %) (Table 1). Citrus pectins are composed predominantly of linear HG, but they also contain a few RG-I regions and minor RG-II regions [39]. The most important structural differences to apple and citrus pectin are that sugar beet pectin has: (i) a shorter galacturonic acid chain length, and thus a lower galacturonic acid amount, (ii) more RG-I regions, and thus a high content of neutral sugars, especially of arabinans, (iii) high degree of acetyl substitution, and (iv) ferulic acid residues ester-linked to arabinose and galactose units [40, 41]. Sugar beet pectin used in this study contained 0.75 ± 0.02 % ferulic acid, whereas no ferulic acid was found in apple and citrus pectins.
There were no significant changes in the electrical charges between the unheated and heated pectins except for Pectin 55 and Pectin 71. At pH > 5.5, heat treated Pectin 55 showed a more negative charge than the unheated one, whereas the ζ-potential of heated Pectin 71 was less negative at pH < 5 compared to the unheated one (Fig. 1). These charge changes may be due to exposure of some charged groups from the denaturing proteins attached to the pectin molecules as the protein content in sugar beet (2–10 %) and citrus pectins (3–3.3 %) is higher than apple pectins (1.6 %) [42–44]. The pectins remained transparent across the entire pH range at both ambient temperature and after heat treatment (Fig. 2), thus indicating that they did not form aggregates that strongly scatter light. This can be contributed to the strong electrostatic repulsion between the pectin molecules. The carboxylic acid content decreases with increasing DE, and thus the electrical charge density in Pectin 50 is expected to be more negative than in the other pectins. Most of the pectins followed this trend, except Pectin 71 (Fig. 1). Unheated Pectin 71 had a more negative ζ-potential value than the other pectins (Fig. 1a) possibly due to different distribution of methyl groups and higher amount of highly complex and branched RG-I and -II regions, thus affecting its conformation [39].
Influence of pH and Pectin Type on WPI-Pectin Complexation
The objective of these experiments was to evaluate pH conditions and influence of pectin type where relatively small protein-polysaccharide electrostatic complexes could be formed. The influence of pH (3.0–7.0) on turbidity in mixed 0.5 % WPI–0.25 % pectin solutions before and after heat treatment (85 °C, 20 min) was measured (Fig. 2). In addition, the impact of initial pH and pectin type on the electrical charge (Fig. 3) and particle size (Table 2) in the mixed biopolymer solutions after heat treatment were determined.
Unheated WPI-Pectin Complexes
The pH dependence on turbidity of mixed unheated WPI-pectin solutions was considerably different from that of the individual biopolymers. At pH 7.0, the electrical charge of both pectin molecules (ζ = between −26 ± 1 and −43 ± 2 mV) and protein molecules (ζ = −21 ± 2 mV) was negative (Fig. 1). Unheated mixtures of WPI and pectins were also optically transparent at pH 5.5–6.5 (data not shown), which was also indicated by the low turbidity values (Fig. 2). This thermodynamic incompatibility due to the strong electrostatic repulsion between pectin and protein molecules prevented their assembly, and indicated that the biopolymer solutions were co-soluble [4]. When the pH was lowered to 5.0, a slow increase in turbidity was observed (Fig. 2), indicating the initial formation of soluble complexes [5, 9, 11]. This is promoted by the pH reduction close to the pI of WPI, thus gaining a significant number of cationic groups (−NH3 +) on its surface [5]. For unheated WPI-pectin complexes an initial pHc was observed at pH 5.0, except for WPI-pectin 55 pHc was around pH 6.5 (data not shown). When pH was lowered below 5.0, the turbidity of the biopolymer mixtures increased, which indicated the formation of WPI-pectin complexes that were large enough to scatter light. Unheated biopolymer complexes containing Pectin 55 and 62 had turbidity maxima at pH 4.0, whereas for Pectin 50 and 70 it was at pH 3.5 (Fig. 2a–d). At pH range from 5.0 to 3.0 the complexes are formed by electrostatic attraction between the anionic groups on the pectin molecules and cationic groups on the protein surface [20, 26]. At pH 3.5, the unheated WPI-pectin 55 aggregated as indicated by the decrease in turbidity (Fig. 2b), and formed a sediment layer on the bottom of the test tube (data not shown). At pH 3.0, the unheated WPI-pectin 50, 62 and 70 showed extensive aggregation and phase separation (data not shown), which can be attributed to the charge neutralization of the insoluble complexes leading to self-association [5, 9]. On the other hand, WPI-pectin 71 had not yet phase separated at pH 3 (data not shown), and showed no decrease in the turbidity (Fig. 2e).
Heat Treated WPI-Pectin Complexes
The turbidity profile of heat treated WPI-pectin mixtures was different from that of the unheated mixtures (Fig. 2). All WPI-pectin mixtures were first adjusted to a specific pH (3.5–7.0) and then heat treated at 85 °C for 20 min. At pH >> pI of the protein, the heat treated WPI-pectin mixtures were optically transparent (Fig. 2) because the molecular interactions of the individual biopolymers were prevented by strong electrostatic repulsion forces consistent with the net negative charge for both protein and polysaccharides at this pH (Fig. 1). Consequently, the system existed as a solution of the individual molecules [5, 9]. At pH 6.9, the ζ-potential values of heated WPI-pectin complexes (Fig. 3) were closer to zero than the charge of the individual biopolymers (Fig. 1). This is because the ζ-potential measurement device is often unable to distinguish between the presence of differently charged species and rather displays an average overall value. In reality, the individual non-interacting molecules in this mixture may have different ζ-potential values.
The increase in turbidity indicated the formation of soluble biopolymer complexes. The initial point of pHc was around pH 6.5 for heated WPI-pectin 55, and at pH ~ 6.0 for heated WPI-pectin 50, 62, 70, 71 as shown by turbidity values and the test tube images (Fig. 2). The reason for increased turbidity of WPI-pectin 55 at pH 6.5 is unknown, but might be due to the structure and/or composition of Pectin 55. At pH 5.5, the turbidity of the heated WPI-pectin complexes greatly increased to their first maxima (Fig. 2), thus indicating also the associative phase separation point (pHϕ1). Simultaneously, the turbidity of the heated WPI also increased (Fig. 2), which can be attributed to increased protein aggregation caused by thermal denaturation [19]. Denaturation causes the protein to fully or partially unfold, thereby exposing the hydrophobic core and increasing the surface hydrophobicity and chemical reactivity. The unfolding of β-lactoglobulin is the key to intra- and intermolecular interactions of WPI through electrostatic, hydrophobic, and hydrogen bonding as well as formation of covalent disulfide bridges. In contrast, α-lactalbumin does not contain any free thiol groups that can induce covalent aggregation reactions upon denaturation and acidification [34]. The role of β-lactoglobulin was also proposed in a study by Weinbreck et al. [11], who suggested that complex coacervation of whey proteins with gum arabic was mainly dominated by β-lactoglobulin compared to α-lactalbumin.
At pH ≤ pI, the number of cationic groups on the surface of the protein increases which leads to stronger electrostatic association of the protein-pectin complexes [5, 9]. The heated WPI-pectin complexes containing Pectin 50, 55, and 70 showed turbidity minima at pH 4.5 (Fig. 2a, b, d), and then increased to another maxima at pH 4 (Pectin 55) and pH 3.5 (Pectin 50 and 70). In contrast, for biopolymer complexes made of Pectin 62 and 71 no turbidity minima were observed (Fig, 2c, e). The results showed that the optimal formation of small and monodispersed (i.e. PDI < 0.2) biopolymer complexes (d ≈ 224–400 nm) occurred at pH 4.0–5.0 (Table 2). This was corroborated with the turbidity (Fig. 2) and ζ-potential results (Fig. 3). The negative charges of protein-polysaccharide complexes (Fig. 3) were similar to ζ-potentials of individual pectins (Fig. 1). Therefore, the particle charge and size suggest that particles with a protein core surrounded by pectin were formed. This “core-shell” structure has been proposed in earlier studies [10, 23]. The smallest biopolymer complexes were formed with Pectin 50 and 55 at pH 4.0–5.0 (d ≈ 224–290 nm), with Pectin 62 at pH 4.5–4.75 (d ≈ 300 nm) as well as with Pectin 70 at pH 4.5–5.0 (d ≈ 280–300 nm) (Table 2a). Interestingly, complexes formed with Pectin 62 exhibited highly monomodal distributions (PDI < 0.1) (Table 2b). These results show that formation of small WPI-pectin complexes was successful. This is in contrast to the study of Bedie et al. [2], who reported that WPI-pectin (DE 36 %) solutions (0.4 % total polymer concentration, r = 2:1, 1:1 and 1:2) formed complexes of ca. 25–300 μm with pH adjustment to 4.0, 3.5, 3.0, and 2.5 either before or after blending. It should be noted though that these complexes were made without heat treatment. In a study by Gentes et al. [12] stable WPI-pectin (DE 30 % with 19 % amidated groups) complexes were formed at pH 4.5 after heat treatment at 85 °C for 15 min or at 90 °C for 2 min. However, there was no information given on the size or charge properties of the formed complexes and interpretation of the results is therefore difficult. Our results are in agreement with earlier studies showing the formation of small, spheroid particles with low polydispersity when using β-lactoglobulin and HM-pectin with DE 54 % (Mw 166 kDa) at pH 4.75 after a heat treatment (85 °C, 15 min) [23]. Moreover, the results of the heat treated WPI-pectin complexes showed that WPI can form complexes with similar sizes as those formed with β-lactoglobulin [16, 23, 24]. This may be attributed to similarities in the aggregation behavior of WPI and β-lactoglobulin at 85 °C [45]. At lower denaturation temperatures though (e.g. 65 °C), α-lactalbumin in WPI may dominate the overall aggregation and differences to β-lactoglobulin become apparent [45].
WPI-Pectin 71, however, formed particles with big particle diameter (d > 400 nm) and high polydispersity (Table 2). This result is in an agreement with a study by Jones et al. [24], who showed the formation of big, non-spherical “fractal aggregates” in an atomic force microscopy image of β-lactoglobulin-pectin complexes (heated at pH 4.75, 80 °C, 20 min) when HM-pectin with DE of 71 % was used. Unfortunately, no additional information about the pectin properties (source or molecular weight) was given. The formation of these “fractal aggregates” particles may be attributed to the high amount of hydrophobic methylester groups in the pectin molecule with increasing DE (71 %), thus decreasing the amount of free carboxyl groups. The repulsive forces between the negative carboxyl groups can prevent the formation of a pectin network. However, when the pH is lowered the carboxylic acid groups lose their negative charges, which subsequently decrease the repulsion between pectin molecules and lower the attraction between pectin and water molecules. Therefore, the pectin molecules can self-associate through hydrogen bonding and hydrophobic interactions. The hydrogen bonding occurs between the free carboxyl groups on the pectin molecules and between the hydroxyl groups of neighboring molecules [29]. On the other hand, citrus pectin has a higher content of galacturonic acid (Table 1) than apple and sugar beet pectins as well as a high degree of complex branching by neutral sugars that affect its conformation and flexibility [29, 46].
At pH 3.0–3.5, biopolymer complexes were close to electrical neutrality (Fig. 3) leading to precipitation and bulk phase separation (Fig. 2). This can be attributed to the low charge density of the pectin below its pKa, thus leading to the dissociation of the WPI-pectin complexes [4–6].
Our original hypothesis was that the pectin type affects the association of the biopolymer complexes. The results showed that various pectin types can form small complexes with WPI, except Pectin 71. The results indicated an increase in the particle size of the WPI-pectin complexes with increasing DE of pectins (Table 2). Therefore, we performed a linear regression analysis for particle size of WPI-pectin complexes versus pectin DE at pH 4.5 and 4.75. Pectin 71 was excluded from the analysis because it did not form small, spherical particles. The regression analysis showed that the r2-value was 0.861 at pH 4.75, and 0.636 at pH 4.5. Thus, the increasing DE of the pectins partly explained the increasing particle sizes of the biopolymer complexes. However, there was no correlation between the molecular weight and the particle sizes. The pectin type has been shown to influence the formation of β-lactoglobulin-pectin complexes. For example, LM-pectins can form smaller complexes (d ≈ 200 nm) with β-lactoglobulin than HM-pectins (d ≈ 300 nm) [16, 23, 24]. However, the overwhelming complexity of pectins has made it difficult to analyze their structure and thus their functionality. The functionality of pectins depends not only on the conformation and flexibility of the pectin molecule but also on the distribution and amount of the functional carboxyl, methyl and acetyl groups [29, 30]. The charge densities of pectins depend on their DE: Pectins with lower DE have higher linear charge densities than pectins with higher DE [47]. Therefore, the protein molecules may have been in closer contact with the pectin molecules with lower DE during heating, thus facilitating their association with each other. However, more research is needed to elucidate the exact effect of the pectin structure.
pH Stability of Heat-Treated Protein-Pectin Complexes
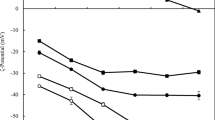
The objective of this series of experiments was to test the pH stability of the heat-treated protein-pectin complexes, which could give important information on their properties and their application possibilities. Only selected protein-pectin complexes of small particle sizes and low PDI were chosen for further testing: WPI-Pectin 50 (d ≈ 250 nm, PDI = 0.145), WPI-Pectin 55 (d ≈ 225 nm, PDI = 0.165) made at pH 4.5, and WPI-Pectin 62 (d ≈ 300 nm, PDI = 0.03) made at pH 4.75 (Table 1). The pH was adjusted either below or above the original pH of the complexes. The influence of pH (3.0 to 6.0) on the heat treated WPI-pectin complexes was examined by measuring the particle sizes (Table 3) and charges (Fig. 4). The particle size of WPI-Pectin 50 was stable at pH range of 3.5–6.0, but at pH 3.0 the particle size clearly increased (Table 3). These results were supported by microscopy images taken of WPI-Pectin 50 revealing that the complexes started aggregating at pH 3.0, whereas above pH 3 the WPI-Pectin 50 complexes were stable (Fig. 4). WPI-Pectin 55 was only stable at pH range of 4.5–5.0. At pH 3.5, WPI-Pectin 55 complex began to break down, which is supported by the electrical charges (Fig. 3): The ζ-potential of WPI-Pectin 55 was near the electrical neutrality (ζ = −10 mV) at pH 3.5, and became positive (ζ = +8 mV) when pH was further reduced to 3.0. This indicates that the pectin was no longer fully adsorbed to the surface of the protein, thus inducing formation of aggregates because of the electrostatic attraction between the positively charged protein molecules and the negatively charged pectin molecules [4–6]. The pH stability of WPI-Pectin 62 was good across the pH range with no changes in particle size compared to the original complex prepared at pH 4.75 (Table 3). In addition, WPI-Pectin 62 also showed remarkably monomodal distributions (Table 3). The electrical charges of the biopolymer complexes decreased with increasing pH and tended to plateau at pH > 5 (Fig. 3). The level of the electrical charge seemed to follow the DE of the pectins: the lower the DE of the pectins, the more negative was the ζ-potential of the biopolymer complex. This is because more carboxylic acid groups than methoxyl groups are present in the pectin molecule when the methylesterification decreases [30].
Conclusions
The results of this study showed that the pectin type, especially the DE, had some impact on the association of WPI-pectin complexes as hypothesized. However, the DE did not fully explain the effect on the particle sizes of the biopolymer complexes. It seems that the pectin structure also plays a role in the complex formation. Most of the pectins formed small particles with WPI. On the other hand, the association of biopolymer complexes greatly depends on the pH. In conclusion, the data indicates that a broad spectrum of pectins with different DE can be used to create small WPI-pectin complexes. In addition, this study provides evidence that complexes can be created from WPI, thus offering food industry the opportunity to formulate particles with accessibility to lower cost raw ingredients instead of using high-end β-lactoglobulin. The WPI-pectin associative complexes formed here demonstrated that they may be used in food or other applications with wide pH range because of their good pH stability. Moreover, their physical stability over time presents possibilities to be used in products with long shelf-life. However, more research is needed to elucidate the chemical stability of biopolymer complexes during processing and storage.
References
J. Weiss, P. Takhistov, D.J. McClements, J. Food Sci. 71, 9 (2006)
G.K. Bédié, S.L. Turgeon, J. Makhlouf, Food Hydrocolloids 22, 5 (2008)
C. Lobato-Calleros, O. Martínez-Torrijos, O. Sandoval-Castilla, J.P. Pérez-Orozco, E.J. Vernon-Carter, Int. Dairy J. 14, 9 (2004)
C.G. de Kruif, R. Tuinier, Food Hydrocoll. 15, 4–6 (2001)
C.G. de Kruif, F. Weinbreck, R. de Vries, Curr. Opin. Coll. Int. Sci. 9, 5 (2004)
S.L. Turgeon, M. Beaulieu, C. Schmitt, C. Sanchez, Curr. Opin. Coll. Int. Sci. 8, 4–5 (2003)
S.L. Turgeon, C. Schmitt, C. Sanchez, Curr. Opin. Coll. Int. Sci. 12, 4–5 (2007)
V. Tolstoguzov, Food Hydrocoll. 17, 1 (2003)
C.L. Cooper, P.L. Dubin, A.B. Kayitmazer, S. Turksen, Curr. Opinion Coll. Interface Sci. 10, 1–2 (2005)
O.G. Jones, D.J. McClements, Adv. Colloid Interface Sci. 167, 1–2 (2011)
F. Weinbreck, R. de Vries, P. Schrooyen, C.G. de Kruif, Biomacromolecules 4, 2 (2003)
M.-C. Gentes, D. St-Gelais, S.L. Turgeon, J. Agric. Food Chem. 58, 11 (2010)
C. Schmitt, C. Sanchez, F. Thomas, J. Hardy, Food Hydrocoll. 13, 6 (1999)
Y.H. Hong, D.J. McClements, Food Biophys. 2, 1 (2007)
Y.H. Hong, D.J. McClements, J. Agric. Food Chem. 55, 14 (2007)
O. Jones, E.A. Decker, D.J. McClements, Food Hydrocoll. 24, 2–3 (2010)
R. Tuinier, C. Rolin, C.G. de Kruif, Biomacromolecules 3, 3 (2002)
Q. Ru, Y. Wang, J. Lee, Y. Ding, Q. Huang, Carbohydr. Polym. 88, 3 (2012)
O.G. Jones, E.A. Decker, D.J. McClements, Food Hydrocoll. 23, 5 (2009)
M. Girard, S.L. Turgeon, S.F. Gauthier, Food Hydrocoll. 16, 6 (2002)
X. Wang, Y. Li, Y.-W. Wang, J. Lal, Q. Huang, J. Phys. Chem. B 111, 3 (2007)
O. Jones, D. McClements, Food Biophys. 3, 2 (2008)
O.G. Jones, E.A. Decker, D.J. McClements, J. Coll. Int. Sci. 344, 1 (2010)
O.G. Jones, U. Lesmes, P. Dubin, D.J. McClements, Food Hydrocoll. 24, 4 (2010)
X. Wang, J. Lee, Y.-W. Wang, Q. Huang, Biomacromolecules 8, 3 (2007)
B.L.H.M. Sperber, H.A. Schols, M.A. Cohen Stuart, W. Norde, A.G.J. Voragen, Food Hydrocolloids 23, 3 (2009)
W. Chanasattru, O.G. Jones, E.A. Decker, D.J. McClements, Food Hydrocolloids 23, 8 (2009)
D.J. McClements, 2nd edn, ed. by D.J. McClements (CRC Press, Boca Raton, 2005).
B.R. Thakur, R.K. Singh, A.K. Handa, M.A. Rao, Crit. Rev. Food Sci. Nutr. 37, 1 (1997)
B.M. Yapo, Carbohydr. Polym. 86, 2 (2011)
C. Schatz, A. Domard, C. Viton, C. Pichot, T. Delair, Biomacromolecules 5, 5 (2004)
X.L. Huang, G.L. Catignani, E.A. Foegeding, H.E. Swaisgood, J. Agric. Food Chem. 42, 5 (1994)
S. Mehalebi, T. Nicolai, D. Durand, Int. J. Biol. Macromol. 43, 2 (2008)
E.A. Permyakov, L.J. Berliner, FEBS Lett. 473, 3 (2000)
H.J. Kim, E.A. Decker, D.J. McClements, Langmuir 18, 20 (2002)
J.I. Boye, C.Y. Ma, A. Ismail, V.R. Harwalkar, M. Kalab, J. Agric. Food Chem. 45, 5 (1997)
C. Schmitt, C. Bovay, A.-M. Vuilliomenet, M. Rouvet, L. Bovetto, R. Barbar, C. Sanchez, Langmuir 25, 14 (2009)
I.G. Plaschina, E.E. Braudo, V.B. Tolstoguzov, Carbohydrate Res. 60, 1 (1978)
B.M. Yapo, P. Lerouge, J.-F. Thibault, M.-C. Ralet, Carbohydr. Polym. 69, 3 (2007)
G.A. Morris, M.-C. Ralet, E. Bonnin, J.-F. Thibault, S.E. Harding, Carbohydr. Polym. 82, 4 (2010)
C.M.G.C. Renard, M.-J. Crépeau, J.-F. Thibault, Eur. J. Biochem 266, 2 (1999)
A.R. Kirby, A.J. MacDougall, V.J. Morris, Carbohydr. Polym. 71, 4 (2008)
J. Leroux, V. Langendorff, G. Schick, V. Vaishnav, J. Mazoyer, Food Hydrocolloids 17, 4 (2003)
T.P. Kravtchenko, A.G.J. Voragen, W. Pilnik, Carbohydr. Polym. 18, 1 (1992)
M. Kazmierski, M. Corredig, Food Hydrocolloids 17, 5 (2003)
G.A. Morris, J.G. de al Torre, A. Ortega, J. Castile, A. Smith, S.E. Harding, Food Hydrocoll. 22, 8 (2008)
B.M. Yapo, Food Res. Int. 42, 8 (2009)
Acknowledgments
We would like to thank Herbstreith & Fox KG (Neuenbürg, Germany) as well as Fonterra GmbH (Hamburg, Germany) for generously providing us with polymer samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salminen, H., Weiss, J. Effect of Pectin Type on Association and pH Stability of Whey Protein—Pectin Complexes. Food Biophysics 9, 29–38 (2014). https://doi.org/10.1007/s11483-013-9314-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-013-9314-3