Abstract
The effect of the sodium chloride reduction from 2 to 1 % and its partial replacement with different level of potassium chloride and yeast extract on durum wheat (Triticum turgidum L subsp. durum Desf.) bread was evaluated through the study of the quality parameters and bread shelf life. Breads were packaged under modified atmosphere conditions and stored at 25 °C for as long as 120 days. No significant differences were observed among the bread samples on the specific volume, crumb porosity, crust thickness, loaf firmness and moisture content. The 5-hydroxymethylfurfural levels ranged in the bread samples, independently from the level of salts and the yeast extract added, from approximately 16 mg/kg to approximately 59 mg/kg of dry matter before storage, and decrease rapidly during storage. The yeast and mould counts showed values lower than 1 log cfu/g until 30 days of storage after a gradual increase was detected in the breads at low sodium chloride content. During storage, an increase of the total viable count in all bread samples was also recorded. The results of the sensory data have shown that the breads had similar evaluation and before storage differed in elasticity, moisture and salt content. After 90 days of storage, only the salt and moisture content differentiated the samples. In fact, moisture content decreased significantly (from about 38 to 23 %) in all bread samples with storage, independently from the salts and the yeast extract content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Durum wheat is used worldwide to produce pasta, couscous, bulgur, etc. And, in many countries of the Mediterranean regions and in Western Asia, the use of durum wheat in bread-making is an old practice. Italy is the main producer of durum wheat of the European Union, and the durum wheat bread is characterised by a yellowish colour, a typical taste and smell, a fine and uniform crumb structure and a more prolonged shelf life due to its greater resistance to the staling process (Quaglia 1988; Nachit 1998; Boyacioglu and D’Appolonia 1994a, b). Moreover, durum bread seems to be less toxic for those who suffer from intolerance to wheat gluten (celiac disease) (Troncone and Auricchio 1991; Silano et al. 2007).
In Sicily, approximately 50 % of the durum wheat produced is used in bread-making, and the characteristic flavour of durum wheat bread is enhanced by adding a high percentage of NaCl. Sodium chloride is an essential ingredient to control the functional properties of wheat dough (McCann and Day 2013) But, today, the dietary salt intake is considerably higher than the recommendations for good health. In the European population, the mean daily salt intake ranges from 8 to 11 g salt/day (3–5 g of sodium), but the recommended dietary requirement is approximately 3–4 g salt/day (EFSA 2006; Lynch et al. 2009). The major adverse effect of excess dietary sodium intake is etiologically related to hypertension and cardiovascular disease (He and MacGregor 2007).
The main source of sodium in the diet is from processed foods (approximately 70–75 % of the total intake) (EFSA 2006). Several initiatives, both in Europe and worldwide, have been undertaken for a reduction in salt intake (WHO Regional Office for Europe 2013). Recently, the Regulation (EC) No 1924/2006 on nutrition and health claims made on foods makes it possible to claims on a label “low sodium/salt”, “very low sodium/salt” and “sodium-free or salt-free” for foods containing low salt levels, according to the fixed limit. In 2007, the Ministry of Health in Italy launched the program ‘Gain in health’ with the aim of preventing obesity and an unfavourable dietary lifestyle. Within this framework, various bakery associations signed a 15 % reduction in the salt content of bread by the end of 2011 and further reductions in the following 2–3 years.
Cereal and cereal products, such as breads, breakfast cereals, biscuits and cake, contribute approximately 30–50 % of the overall salt intake (WHO 2007). The use of KCl, CaCl2 and MgSO4 to replace the NaCl in food products has been reported (Van Der Klaauw and Smith 1995). No difficulties in the baking performance were reported even when NaCl was completely replaced with KCl (Salovaara 1982a), but the replacement of NaCl with KCl higher than 30 % made foods unpalatable (Salovaara 1982b; Charlton et al. 2007). Another strategy is the addition of yeast extracts containing nucleotides that amplify the salty taste of NaCl (Bolhuis et al. 2011). To the best of our knowledge, no data were reported in literature on the effect of NaCl reduction on the durum wheat bread properties. Durum wheat (Triticum turgidum L. subsp. Durum Desf) is characterised by peculiar chemical, rheological and baking properties (Boyacioglu and D’Appolonia 1994a; 1994b; Liu et al. 1996).
The molecule 5-hydroxymethylfurfural (HMF) is used as a quality parameter in several processed foods (Rada-Mendoza et al. 2002, 2004; Arena et al. 2001; Fallico et al. 2004; Zappalà et al. 2005). In cereal and baking products, it makes possible to monitor the heating processes (Resmini et al. 1993; Ramírez-Jiménez et al. 2000a, 2003; Fernàndez-Artigas et al. 1999). Notwithstanding the fact that the HMF concentrations in foods can vary greatly, bread and coffee are the most important contributors to the dietary HMF intake (Murkovic and Pichler 2006). Moreover, HMF has been shown to be converted in vitro and in vivo into 5-sulfoxymethylfurfural, which has been reported to be mutagenic (Lee et al. 1995). To this point, the strategies to reduce the HMF level in bakery products have been to reduce the time/temperature profile, which causes a minor colour and taste modification (Ramirez Jemenz et al. 2000b; Capuano et al. 2008); increase the pH of the dough (Gökmen et al. 2007); change the sugar profile and the leavening agents of the recipe (Ait-Ameur et al. 2007; Gökmen et al. 2008; Kowalski et al. 2013); and use sodium metabisulfite (Erbas et al. 2012).
The aim of this work was the evaluation of the effect of the NaCl reduction and its partial replacement with KCl and yeast extract on durum wheat bread properties: specific volume, crumb porosity, crust thickness, loaf firmness, moisture, crust and crumb colour, pH, total titratable acidity, 5-hydroxymethylfurfural, microbiological assay and sensory evaluation. Moreover, the evolution both of the quality parameters and the sensory evaluation were studied during a long storage time.
Materials and Methods
Materials
Durum wheat semolina was provided by the ‘Valle del Dittaino’ Agricultural Cooperative Society a.r.l. (Assoro, Enna, Italy). The bread ingredients were food grade. The compressed yeast (Mauri Extra Classic) was obtained from ’AB Mauri Italy S.p.A., Pavia, Italy; the NaCl from ‘Mulino S. Giuseppe’, Catenanuova, Enna, Italy, and the KCl from Fluka. The yeast extract (Maxarome Pure Powder AGGL) was donated by DSM Food Specialties. The yeast extract contains a high level of 5’GMP + 5’IMP nucleotides (20 %) that provide a taste enhancement in the salt-reduced breads (DSM, product data sheet).
Physicochemical Analysis of the Semolina
Ash content was obtained following the ISO method 2171 (2007). Protein content (% dry matter) was determined with Infratec 1241 Grain Analyzer (Foss Tecator, Höganas, Sweden) by Near Infrared Transmittance, using a calibration (range 8.3–15.3) based on Kjeldahl nitrogen method (UNI EN ISO 20483). Calibration was validated in accordance with UNI EN ISO 12099 using different sets of test samples of durum wheat grain and semolina with linear correlation coefficient of r = 0.99. The gluten index was determined using a Glutomatic 2200 apparatus (Perten Instruments AB, Huddinge, Sweden) according to the method UNI 10690 (1979). The α-amylase activity was determined using the Falling Number 1500 apparatus (Perten Instruments AB, Huddinge, Sweden), following the method UNI EN ISO 3093 (2010). The yellow index was determined by a CR 200 Minolta colorimeter Chroma (Minolta, Osaka, Japan), with illuminant D65.
Rheological Dough Properties
A mixograph curve was obtained using the National Mfg. Co. (Nebraska, USA) standard, according to AACC method 54–40.02 (2000). The farinograph curves were obtained in a 300-g farinograph bowl (Brabender instrument, Duisburg, Germany) according AACC method 54–21.02 (2000). The alveograph trial was performed using an Alveograph MA 82 (Tripette et Renaud, Villeneuve-la-Garenne, France), following the standard method (UNI 10453 1995).
Bread Making Process and Storage Conditions
The breads were produced in an industrial bakery (Valle del Dittaino, Agricultural Cooperative Society a.r.l., Assoro, Enna, Italy). For each dough, 50 kg of durum wheat semolina was mixed with tap water (66 %), compressed yeast (0.5 %) and NaCl for 15 min using a mixer with beater arms (IBT 300, Pietro Berto S.p.A, Marano Vicentino, Vicenza, Italy). In the control dough, NaCl was 2.0 %, whereas in the other four doughs, NaCl was set at 1.0 % and variable amounts (expressed on the weight of durum wheat semolina) of KCl (0.5, 0.3, 0.2 %) and/or yeast extract (0.5, 0.3, 0.2 %) were added (Table 1). The tap water temperature was 16 °C, and at the end of the mixing, the dough temperature was 26.7 ± 0.5 °C. Afterward, the dough was broken into pieces of approximately 1,080 g using volumetric dividing, obtaining for each dough about 100 loaves, (Omega, Pietro Berto S.p.A, Marano Vicentino, Vicenza, Italy). The loaves were rounded in a conical rounder (CO 3000, 1500 pz/h, Turri F.lli s.r.l., Costa di Rovigo, Rovigo, Italy) and leavened in a tray proofing cell (Pavailler Engineering S.R.L., Galliate, Novara, Italy) for 2 h and 45 min at 31 °C, with an relative humidity (RH) of approximately 65 %. The loaves were baked for 1 h at 210 °C in a continuous oven (Pavailler Engineering S.R.L., Galliate, Novara, Italy). After 2 h of cooling in a conditioned room at 20 °C with an RH of 60 %, the breads were packaged under a modified atmosphere in T6011B (275 μm thickness) (Sealed Air, Cryovac, Italy), and a film T9250B (125 μm thickness) (Sealed Air, Cryovac, Italy) was used to cover and seal the package. The containers were vacuumised, filled with mixed gas and sealed with a Multivac R-230 packaging machine (Germany) equipped with a modified atmosphere (MAP) mix 9000 gas-mixer (PBI–Dansensor A/S, Ringsted, Denmark).
The trays were packed using 70 % N2/30 % CO2 gas combinations. All packaged bread samples were stored at 25 ± 2 °C and 60 ± 2 % RH for 4 months and periodically (0–30–60–90–120 days of storage) sampled. Nine loaves for each formulation were withdrawn each month and immediately analysed. The CO2 and O2 concentrations in the samples were measured using the gas-analysing equipment Check Point, Dansensor (PBI, Ringsted, Denmark).
The following properties were tested for each bread sample during each sampling event: volume, height, weight, crumb porosity, internal structure, loaf firmness, crust and crumb colour, moisture, pH, acidity, HMF content, microbiological analysis and sensory evaluation.
Bread Quality Evaluation
Determination of the Physical Properties of Breads
The volume was determined according to the rapeseed displacement in a loaf volume meter, and the specific volume (cubic meters per gram) was calculated as a ratio of the loaf volume and the bread weight. The internal structure was visually estimated, and the crumb porosity was estimated using the Mohs scale. Loaf crust thickness was measured using a digital caliper (Digi-Max™, Scienceware®, NJ, U.S.A.), on the loaf basis of three central slices, after removing the crumb. The loaf firmness was measured using a texture analyser (Zwick Z 0.5 Roëll, Germany) equipped with an aluminium 8-mm-diameter cylindrical probe. The resulting peak force was measured in kilograms per cubic centimeter.
The CIE L*a*b* colour parameters were measured for the crumb, in the transversely cut bread and on the crust surface, averaging ten distinct points in each case, using a Chroma Meter (CR-200, Konica Minolta, Osaka, Japan) with illuminant D65.
The moisture content was determined in triplicate by gravimetric analysis. The bread samples were ground in a home grinder (La Moulinette, Moulinex, 2002), and then portions of the ground bread sample were placed in an oven at 105 °C until the dry weight was constant.
Determination of the pH and the Total Titratable Acidity
The pH and the total titratable acidity (TTA) were measured according to Lefebvre et al. (2002) using a pHmeter (Mettler Toledo, MP 220). The TTA results were expressed, for the dry matter, as milliliters of 0.1 M NaOH consumed.
HMF Extraction and HPLC Analysis
The bread samples were ground in a home grinder (La Moulinette, Moulinex, 2002); subsequently, an aliquot of the ground sample (5 g) was transferred into a volumetric flask (50 ml), and 25 ml of water was added (JT. Baker, Deventer, Holland). The solution was stirred for 10 min, and then the sample was diluted up to 50 ml with water (JT. Baker, Deventer, Holland) and centrifuged for 45 min at 5,000 rpm. An aliquot of the supernatant was filtered through a 0.45-μm filter (Albet) and injected into an HPLC system (Shimadzu Class VP LC-10ADvp) equipped with a DAD (Shimadzu SPD-M10Avp). The column was a Gemini NX C18 (150 mm × 4.6 mm, 5 μm) (Phenomenex), fitted with a guard cartridge packed with the same stationary phase. The HPLC conditions were according to Fallico et al. (2004). All of the solvents used were HPLC purity grade: water from J.T. Baker, and acetic acid and methanol from Merck. The wavelength range was 220–660 nm, and the chromatograms were monitored at 283 nm.
HMF was identified by splitting the peak of the HMF from the bread-solution sample with a standard of HMF (P > 98 % Sigma-Aldrich, St. Louis, Mo., U.S.A.) and by comparison of the UV spectra of the HMF standard with that of the bread samples. All analyses were performer in duplicate, including the extraction procedure, and the reported HMF concentration is therefore the average of four values. The results were expressed as mg of HMF per kilogram of the bread dry matter.
Microbiological Analysis
The microbiological analysis of the bread samples was performed in triplicate at 0, 30, 60, 90 and 120 days of storage. Ten grams of each loaf were aseptically weighed and homogenized using a Stomacher (Brinkmann, Westbury, NY, USA) for 5 min and were serially diluted in a sterile physiological solution (0.9 % NaCl). Serial dilutions of the suspension were pour-plated in duplicate onto the following media: plate count agar (PCA) (Oxoid) for total viable count; violet red bile glucose agar (VRBGA) (Oxoid) for Enterobacteriaceae; and Saboraud dextrose agar for yeasts and moulds. The VRBGA plates were incubated at 32 °C for 48 h under aerobic conditions. The PCA plates were incubated at 30 °C for 4 days, and the yeast-count plates were incubated at 25 °C for 4 days.
Statistical Analysis
The statistical analysis of the results was performed using the STATSOFT 6.0 program (Vigonza, Padova, Italy). The significant differences were evaluated by variance analysis (ANOVA), and the mean separation was determined using the Tukey’s test. Significant differences were determined at the alpha level of 0.01 and 0.05. The data reported were expressed as the means ± standard deviation.
Sensory Evaluation
The sensory profile was determined according to Lanza et al. (2011) and was defined by a trained panel of 12 judges (six females and six males) submitted to further training over 4 weeks to generate attributes using handmade and industrial breads and to familiarise themselves with the scales and procedures (UNI EN ISO 8586 2008).
The judges, using a scale between 1 (absence of the sensation) and 9 (extremely intense), have evaluated the intensity of the 19 sensory attributes selected on the basis of frequency (%), following the definitions given by Raffo et al. (2003) (Table 2). The evaluation sessions, performed at 0, 30, 60, 90, and 120 days of storage, were conducted in a sensory laboratory (UNI EN ISO 8589, 2010) from 11:00 am to 12:00 am in individual booths illuminated with white light. The loaves of bread, preliminarily cut into slices, were served on plates, coded with three-digit numbers, and water was provided to judges for rinsing between samples. All data were acquired by a direct computerised registration system (FIZZ Byosistemes. ver. 2.00 M, Couternon, France).
The sensory data for each attribute were submitted to one-way ANOVA by the software package Statgraphics® Centurion XVI (Statpoint Technologies, INC.) using samples as factors. The significance was tested by means of the F-test. To differentiate the samples, the mean values were submitted to the multiple comparison test using the least significant difference (LSD) procedure.
Results and Discussion
Characterisation of the Durum Wheat Semolina
Table 3 reports the qualitative and rheological characteristics of the durum wheat semolina. Similar findings were reported in literature (Boyacioglu and D’Appolonia 1994a; Caballero et al. 2007; Giannone et al. 2010). The semolina protein, ash and moisture contents were within the Italian legal limits (D.P.R. 187 (2001)) for durum wheat flours. For the gluten index, the semolina sample exhibited good quality. The falling index showed that the semolina has a reduced activity of α-amylase due to frequent drought conditions that occur in Sicily during the period of the ripening grain, which contributes to improving the basic amylase.
The yellow index showed a proper value ensuring an adequate crumb colour, which is a positive index for durum wheat bread.
As regards the mixograph curve, the semolina sample showed a high mixing time. The peak dough height and the classification scale were in line with data reported in literature for semolina (Boyacioglu and D’Appolonia 1994a).
As for the farinograph analysis, the values obtained both for development time and dough stability parameters were strongly correlated with a good dough resistance to the fermentation and were similar to those reported in literature for semolina (Palumbo et al. 2002; Spina et al. 2003). The water absorption determined indicates that the dough, compared with the values normally found for durum wheat, has a medium ability to absorb water, which is closely related to the protein content, to the size of particles and to the percentage of damaged starch.
The alveograph trial showed a sufficient dough strength (177.50 × 10−4 joule), associated with a high relationship between the tenacity and the extensibility (2.86), values frequently reported in durum wheat by multiple authors (Boyacioglu and D’Appolonia 1994a; Palumbo et al. 2002; Spina et al. 2003; Sissons 2008). Overall, the chemical and rheological parameters studied correspond to a semolina suitable for the bread-making process.
Quality Parameters of the Industrial Bread and the Evolution During Storage
Table 4 reports the physical properties of the industrial breads during 120 days of storage. Regarding the specific volume, no significant differences were recorded among the bread samples and during the storage time, independently from the level of salts and yeast extract added. These findings are in agreement with those reported by Lynch et al. (2009). The ratio between the height (h) and the diameter (d) of the loaves is used in the baking industry to parameterise any failure of the dough. The control was found to have the highest h/d ratio (approximately 3.0) due to the salt concentration in the recipe that leads to a high dough tenacity (McCann and Day 2013). All of the other bread samples, as expected, showed a lower ratio. Regarding the crumb porosity, the control and bread sample 2 showed a less developed crumb porosity (value 7) during the entire storage period, whereas bread sample 3 showed a porosity with larger alveo (value 5). Regarding the internal structure, the bread samples with the lowest total salt content and the control bread had an irregular structure. The sample loaves 1 and 3 exhibited, at the beginning, the lowest crust thickness, measured at the loaf basis (approximately 3 mm). The other samples exhibited a higher crust thickness (greater than 4 mm). After 60 days of storage, no remarkable differences were recorded among these, and the values decreased up to 3.0–3.5 mm. The storage time did not significantly affect the bread firmness. The differences among the samples were not statistically significant. The modified atmosphere condition preserve the initial characteristics of the breads, while is reported that during the staling process in bread stored in a paper box at room temperature, the firmness increases significantly in 7 days (Lynch et al. 2009; Giannone et al. 2010).
Table 5 reports the moisture content, the pH, the acidity and the HMF content of the bread samples. At the beginning, the moisture content was approximately 38 % in all of the bread samples. No significant differences were found in the moisture content between the control bread and the other bread samples, indicating that the NaCl replacement did not modify the capacity to retain water from the dough. The moisture content remains almost constant up to 60 days of storage in all bread samples. At 90 days of storage, the moisture content decreases up to approximately 24 % in all bread samples independently from the salts and the yeast-extract content; this level was maintained up to 120 days of storage.
The effects of NaCl replacement with various levels of KCl and yeast extract on the pH and the titratable acidity seems to be more linked to the storage time rather than the recipe. After 90 days of storage, in some bread samples, a slight reduction in the pH and the titratable acidity was detected (Table 5). The various NaCl replacements did not influence these parameters according to Salovaara et al. (1982a), even after prolonged storage.
The HMF levels ranged from approximately 16 mg/kg to approximately 59 mg/kg of dry matter, in the bread samples prior to storage (Table 5), but it is difficult to attribute these differences in the HMF levels between samples to the NaCl replacement (Table 1).
In fact, the high level of NaCl induced a higher starch degradation with an increased availability of small molecules to the Maillard reaction and limited the yeast growth, reducing the sugar consumption, resulting in both cases in higher levels of Maillard indicators (Moreau et al. 2011a).
However, our data did not support these findings. At time 0, the control bread had an HMF level of approximately 39.7 mg/kg, lower than that of the other breads samples, with the exception of sample 3. Sample 3 had an NaCl content that was half that of the control bread, a KCl content similar to that of sample 2 and the same yeast percentage as sample 4YE, but the HMF level was dissimilar from that of the others. Moreover, no significant differences in the HMF levels were found between samples 1, 2 and 4YE. Ramírez-Jiménez et al. (2000a) report different HMF levels (3.4–15.7 mg/kg) in bread with the same recipe and similar manufacturing conditions. Factors such as small differences in the water content, in the leavening or baking time and the ratio between the crumb and the crust of the bread could influence the HMF content. In our bread samples, no significant differences were found in the moisture content (Table 5), and the manufacturing conditions were identical for all bread samples. The ratio between the crumb and the crust ranged from 2.6 in the control bread and 2.3, 2.4, 2.7 and 2.4 in bread samples 1, 2, 3 and 4YE, respectively. This probably could explain the lowest HMF levels in the control bread and in bread sample 3.
Storage causes significant differences in the HMF levels in all bread samples. During storage, HMF decreases rapidly, and after 120 days of storage, its levels range from approximately 2 to 25 mg/kg of dry matter and, in sample 2, was not detected at all. The highest HMF degradation processes were observed in breads in which NaCl was replaced with KCl and yeast extract. In fact, the control bread had a generally higher level of HMF with respect to the other bread samples, and at the end of storage, its HMF content ranged from approximately 5 to 25 times higher than those of the other bread samples. Moreover, in the control bread and less evidently in sample 1, the trend of decreasing HMF was not regular.
Probably, the effect of NaCl on the starch degradation and the yeast growth causes a major increase in the availability of the molecules that can react during storage to form HMF. In fact, the HMF level during storage is the macroscopic result of formation and degradation reactions (Fallico et al. 2009), and the final HMF is the result of the equilibrium between the formation and the degradation pathways, depending on the pH, the temperature and the presence and concentration of analytes (Belitz et al. 2004). Further studies are required in order to clarify the mechanisms involved in the HMF accumulation.
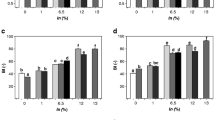
Table 6 reports the colour parameters of the crust and the crumb of bread samples and their trends during storage. At time 0, the control bread and sample 4YE present the lowest L* crust values, whereas the other bread samples showed the highest L* values. The addition of KCl induced a slight increase of samples lightness (L*). A proportional colour formation to the amount of NaCl in the model system containing ‘wx’ maize starch and distilled water was reported, and a slight effect on the browning of KCl with respect to NaCl was highlighted (Moreau et al. 2011a, b). No significant differences were found for parameters a* and b* between the crust of bread samples. The lightness of the control bread remains almost constant during storage. In samples 1 and 2, a decrease in the lightness value was detected at 120 and 90 days of storage, respectively. In the samples 4YE after a prolonged storage, an increase of the L* value was observed (Table 6).
No linear correlation was found between HMF and 100 − L* (r 2 = 0.236), a parameter considered to be the browning index (Resmini et al. 1993; Fernàndez-Artigas et al. 1999). Ramírez-Jiménez et al. (2000b), found a linear correlation (r 2) between 100− L*and HMF above 0.70 for common, special and snack breads and in a dough baked at various times. They also found that products with the same colours can have different HMF values, suggesting that HMF may be a better indicator than the colour.
The effects of NaCl replacement with KCl and yeast extract on the colour parameters of crumbs during 30 days of storage were insignificant. Afterward, several differences in the L*, a*, and b* parameters were found, generally associated with the storage time rather than the different recipe (Table 6).
Microbiological Assay
The values of CO2 and O2 in the samples packaged in ATM remained unvaried for the entire storage period (data not shown).
No mould growth was visible in the well-sealed packages. If the O2% in the headspace is lower than 1 %, no mould growth is possible, and the antimicrobial effect of N2 is complete (Rodríguez et al. 2000). The microbial dynamics of the different groups throughout the storage period are shown in Table 7. Results of ANOVA analysis, performed on samples, are shown in the same table. The yeast and mould counts exhibited values lower than 1 log10 cfu/g for all of the bread samples until 30 days of storage, with the exception of sample 2 (Table 7). Between 30 and 60 days of storage, a gradual increase of yeast and mould counts was detected in samples 1, 2 and 3 whereas, in the sample 4YE, an increase was detected between 60 and 90 days of storage. In control bread, the yeast and mould counts remain about to 0 log10 cfu/g during all storage time, due to the highest NaCl content. At 120 days of storage samples 1, 2, 3 and 4YE reached the highest yeast and mould counts.
A different trend was highlight for the total viable count (Table 7). At the beginning, the total viable count was low in all bread samples (0.10–0.50 log cfu/g), and during storage, a steady increase of the total viable count was recorded (Table 7). Between 30 and 60 days of storage in all samples, there was a significant increase of the total viable count. At the end of storage, the highest total viable count was reached.
Sample at 2.0 % of NaCl (control bread) had an inhibition effect for yeast growth while its effectiveness was lower for bacteria proliferation.
The antimicrobial effectiveness of NaCl and KCl has been studied by Bidlas and Lambert (2008), and the partial replacement has not displayed affected microbial growth of pathogenic bacteria such as Enterobacter and Staphylococcus.
The MAP condition resulted in good loaf conservation for the entire period, with standard TVC and yeast counts, equivalent to those in the studies of Dergirmencioglu et al. (2011), in which 0.15 and 0.30 % of potassium sorbate was used for its antimicrobial activity at the same CO2/N2 ratio.
Sensory Evaluation
Tables 8 and 9 report sensory attributes which significantly differentiated, at each sampling for bread formulation or during storage, respectively. The intensity (mean score) was reported only for the significantly different attributes.
At time 0, bread samples were evaluated similar by panelists, with the exception of the following attributes: elasticity, humidity and saltiness. Obviously, the control bread had the highest saltiness score and elasticity, whereas the bread samples with the highest level of yeast extract (3 and 4YE) had the lowest elasticity scores. It is well known the role of NaCl on the interaction between the glutenin protein chain and consequently on the elasticity of the dough (Lynch et al. 2009). At 30 days of storage, no significant differences were found between the bread samples. At 60 days of storage, the control had the highest intensity of the elasticity, humidity, saltiness and softness. Additionally, at 60 days of storage, the bread samples with the highest yeast extract level had the lowest intensity of elasticity and softness attributes. At 120 days, the control and sample 1 showed a high intensity of humidity, whereas bread samples 3 and 4YE had the lowest intensity for humidity. Notwithstanding these differences, the overall evaluation of the breads at low levels of NaCl were similar, at each sampling, to that of the control bread.
During the storage period, four attribute change in bread samples as reported in Table 9. All bread samples show significantly decrease, during the 120 days of storage, of elasticity and softness, generally associated to the related reduction of the moisture content (Table 5). The samples 1, 2 and 4YE show significant decrease also, for the attribute humidity. The bread samples, during storage, did not develop off-odour and off-flavour, and the attributes such as the aroma/flavour of bread and the aroma/flavour of wheat (characteristic of the bread) were not changed. Obviously, the overall assessment decreases during the long storage period.
Conclusion
The results of this study showed that the reduction of the NaCl from 2 to 1 % and its partial replacement with KCl and/or yeast extract in durum wheat bread is a possible strategy for the reduction of salt intake. Durum wheat bread at low salt level had generally similar properties to bread with the highest NaCl content. HMF shows a peculiar trend not related to salt content. For this purpose, further studies needed to explain the scientific mechanisms involved in the HMF formation through baking.
During the long storage time in MAP conditions, the trend of the main bread quality parameters, both physicochemical and sensorial, did not recorded differences attributable to the different levels of salts and yeast extract added, but generally the differences found should be associated to storage time. These results should be interesting for the possibility to produce a low salt bread with a long shelf-life. The bread was microbiologically acceptable for the entire storage period, evidencing the importance of the packaging conditions.
Finally, the bread samples at low NaCl contents were acceptable when considering the main sensorial attributes. This was one of the goals of our work, considering the increasing interest on durum wheat bread from consumers and the guidelines for a healthy diet.
References
AACC International. Approved Methods of Analysis, 11th Ed. Method 54–21.02. Rheological behaviour of flour by farinograph: constant flour weight procedure. January 6, 2011. AACC International, St. Paul, MN, U.S.A. http://dx.doi.org/10.1094/AACCIntMethod-54-21.02.
AACC. International. Approved Methods of Analysis, 11th Ed. Method 54–40.02. Mixograph method. Approved November 3, 1999. AACC International, St. Paul, MN, U.S.A. http://dx.doi.org/10.1094/AACCIntMethod-54-40.02.
Ait-Ameur, L., Mathieu, O., Lalanne, V., Trystram, G., & Birlouez-Aragon, I. (2007). Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures. Food Chemistry, 101(4), 1407–1416.
Arena, E., Fallico, B., & Maccarone, E. (2001). Thermal damage in blood orange juice: kinetics of 5-hydroxymethyl-2-furancarboxaldehyde formation. International Journal of Food Science & Technology, 36, 145–151.
Belitz, H. B., Grosch, W., & Schieberle, P. (2004). Food chemistry (3rd ed., pp. 258–282). Berlin: Springer.
Bidlas, E., & Lambert, R. J. W. (2008). Comparing the antimicrobial effectiveness of NaCl and KCl with a view to salt/sodium replacement. International Journal of Food Microbiology, 124, 98–102.
Bolhuis, D. P., Temme, E. H. M., Koeman, F. T., Noort, M. W. J., Kremer, S., & Janssen, A. M. (2011). A salt reduction of 50% in bread does not decrease bread consumption or increase sodium intake by the choice of sandwich fillings. The Journal of Nutrition, 141, 2249–2255.
Boyacioglu, M. H., & D’Appolonia, B. L. (1994a). Characterization and utilization of durum wheat for breadmaking. I. Comparison of chemical, rheological and baking properties between bread wheat flours and durum wheat flours. Cereal Chemistry, 71(1), 21–28.
Boyacioglu, M. H., & D’Appolonia, B. L. (1994b). Characterization and utilization of durum wheat for breadmaking. III. Staling properties of bread baked from bread wheat flours and durum wheat flours. Cereal Chemistry, 71(1), 34–41.
Caballero, P. A., Gómez, M., & Rosell, C. M. (2007). Improvement of dough rheology, bread quality and bread shelf-life by enzymes combination. Journal of Food Engineering, 81(1), 42–53.
Capuano, E., Ferrigno, A., Acampa, I., Ait-Ameur, L., & Fogliano, V. (2008). Characterization of Maillard reaction in bread crisps. European Food Research and Technology, 228, 311–319.
Charlton, K. E., MacGregor, E., Vorster, N. H., Levitt, N. S., & Steyn, K. (2007). Partial replacement of NaCl can be achieved with potassium, magnesium and calcium salts in brown bread. International Journal of Food Science and Nutrition, 58(7), 508–521.
D.P.R. 187 (2001). Regulation for the revision of the laws on the production and marketing of flours and pasta.
Degirmencioglu, N., Göcmen, D., Inkaya, A. N., Aydin, E., Guldas, M., & Gonenc, S. (2011). Influence of modified atmosphere packaging and potassium sorbate on microbiological characteristics of sliced bread. Journal of Food Science and Technology, 48, 236–241.
EFSA (2006). Tolerable upper intake levels for vitamins and minerals. Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and Allergies 429–446 pp.
Erbas, M., Sekerci, H., Arslan, S., & Durak, N. (2012). Effect of sodium metabisulfite addition and baking temperature on Maillard reaction in bread. Journal of Food Quality, 35, 144–151.
Fallico, B., Zappalà, M., Arena, E., & Verzera, A. (2004). Effect of conditioning on HMF content in unifloral honeys. Food Chemistry, 85, 305–313.
Fallico, B., Arena, E., & Zappalà, M. (2009). Prediction of honey shelf life. Journal of Food Quality, 32, 352–368.
Fernàndez-Artigas, P., Guerra-Hernàndez, E., & Garcìa-Villanova, B. (1999). Browning indicators in model systems and baby cereals. Journal of Agricultural and Food Chemistry, 47, 2872–2878.
Giannone, V., Longo, C., Damigella, A., Raspagliesi, D., Spina, A., & Palumbo, M. (2010). Technological properties of bakers’ yeasts in durum wheat semolina dough. Journal Industrial Microbiology and Biotechnology, 37, 371–379.
Gökmen, V., Açar, Ö. C., Köksel, H., & Açar, J. (2007). Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies. Food Chemistry, 104, 1136–1142.
Gökmen, V., Açar, Ö. C., Serpen, A., & Morales, F. J. (2008). Effect of leavening agents and sugars on the formation of hydroxymethylfurfural in cookies during baking. European Food Research and Technology, 226, 1031–1037.
He, F. J., & MacGregor, G. A. (2007). Dietary salt, high blood pressure and other harmful effects on health. In D. Kilcast & F. Angus (Eds.), Reducing salt in foods: practical strategies (pp. 18–54). Cambridge: CRC Press, Woodhead Publishing Limited.
Kowalski, S., Lukasiewicz, M., Juszczak, L., & Kutyła-Kupidura, E. M. (2013). Dynamics of 5-hydroxymethylfurfural formation in shortbreads during thermal processing. Czech Journal of Food Sciences, 31(1), 33–42.
Lanza, C. M., Mazzaglia, A., Scacco, A., & Pecorino, B. (2011). Changes in sensory and instrumental features of industrial Sicilian bread during storage. Italian Journal of Food Science, 23, 6–12.
Lee, Y. C., Shlyankevich, M., Jeong, H. K., Douglas, J. S., & Surh, Y. J. (1995). Bioactivation of 5-hydroxymethyl-2-furaldehyde to an electrophilic and mutagenic allylicsulfuric acid ester. Biochemical and Biophysical Research Communications, 209, 996–1002.
Lefebvre, D., Gabriel, V., Vayssier, Y., & Fontagne’-Faucher, C. (2002). Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. Lebensm.-Wiss. u.-Technology, 35, 407–414.
Liu, C. Y., Shepherd, K. W., & Rathjen, A. J. (1996). Improvement of durum wheat pastamaking and breadmaking qualities. Cereal Chemistry, 73, 155–166.
Lynch, E. J., Dal Bello, F., Sheehan, E. M., Cashman, K. D., & Arendt, E. K. (2009). Fundamental studies on the reduction of salt on dough and bread characteristics. Food Research International, 42, 885–891.
McCann, T. H., & Day, L. (2013). Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking. Journal of Cereal Science, 57, 444–452.
Moreau, L., Bindzus, W., & Hill, S. (2011a). Influence of salts on starch degradation: part I—sodium chloride. Starch-Starke, 63(11), 669–675.
Moreau, L., Bindzus, W., & Hill, S. (2011b). Influence of salts on starch degradation: part II—salt classification and caramelisation. Starch-Starke, 63(11), 676–682.
Murkovic, M., & Pichler, N. (2006). Analysis of 5-hydroxymethylfurfural in coffee, dried fruits and urine. Molecular Nutrition and Food Research, 50, 842–846.
Nachit, M.M., (1998). Durum breeding research to improve dry-land productivityin the Mediterranean region. In: Nachit, M.M., et al. (Eds.), SEWANADurum Research Network, ICARDA editions:1–15.
Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods.
Palumbo, M., Spina, A., & Boggini, G. (2002). Bread-making quality of Italian durum wheat (Triticum durum Desf.) cultivars. Italian Journal of Food Science, 14(2), 123–133.
Quaglia, G. B. (1988). Other durum wheat products. In G. Fabriani & C. Lintas (Eds.), Durum wheat chemistry and technology (pp. 263–282). St. Paul: American Association of Cereal Chemists.
Rada-Mendoza, M., Olano, A., & Villamiel, M. (2002). Determination of hydroxymethylfurfural in commercial jams and in fruit-based infant foods. Food Chemistry, 79, 513–516.
Rada-Mendoza, M., LuzSanz, M., Olano, A., & Villamiel, M. (2004). Formation of hydroxymethylfurfural and furosine during the storage of jams and fruit-based infant foods. Food Chemistry, 85, 605–609.
Raffo, A., Pasqualone, A., Sinesio, F., Paoletti, F., Quaglia, G., & Simeone, R. (2003). Influence of durum wheat cultivar on the sensory profile and staling rate of Altamura bread. European Food Research and Technology, 218, 49–55.
Ramírez-Jiménez, A., García-Villanova, B., & Guerra-Hernández, E. (2000). Hydroxymethylfurfural and methylfurfural content of selected bakery products. Food Research International, 33, 833–838.
Ramirez-Jiménez, A., Guerra-Hernàndez, E., & Garcìa-Villanova, B. (2000). Browning indicators in bread. Journal of Agricultural and Food Chemistry, 48, 4176–4181.
Ramírez-Jiménez, A., Guerra-Hernández, E., & García-Villanova, B. (2003). Evolution of non enzymatic browning during storage of infant rice cereal. Food Chemistry, 3, 219–225.
Resmini, P., Pellegrino, L., Pagani, M. A., & De Noni, I. (1993). Formation of 2-acetyl-3-d-glucopyranosylfuran (glucosylisomaltol) from nonenzymatic browning in pasta drying. Italian Journal of Food Science, 5(4), 341–353.
Rodríguez, M., Medina, L. M., & Jordano, R. (2000). Effect of modified atmosphere packaging on the shelf life of sliced wheat flour bread. Nahrung, 44, 247–252.
Salovaara, H. (1982a). Effect of partial sodium chloride replacement by other salts on wheat dough rheology and breadmaking. Cereal Chemistry, 59(5), 422–426.
Salovaara, H. (1982b). Sensory limitations to replacement of sodium with potassium and magnesium in bread. Cereal Chemistry, 59(5), 427–430.
Silano, M., Di Benedetto, R., Trecca, A., Arrabito, G., Leonardi, F., & De Vincenzi, M. (2007). A decapetide from durum wheat prevents celiac peripheral blood lymphocytes from activation by gliadin peptides. Pediatric Research, 61, 67–71.
Sissons, M. (2008). Role of durum wheat composition on the quality of pasta and bread. Food, 2(2), 75–90.
Spina, A., Palumbo, M., Boggini, G. (2003). Bread-making quality of new durum wheat cultivars. Proceedings of the Tenth International Wheat Genetics Symposium, 1394–1396.
Troncone, R., & Auricchio, S. (1991). Gluten-sensitive enterophathy (celiac disease). Food Reviews. International, 7, 205-.
Van Der Klaauw, N. J., & Smith, D. V. (1995). Taste quality profiles for fifteen organic and inorganic salts. Physiology & Behavior, 58, 295–306.
WHO Regional Office for Europe (2013). Mapping salt reduction initiatives in the WHO European Region. ISBN 978 92 890 0017 8.
World Health Organization. (2007). Reducing salt intake in populations. Report of a WHO forum and technical meeting, 5–7 October 2006. Paris, France.
Zappalà, M., Fallico, B., Arena, E., & Verzera, A. (2005). Methods for the determination of HMF in honey: a comparison. Food Control, 16(3), 273–277.
Acknowledgements
This work was financially supported by the Regional Operating Programme of REGIONE SICILIANA ‘PO FESR 2007–2013 Avviso 2011—Asse IV, Obiettivo 4.1.1, Linea di Intervento 4.1.1.2’ with the title of the research program ‘Impiego e valutazione di fibre e sostanze nutraceutiche per l’ottenimento di prodotti da forno salutistici’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spina, A., Brighina, S., Muccilli, S. et al. Partial Replacement of NaCl in Bread from Durum Wheat (Triticum turgidum L subsp. durum Desf.) with KCl and Yeast Extract: Evaluation of Quality Parameters During Long Storage. Food Bioprocess Technol 8, 1089–1101 (2015). https://doi.org/10.1007/s11947-015-1476-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1476-1