Abstract
The aim of this study was to evaluate the effect of high-pressure processing (HPP) on the quality of fresh sea bass fillets using two pressure levels (250 and 400 MPa; 5 min pressure holding time). Vacuum-packed fillets were used as control samples, and all fillets were stored under refrigerated conditions for 18 days. The microbiological, chemical, physical, and sensory parameters were followed. Both HPP treatments increased the microbiological shelf life of sea bass fillets. In day 14, control samples reached the upper acceptability limit (7 log colony-forming units (CFU) g−1), while fillets treated at 250 and 400 MPa had 3.2 and 1.4 log CFU g−1, respectively. In general, hydrogen sulphide-producing bacteria and Enterobacteriaceae loads were below the detection limit in HPP treatments. Results from nucleotide analysis indicate that HPP treatments reduced the conversion of inosine 5′-monophosphate to inosine. HPP also influenced fillet sensory characteristics. The most evident changes in fillets were the increase in whiteness, the loss of translucency, and a firmer consistency. The effect was more pronounced in the treatment at 400 MPa. Lipid oxidation increased in HPP-treated samples, being more accentuated in the treatment at 400 MPa. Instrumental smell intensity increased in both HPP treatments, though the sensory panel did not detect any rancid or other unpleasant odours. No effect was observed in the amount of volatile bases or in pH values. In conclusion, HPP treatments showed potential application for new fish product development with increased microbiological safety and shelf life, longer freshness, and unique characteristics (e.g. firmer and whitish).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increase in the consumer's preference for fresh and minimally processed food products, rather than processed and frozen ones, demands for research on new processing and preservation methods, especially for highly perishable products such as fish. Fish freshness is rapidly lost during post-mortem phase due to autolytic degradation by the action of endogenous enzymes, leading to prime quality loss (Nielsen and Nielsen 2006). This creates ideal conditions for the growth of microorganisms responsible for spoilage (Chéret et al. 2005; Oehlenschläger and Rehbein 2009).
High-pressure processing (HPP) is a technology of growing interest for the processing and preservation of food. This technology has the potential to better retain the food's nutritional and organoleptic characteristics, compared with traditional thermal processing (Patterson et al. 2007). HPP can also be used by the food industry to create new product textures since it induces modifications on food functional properties (Chapleau & Lamballerie-Anton 2003). HPP treatments have also the ability to inactivate spoilage and pathogenic microorganisms, thus extending food shelf life (Patterson et al. 2007).
Several studies with different HPP conditions have been conducted in seafood, including red mullet (Erkan et al. 2010), squid (Gou et al. 2010), gilthead sea bream (Erkan and Üretener 2010), and salmon (Yagiz et al. 2009). Reported results are not always in agreement due to differences in conditions: HPP (e.g. pressure level and holding time), species under study, packaging, and storage. In general, microbiological shelf life increased in HPP treatments, while the effect of HPP in other quality parameters varied between studies.
Sea bass Dicentrarchus labrax (Linnaeus, 1758) is a teleost species widely distributed in the Mediterranean Sea and Atlantic Ocean and intensively farmed in several Mediterranean countries, e.g. Greece, Spain, and Italy, being highly appreciated due to its excellent organoleptic properties and reasonable price (Ayala et al. 2005). In retail stores, sea bass is most commonly sold as whole fish and stored in ice, presenting a shelf life of 12–13 days (Taliadourou et al. 2003). Still, according to FAO, fish losses caused by spoilage account for around 10 % of the total production from capture fisheries and aquaculture (FAO 2005–2012). In this sense, different attempts have been done to extend sea bass shelf life (Mendes and Gonçalves 2008a, 2008b; Özden et al. 2007).
The aim of this work was to study the effect of HPP treatments on the quality and shelf life of fresh sea bass fillets vacuum-packed and stored under refrigerated conditions. In particular, two pressure levels (250 and 400 MPa; 5 min) were selected based on previous experiments with sea bass fillets (Teixeira et al. 2013). HPP-treated samples were compared with non-treated samples to evaluate their influence on microbiological, chemical, physical, and sensory parameters.
Materials and Methods
Preparation of Samples and Treatments
Fresh farmed sea bass D. labrax (Linnaeus, 1758) specimens were acquired from a local aquaculture farm (maximum post-mortem time, 24 h). The average weight of each fish was 434 ± 78 g, and the total length was 33 ± 2 cm. Fish individuals (n = 27) were filleted, and the skin was removed. All fillets (including those of control treatment) were vacuum-packed individually in low-oxygen permeable barrier bags (Colamin XX 100e, Obermühle, Pössneck, Germany) with a vacuum packager (Packman, Albipack, Águeda, Portugal) before pressurization treatments.
HPP treatments were carried out in a hydrostatic press (high-pressure system U33, Unipress Equipment, Poland). This equipment has a pressure vessel of 35-mm diameter and 100-mm height surrounded by an external jacket, connected to a thermostatic bath to control the temperature (6 °C), using a mixture of propylene glycol and water (1:1) as pressurizing fluid. Fillets were processed individually at pressure levels of 250 and 400 MPa (1 MPa ≈ 145 psi) for 5 min using a pressurization rate of ca. 8 MPa s−1 and a depressurization time of <20 s. Control samples (0.1 MPa) were also included in the study. After treatments, samples (vacuum-packed) were stored under refrigeration (1.9 ± 0.3 °C) for 18 days. During storage, three packages from each treatment (0.1, 250, and 400 MPa) were taken for microbiological, chemical, physical, and sensory analyses at predetermined intervals: 1, 4, 7, 11, 14, and 18 days of storage.
Chemicals
Potassium hydroxide, adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), inosine 5′-monophosphate (IMP), hypoxanthine (Hx), and 1,1,3,3-tetraethoxypropane (TEP) were purchased from Sigma-Aldrich (Sigma-Aldrich Chemie GmbH, Germany); potassium dihydrogen phosphate, dipotassium hydrogen phosphate, 2-thiobarbituric acid (TBA), ethylenediaminetetraacetic acid (EDTA), propyl gallate, methanol, acetonitrile, and plate count agar from Merck (Darmstadt, Germany); perchloric acid and trichloroacetic acid (TCA) from Panreac Química S.A.U. (Barcelona, Spain); inosine (HxR) from BDH Chemicals Ltd (Poole, England); and maximum recovery diluent, violet red bile glucose agar, Pseudomonas agar base, and supplement SR 103 from Oxoid (Basingstoke, Hampshire, England); Lyngby iron agar from Scharlau Chemie (Sentmenat, Spain). The water used was distilled and Milli Q-purified.
Microbiological Analysis
Microbiological analysis was performed following the methodologies described by Anacleto et al. (2011). Sea bass muscle (10 g) was aseptically collected to a Stomacher bag with filter. A primary tenfold dilution was made with maximum recovery diluent and homogenized for 1 min at medium speed (230 rpm) using a Stomacher homogenizer (laboratory blender STOMACHER 400, Seward Laboratory Systems Inc., FL, USA). Appropriate series of decimal dilutions were then prepared. Homogenates were spread on agar for Pseudomonas spp. and hydrogen sulphide-producing bacteria enumeration or poured into molten agar for psychrotrophic bacteria and Enterobacteriaceae (with double layer to ensure anaerobic conditions) enumeration.
Pseudomonas spp. were enumerated on Pseudomonas agar base supplemented with SR 103, hydrogen sulphide-producing bacteria (including Shewanella putrefaciens) on Lyngby iron agar, and psychrotrophic bacteria on plate count agar after incubation at 20 °C for 4 days. Enterobacteriaceae were enumerated on violet red bile glucose agar after incubation at 30 °C for 1 day. For the hydrogen sulphide-producing bacteria, only the black colonies or those with a black centre were counted, whereas for Enterobacteriaceae, the large colonies with purple haloes were counted and a representative number were tested for oxidation and fermentation reactions. Petri dishes containing 30–300 colony-forming units (CFU) were selected for counting, and the results were expressed in logarithms of the CFU number per gram of muscle (log CFU g−1). Determinations were performed in duplicate.
Chemical Analysis
Nucleotide Degradation
Nucleotide and its breakdown products were extracted according to the method of Ryder (1985). During extract preparation, samples were kept in ice bath. Sea bass muscle was minced with a grinder (Retsch Grindomix GM200, Düsseldorf, Germany; 5,000 rpm). Minced muscle (5 g) was homogenized with perchloric acid (25 mL; 0.6 M) using an Ultra Turrax homogenizer (1 min; 6,500 rpm; Ultra Turrax T25, Janke & Kunkel IKA®-Labortechnik). The homogenate was centrifuged (20,000×g, 10 min, 0 °C; centrifuge 3K30, Sigma, Osterode, Germany), and 10 mL of the supernatant was neutralized with potassium hydroxide (1 M) to a pH of 6.90. The neutralized supernatant stood for 30 min at 2 °C to precipitate most of the potassium perchlorate, which was then removed by filtration through sintered glass. The filtrate solution was made up to 20 mL with water, filtered (0.22 μm pore size), and kept at −80 °C until injection.
An aliquot (20 μL) was injected into a high-performance liquid chromatograph (HP Agilent 1100 Series, Agilent, USA) equipped with a LiChrosorb RP-18 reverse-phase column (250 × 4.6 mm; 10 μm; VDS Optilab) operating isocratically with a mobile phase pumped at 1.6 mL min−1, and the detection wavelength set at 254 nm. The mobile phase was composed of potassium dihydrogen phosphate (0.04 M) and dipotassium hydrogen phosphate (0.06 M), with a pH of 6.90.
Nucleotide and its breakdown products were identified and quantified by comparison with standards. Standard curves for ATP and each compound involved in its degradation pathway, ADP, AMP, IMP, HxR, and Hx, were constructed in the 0.02–0.8 mM range. The peak areas were obtained with the software Agilent ChemStation for LC (Agilent, USA). All determinations were performed in triplicate. The K I index, which is a simplified form of the freshness indicator K index, was estimated according to the following equation (Karube et al. 1984):
Malondialdehyde
Malondialdehyde (MDA) was quantified according to the method described by Seljeskog et al. (2006) with modifications in the sample deproteinisation as described by Mendes et al. (2009). Briefly, minced sea bass muscle (5 g) was homogenized with TCA solution (10 mL; 75 g L−1 TCA, 1 g L−1 EDTA, 1 g L−1 propyl gallate) using an Ultra Turrax homogenizer (1 min, 6,500 rpm). Then, the homogenate was filtered (Whatman #1), and the filtrate was centrifuged (5,000 rpm, 10 min). Sample supernatant (0.5 mL) was mixed with TBA (1.5 mL, 40 mM), heated (97 °C, 60 min), and cooled in the freezer (−20 °C) for 20 min. Methanol (3 mL) was added, and the resulting solution was filtered (0.22 μm pore size) and kept at −80 °C until injection.
An aliquot (10 μL) was injected into a high-performance liquid chromatograph (Agilent 1100 Series, Agilent, USA). Separation of the MDA–TBA adduct was done using a reversed-phase column (4.6 × 150 mm; 5 μm; Phenomenex Gemini ODS C18 110 Å, Phenomenex, Torrance, CA, USA), operating isocratically with a mobile phase pumped at 1.0 mL min−1, and the spectrofluorimetric detector wavelengths were set at 525 nm (excitation) and 560 nm (emission). The mobile phase was composed of potassium dihydrogen phosphate (50 mM), methanol, and acetonitrile in the proportion of 72:17:11 (v/v/v).
The MDA–TBA adduct was identified and quantified by comparison with TEP which was used as MDA standard. A standard curve was made for TEP diluted in TCA solution at concentrations ranging 0.6–10.0 μM without hydrolysis prior to the TBA reaction. The peak areas were obtained with the software Agilent ChemStation for LC (Agilent, USA). All determinations were performed in triplicate.
Total Volatile Basic Nitrogen and Trimethylamine Nitrogen
The total volatile basic nitrogen (TVB-N) and trimethylamine nitrogen (TMA-N) contents were determined with the microdiffusion method using Conway diffusion cells (Conway and Byrne 1933). Briefly, minced sea bass muscle (5 g) was homogenized with TCA (10 mL; 5 % w/v) using an Ultra Turrax homogenizer (2 min; 6,500 rpm), and the homogenate was filtered (Whatman #1). After alkalinisation with saturated potassium carbonate, the volatile base components were absorbed by boric acid, which was then titrated with hydrochloric acid (0.01 N). For TMA-N determination, formaldehyde was added to the samples. Results were expressed as milligrams of nitrogen per 100 g of muscle. All determinations were performed in triplicate.
pH
The pH was measured directly on minced sea bass muscle using a surface calibrated pH electrode (SenTix 21, WTW, Weilheim, Germany) connected to a pH meter (microprocessor pH meter 539, WTW, Weilheim, Germany), at room temperature. All determinations were performed in duplicate.
Physical Analysis
Colour
Colour measurements were assessed with a colourimeter (CR-410, Konica Minolta Camera, Co, Japan) in minced sea bass muscle to avoid colour heterogeneity of fillets. The colourimeter was calibrated against a white standard plate (CIE L*a*b* system: L* = 97.79; a* = −0.02; b* = 1.84). Lightness (L*), red–green value (a*), and yellow–blue value (b*) were measured. All determinations were performed in triplicate. Chroma (C*), hue (h*), and whiteness (W) were estimated according to Schubring (2009), as follows:
Smell Intensity
Instrumental smell intensity (number of molecules) was determined with a portable odour level indicator (Cosmos XP-329 III R, New Cosmos Electric Co. Ltd, Osaka, Japan) that uses platinum heat coil covered with a high-sensitivity metal oxide (SNO2/ZnO) semiconductor as a sensor, kept at high temperature during use. Packages were carefully perforated in one end by insertion of a pointed Teflon tube and a second hole made in the opposite side of the bag in order to allow a stream of air through the product into the Cosmos unit. Results were expressed as Cosmos units of smell intensity. Determinations were performed in each package (three packages for each treatment and sampling day). All determinations were performed in triplicate.
Sensory Analysis
Sensory evaluation was conducted in a specific room equipped with individual booths under controlled conditions of light and temperature. The attributes of raw sea bass fillets were evaluated by a panel consisting of at least six experienced judges. A description of attributes and terminology used were discussed with the panel members. HPP-treated (250 and 400 MPa) and control fillets were presented individually on each sampling day. Panellists were asked to score odour, appearance, and texture (firmness) of samples. The intensity of odour, appearance, and texture (firmness) of fillets was assessed using a category scale, which ranged from 0 to 4 (Meilgaard et al. 1999), in which 0 indicates “absence”, 2 indicates “moderate”, and 4 indicates “very intense”. The results were reported as the average of scores.
Statistical Analysis
The effects of treatments and storage time were tested with a two-way analysis of variance, followed by a multiple comparisons test (Tukey's honestly significant difference (HSD)) to identify the differences. In sensory analysis, the results of colour (rosy) in the control treatment were evaluated using one-way analysis of variance, followed by Tukey's HSD to identify the differences between storage days. All statistical analyses were tested at a 0.05 level of probability with the software STATISTICA™ 6.1 (Statsoft, Inc., Tulsa, OK, USA).
Results and Discussion
Microbiological Analysis
In live and healthy fish, microorganisms are found on the outer surface (skin and slime), and in some inner surfaces like gills and gastrointestinal tract, while the remaining tissues are sterile (Gram and Huss 1996; Jay et al. 2005). After death, the presence of bacteria in the fish muscle results from contamination during processing and handling, where 5 log CFU g−1 is the limit for a product to be considered with good quality (ICMSF 1986).
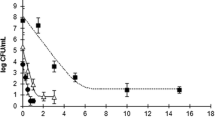
The initial bacterial load (psychrotrophic bacteria) was 2.3 log CFU g−1 and values reached 7 log CFU g−1, the upper acceptability limit for fresh marine species (ICMSF 1986), after 14 days of storage in control samples (Fig. 1). HPP treatments at 250 and 400 MPa resulted in a considerable reduction in bacterial load, in the beginning of storage (Fig. 1). In samples treated at 250 and 400 MPa, counts increased to 3.7 and 2.6 log CFU g−1, respectively, in the end of experiment.
Changes in psychrotrophic bacteria, Pseudomonas spp., hydrogen sulphide-producing bacteria, and Enterobacteriaceae during refrigerated storage of sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. Vertical error bars represent the standard deviation. Different letters denote significant differences (p < 0.05) between treatments (x–z) or between sampling days (a–e). The grey dashed line indicates the upper acceptability limit for fresh marine species. CFU colony-forming units
Despite the evident reduction in bacterial load induced by HPP, the initial contamination of fish muscle seems also to be of great importance to extend shelf life. In a previous study, a 3 log reduction in total aerobic counts was obtained at 400 MPa, but 6 log CFU g−1 was reached after only 7 days of storage (Chéret et al. 2005). In a study carried out by Yagiz et al. (2009), fresh salmon treated with 300 MPa (15 min pressure holding time) showed similar results as those obtained in 400 MPa treatments tested in the present study.
The initial microflora of fillets consisted mainly of Pseudomonas spp. (Fig. 1). This group of bacteria followed a similar trend to the one described for psychrotrophic bacteria during storage for all treatments. In what concerns hydrogen sulphide-producing bacteria, counts in control samples were below the detection limit in the beginning of storage and, during storage, increased up to 6.9 log CFU g−1 in day 18 (Fig. 1). In contrast, HPP caused a different evolution in the microflora composition of fillets, as hydrogen sulphide-producing bacteria were kept below the detection limit during all storage time. Enterobacteriaceae load was low in all treatments during the first 14 days of storage, only reaching 3.1 log CFU g−1 at day 18 in control samples (Fig. 1).
In previous studies, it was observed that HPP treatments of 50 and 100 MPa were effective in reducing Pseudomonas spp. than hydrogen sulphide-producing bacteria (Amanatidou et al. 2000). These authors also observed that treatments at 200 MPa (pressure holding times of 10–60 min) were able to reduce counts of Pseudomonas spp. and hydrogen sulphide-producing bacteria ca. 2 log units.
In the current study, because maximum microbiological limits were never reached in fillets treated with HPP, it was not possible to determine the microbiological shelf life, but it was extended at least 4 days. Data published previously also indicate that HPP can extend the shelf life of fresh fish, including sea bass (Chéret et al. 2005), gilthead sea bream (Campus et al. 2010; Erkan and Üretener 2010), red mullet (Erkan et al. 2010), and salmon (Yagiz et al. 2009). Still, it depended on the pressure level applied, as only the treatment at the highest pressure level (500 MPa) increased the microbiological shelf life of sea bass (Chéret et al. 2005).
Chemical Analysis
Nucleotide Degradation
ATP breakdown products formed in fish after death result from a chain reaction in which metabolites typically rise and then fall as the next metabolite in the degradative chain begins to rise (Gill 2000). The initial stages in nucleotide degradation are thought to be mainly due to enzymatic reactions, while the degradation of HxR to Hx is also due to bacterial action (Gill 2000). IMP has been recognized as having flavour-enhancing properties, while Hx contributes to the off-flavours typical of spoiled fish (Gill 2000).
Low levels of ATP, ADP, and AMP were found in the beginning of storage (concentration levels were below 0.15 μmol g−1; Fig. 2), which is in accordance with baseline levels obtained for several fish species (Mendes et al. 2001). Taking into account that ATP, ADP, and AMP concentrations rapidly decrease and are negligible within 25 h after death (Karube et al. 1984), the simplified freshness indicator K I was used with IMP and its breakdown products.
Changes in K I value and IMP concentration during refrigerated storage of sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. Vertical error bars represent the standard deviation. Different letters denote significant differences (p < 0.05) between treatments (x–y) or between sampling days (a–f). K I index freshness indicator index, IMP inosine 5′-monophosphate
Initially, fillets from all treatments showed a K I index of ca. 33 % and IMP concentrations around 1.5 μmol g−1 (Fig. 2). K I index values increased with storage time and, by the end of storage, reached 87 %, while IMP decreased to 0.3 ± 0.1 μmol g−1 in control samples. HPP treatments reduced nucleotide degradation as observed by the lower K I index values, principally at the end of storage (Fig. 2). From day 7, differences were clear in treatments at 400 MPa, while at 250 MPa, differences were only evident after day 14. In fact, no significant differences were found in IMP concentration between days 11, 14, and 18 in 250 and 400 MPa treatments, while in control samples IMP concentration continued to decrease with storage time. The lower degradation of IMP to HxR, observed in HPP treatments at 250 and 400 MPa, indicates that pleasant flavours of fresh fish might be kept for longer periods compared with control samples.
In a previous study, the slowdown in nucleotide degradation was not observed in salmon treated with HPP, possibly as a consequence of employing weaker pressure levels (135–200 MPa) and lower holding time (30 s) conditions (Ortea et al. 2010).
Acid phosphatase together with 5′-nucleotidase and nucleoside phosphorylase are the enzymes responsible for the conversion of IMP to HxR and HxR to Hx in fish muscle (Gill 2000). The results of the current study indicates that HPP treatments might reduce the activity of some enzymes involved in the nucleotide degradation process, and this effect might be more pronounced at higher pressure levels (400 MPa). This evidence is in accordance with previous studies that we carried out on the effect of HPP treatments in the activity of acid phosphatase (Teixeira et al. 2013). Additionally, the reduction in microflora observed in HPP-treated fillets might have also contributed for the slower nucleotide degradation.
Lipid Oxidation
Fish is highly susceptible to lipid oxidation because its muscle is characterized by a high content of polyunsaturated fatty acids (Erickson 2002). As a consequence, lipid hydroperoxides are formed and then decomposed to alcohols, aldehydes, ketones, and hydrocarbons (Erickson 2002).
MDA levels were monitored during storage, as a measure of secondary oxidation products formed in fillets. In the beginning of storage, MDA concentration was 0.2 mg kg−1 in control samples. After HPP treatments, lipid oxidation statistically increased in both treatments (Fig. 3). During storage, MDA increased in all treatments, being more pronounced at 400 MPa where 1.7 mg kg−1 was reached by the end of storage.
Changes in malondialdehyde (MDA) during refrigerated storage of sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. Vertical error bars represent the standard deviation. Different letters denote significant differences (p < 0.05) between treatments (x–z) or between sampling days (a–d). The grey dashed line represents the limit from which rancid flavours might develop
According to guidelines for MDA concentration in seafood, fish muscle with values above 0.72 mg kg−1 will probably develop rancid flavours (Ke et al. 1976). This limit was exceeded at days 7, 11, and 18 in samples treated at 400 and 250 MPa, and in control samples, respectively. However, panellists of sensory analysis did not detect rancid odours in fillets from any treatment during storage.
Similar variations in MDA concentration occurred in a previous study with cod treated at higher pressure levels (Angsupanich and Ledward 1998). However, Amanatidou et al. (2000) did not report an increase in MDA concentration in salmon treated at a lower pressure level (150 MPa, pressure holding time of 10 min). Lipid oxidation was reported to increase at a lower rate with storage time in HPP treatments at 220–330 MPa, compared with non-treated samples (Erkan and Üretener 2010; Erkan et al. 2010; Yagiz et al. 2009). Differences observed between studies might be related to different fish species, HPP conditions (e.g. pressure holding time), package characteristics, and storage conditions.
In previous studies, the differences in the effect of HPP in lipid oxidation were explained by possible differences in the fat characteristics, as well as in the content of unsaturated fats in different fish species that may be responsible for the non-uniform sensitivity of fats from different fish sources to pressure (Erkan and Üretener 2010). The accelerated oxidation in pressurized fish muscle may be due to denaturation of haem protein by pressure which releases metal ions promoting lipid auto-oxidation (Chevalier et al. 2001).
Total Volatile Basic Nitrogen and Trimethylamine Nitrogen
Total volatile bases comprise amines with one atom of nitrogen per molecule and are one of the most widely used measurements of spoilage evaluation of fishery products (Howgate 2009). Total volatile bases include trimethylamine, produced by spoilage bacteria; dimethylamine, produced by autolytic enzymes; ammonia, produced by the deamination of amino acids and nucleotide catabolites; and other volatile basic nitrogenous compounds associated with seafood spoilage (Howgate 2009; Huss 1995).
The initial TVB-N concentration in control samples was 17.0 mg N 100 g−1 (Table 1). The HPP treatments did not significantly change TVB-N values, and during storage, values did not increase significantly. Still, lower values were observed in HPP samples in days 14 and 18. TVB-N values did not reach the maximum allowable value (25–35 mg 100 g−1) established by the European Commission (2005) in any treatment. In previous studies, HPP treatments of 250 MPa increased TVB-N values of red mullet and decreased those of gilthead sea bream (Erkan and Üretener 2010; Erkan et al. 2010).
Trimethylamine oxide is naturally present in the living tissue of many marine fish species as part of the non-protein nitrogen fraction and is an important compound for maintenance of several physiological functions in fish (Sotelo and Rehbein 2000). Trimethylamine oxide reduction by bacteria forms trimethylamine, which is often associated with the typical fishy odour of spoiled seafood (Huss 1995).
At the beginning of the storage period, TMA-N values of control and HPP samples were lower than 0.5 mg N 100 g−1 (data not shown). The HPP treatments did not significantly change TMA-N values, and during storage, values did not change, being in all cases below the several limits of acceptability (5–12 mg N 100 g−1) proposed in previous studies (Erkan and Özden 2008; Ruiz-Capillas and Moral 2005). In studies with red mullet and gilthead sea bream, HPP treatments of 250 MPa (pressure holding time of 5 min at 7 °C) did not cause significant changes in TMA-N values, but other combinations of pressure level, temperature, and pressure holding time affected TMA-N values differently (Erkan and Üretener 2010; Erkan et al. 2010).
pH
Post-mortem glycolysis of fish muscle results in the accumulation of lactate and H+, which in turn lowers muscle pH (Erickson 2002), reduces the net surface charge on muscle proteins, and causes their partial denaturation (Huss 1995). The decrease in pH can lead to some water holding capacity loss (Huss 1995). Additionally, pH strongly influences the microflora of fish muscle, specially pH-sensitive spoilage bacteria (Gram and Huss 1996).
In the beginning of the experiment, pH values were 6.3 in control samples (Table 1). HPP treatment caused a slight increase in initial pH values, although not statistically (Table 1). In day 4, pH values dropped in HPP treatments, but during storage, pH values increased to values of ca. 6.5–6.6, without revealing differences between treatments.
In previous studies, HPP treatments with longer pressure holding times (20 min) did not affect the initial pH values of samples treated at 200 or 400 MPa, but after 7 days of storage, pH values were lower in the 200 MPa treatment than in other treatments (Angsupanich and Ledward 1998). In contrast, Erkan et al. (2010) observed a reduction in pH values in the beginning of storage time and an increase by the end in samples treated at 330 MPa. In gilthead sea bream, temperature used during HPP treatment influenced the evolution of pH during storage (Erkan and Üretener 2010).
Physical Analysis
Colour
Colour is an important quality attribute of food as it influences appearance and presentation, and might determine the acceptability by consumers and purchasing decision (Sahin and Sumnu 2006). The colour of fish muscle is related with carotenoids and hemepigments (Hui et al. 2006), and with the muscle physical structure and the amount of unbound water that influences light scattering (Chéret et al. 2005).
Control fillets showed whiteness, chroma, and hue values of 70.9, 10.0, and 55.9, respectively (Fig. 4). Both HPP treatments caused an increase in whiteness and a decrease in chroma; thus, samples became more opaque white and revealed a cooked appearance specially at 400 MPa. During storage, some fluctuations around the initial values were observed in colour parameters in all treatments (Fig. 4). By the end of storage, fillets did not get darker in any treatment, hue increased in control samples, and chroma increased in the 400 MPa treatment. Comparing both HPP treatments, whiteness and hue were lower in the 250 MPa treatment and chroma was higher. Still, by the end of the experiment, no significant differences were found in chroma between both treatments.
Changes in colour parameters (whiteness, chroma, and hue) during refrigerated storage of sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. Vertical error bars represent the standard deviation. Different letters denote significant differences (p < 0.05) between treatments (x–z) or between sampling days (a–b)
Colour changes in sea bass muscle caused by HPP are mainly due to modifications in the protein matrix (Chéret et al. 2005). Similar colour changes occur in cooked fish muscle due to denaturation of myofibrillar and sarcoplasmic proteins (Erkan et al. 2010; Yagiz et al. 2009).
In previous studies, no major changes were observed in colour parameters with storage time in HPP treatments in fresh salmon (Amanatidou et al. 2000; Yagiz et al. 2009). However, the effect of HPP in colour parameter evolution was reported to depend on the pressure level applied in red mullet (Erkan et al. 2010) and on the temperature used during HPP treatment in gilthead sea bream (Erkan and Üretener 2010).
Smell Intensity
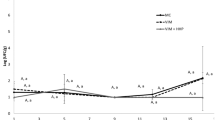
Initially smell intensity values were ca. 200 Cosmos units in control fillets (Fig. 5). The application of HPP at 250 MPa caused an increase on the smell intensity, attaining ca. 470 Cosmos units (Fig. 5). With the increase in storage time, smell intensity increased to levels of 800–900 in day 7, and the differences between treatments disappeared. Then, smell intensity remained constant throughout storage, except in day 18 that the 250 MPa treatment showed lower values than other treatments.
Changes in smell intensity during refrigerated storage of sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. Vertical error bars represent the standard deviation. Different letters denote significant differences (p < 0.05) between treatments (x–y) or between sampling days (a–c)
In a previous study, a different pattern was observed: the smell intensity of several fresh fish species (Bidyanus bidyanus, Salmo trutta, Oreochromis niloticus × Tilapia aurea, and Lates calcarifer) remained almost constant during the first 15 days of refrigerated storage, and the increase observed later reflected a decrease in organoleptic properties (Gelman et al. 2003).
The increase in MDA concentration with pressure level and with storage time could explain part of the variations observed in smell intensity. However, the smell intensity evolution obtained by instrumental analysis did not follow the same tendency as sensory analysis. This evidence happened possibly because the odour disappear with package opening during sensory analysis and ultimately because panellists did not notice the small variations that could remain in the headspace of fillets.
Sensory Analysis
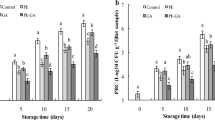
Initially, raw control fillets were rosy, translucent, and bright, with a fresh odour characteristic of raw fish, and the muscle was firm recovering the shape after the finger test. HPP promoted changes in fillets that influenced sensory characteristics (Fig. 6). The characteristic colour was lost in HPP fillets as denoted by the white colour revealed, quite similar to cooked fish, which is in accordance with the colour results obtained by instrumental analysis (Figs. 4 and 6). Additionally, HPP treatments caused a considerable loss of translucency and the brightness looked like glassy/vitreous. In samples treated at 400 MPa, the brightness was intensified, and fillets become firmer. Fresh odour decreased in HPP treatments, thought not statistically. Overall, the sensory acceptance of the HPP products was high.
Sensory appreciation of raw sea bass fillets treated with high-pressure processing at 250 and 400 MPa. Control samples are indicated as 0.1 MPa. The unfilled markers represent day 1, and the filled markers correspond to days 11 (grey) and 18 (black) of refrigerated storage. Category scale (0–4): 0, absence; 2, moderate; 4, very intense. Standard deviation was lower than 1.5
Panellists decreased the score given to colour, translucency, brightness, fresh odour, and firmness with storage time in control fillets (Fig. 6). Other attributes such as yellowish and brownish were scored as “absence” or “weak intensity” during storage. Unpleasant odours like fermented, sour, and putrid were not detected by most panellists. At the end of storage, control samples revealed changes in all attributes evaluated.
During storage of fillets treated with HPP, panellists decreased the score given to the attribute white in samples treated at 400 MPa, although this evidence is not in accordance with the instrumental measurements. In fillets treated at 250 MPa, the initial characteristics were kept for a longer period (Fig. 6). In the end of storage, the fillets treated at 250 MPa showed some changes in appearance, and those treated at 400 MPa lost firmness and the gapping increased.
In previous studies with gilthead sea bream and red mullet, sensory scores for appearance seems not to have been affected by HPP treatments in the first day of storage, and samples treated with HPP were better scored than control samples during storage (Erkan and Üretener 2010; Erkan et al. 2010). However, these authors also reported differences in instrumental colour results, in a similar way to the current study. The difference in the results can be explained by the use of a different sensory scale, where a score of 10–9 indicated “very good” quality and a score of 3.9–0 denoted as spoiled.
Conclusions
Taking into account microbiological criteria, HPP increases the shelf life of sea bass fillets for at least four extra days under refrigerated conditions. Both HPP treatments (250 and 400 MPa; 5 min) induced a reduction in the conversion of IMP to HxR and a slower decrease of freshness. HPP promoted changes in fillets that influenced sensory characteristics, conferring unique characteristics such as whiteness increase, translucency loss, and firmer consistency. Lipid oxidation and smell intensity (instrumental measurement) also increased in HPP-treated sea bass. Although MDA concentration in samples exceeded the established limit, no rancid or unpleasant odours were detected in sensory analysis. In general, the effects of HPP treatments were more pronounced in the treatment at 400 MPa. No effect of HPP treatments was observed in the amount of volatile bases neither in pH values. Both HPP treatments showed potential for preservation of new fish products with increased microbiological safety and shelf life, longer freshness, and unique characteristics (e.g. firmer and whitish).
References
Amanatidou, A., Schlüter, O., Lemkau, K., Gorris, L. G. M., Smid, E. J., & Knorr, D. (2000). Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic salmon. Innovative Food Science & Emerging Technologies, 1(2), 87–98.
Anacleto, P., Teixeira, B., Marques, P., Pedro, S., Nunes, M. L., & Marques, A. (2011). Shelf-life of cooked edible crab (Cancer pagurus) stored under refrigerated conditions. LWT - Food Science and Technology, 44(6), 1376–1382.
Angsupanich, K., & Ledward, D. A. (1998). High pressure treatment effects on cod (Gadus morhua) muscle. Food Chemistry, 63(1), 39–50.
Ayala, M. D., Albors, O. L., Blanco, A., Alcázar, A. G., Abellán, E., Zarzosa, G. R., et al. (2005). Structural and ultrastructural changes on muscle tissue of sea bass, Dicentrarchus labrax L., after cooking and freezing. Aquaculture, 250(1–2), 215–231.
Campus, M., Addis, M. F., Cappuccinelli, R., Porcu, M. C., Pretti, L., Tedde, V., et al. (2010). Stress relaxation behaviour and structural changes of muscle tissues from gilthead sea bream (Sparus aurata L.) following high pressure treatment. Journal of Food Engineering, 96(2), 192–198.
Chapleau, N. J., & de Lamballerie-Anton, M. I. (2003). Changes in myofibrillar proteins interactions and rheological properties induced by high-pressure processing. European Food Research and Technology, 216(6), 470–476.
Chéret, R., Chapleau, N., Delbarre-Ladrat, C., Verrez-Bagnis, V., & d. Lamballerie, M. (2005). Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. Journal of Food Science, 70(8), e477–e483.
Chevalier, D., Bail, A. L., & Ghoul, M. (2001). Effects of high pressure treatment (100–200 MPa) at low temperature on turbot (Scophthalmus maximus) muscle. Food Research International, 34(5), 425–429.
Conway, E. J., & Byrne, A. (1933). An absorption apparatus for the micro-determination of certain volatile substances: the micro-determination of ammonia. Biochemical Journal, 27(2), 419–429.
European Commission (2005). Commission Regulation No 2074/2005 of 5 December 2005 laying down implementing measures for certain products under Regulation No 853/2004 of the European Parliament and of the Council and for the organisation of official controls under Regulation No 854/2004 of the European Parliament and of the Council and Regulation No 882/2004 of the European Parliament and of the Council, derogating from Regulation No 852/2004 of the European Parliament and of the Council and amending Regulations No 853/2004 and No 854/2004. L 338, 22 December 2005 (pp. 27–59). Official Journal.
Erickson, M. C. (2002). Lipid oxidation of muscle foods. In C. C. Akoh & D. B. Min (Eds.), Food lipids—chemistry, nutrition, and biotechnology (pp. 365–411). New York: Marcel Dekker, Inc.
Erkan, N., & Özden, Ö. (2008). Quality assessment of whole and gutted sardines (Sardina pilchardus) stored in ice. International Journal of Food Science & Technology, 43(9), 1549–1559.
Erkan, N., & Üretener, G. (2010). The effect of high hydrostatic pressure on the microbiological, chemical and sensory quality of fresh gilthead sea bream (Sparus aurata). European Food Research and Technology, 230(4), 533–542.
Erkan, N., Üretener, G., & Alpas, H. (2010). Effect of high pressure (HP) on the quality and shelf life of red mullet (Mullus surmelutus). Innovative Food Science & Emerging Technologies, 11(2), 259–264.
FAO (2005–2012). World inventory of fisheries. Reducing post-harvest losses. Issues fact sheets. Text by Lahsen Ababouch. http://www.fao.org/fishery/topic/12369/en. FAO Fisheries and Aquaculture Department. Rome. Accessed 19 October 2012.
Gelman, A., Drabkin, V., & Glatman, L. (2003). A rapid non-destructive method for fish quality control by determination of smell intensity. Journal of the Science of Food and Agriculture, 83(6), 580–585.
Gill, T. (2000). Nucleotide-degrading enzymes. In N. F. Haard & B. K. Simpson (Eds.), Seafood enzymes—utilization and influence on postharvest seafood quality (pp. 37–68). Basel: Marcel Dekker Inc.
Gou, J., Lee, H.-Y., & Ahn, J. (2010). Effect of high pressure processing on the quality of squid (Todarodes pacificus) during refrigerated storage. Food Chemistry, 119(2), 471–476.
Gram, L., & Huss, H. H. (1996). Microbiological spoilage of fish and fish products. International Journal of Food Microbiology, 33(1), 121–137.
Howgate, P. (2009). Traditional methods. In H. Rehbein & J. Oehlenschläger (Eds.), Fishery products: quality, safety and authenticity (pp. 19–41). Oxford: Wiley-Blackwell.
Hui, Y. H., Cross, N., Kristinsson, H. G., Lim, M. H., Nip, W. K., Siow, L. F., et al. (2006). Biochemistry in seafood processing. In Y. H. Hui, W. Nip, L. M. L. Nollet, G. Paliyath, & B. K. Simpson (Eds.), Food biochemistry and food processing (pp. 351–378). Ames: Blackwell.
Huss, H. H. (1995). Quality and quality changes in fresh fish. Rome: Food and Agriculture Organization of the United Nations.
ICMSF. (1986). Microorganisms in foods. 2. Sampling for microbiological analysis: principles and specific applications. Buffalo: University of Toronto Press.
Jay, J. M., Loessner, M. J., & Golden, D. A. (2005). Modern food microbiology. New York: Springer.
Karube, I., Matsuoka, H., Suzuki, S., Watanabe, E., & Toyama, K. (1984). Determination of fish freshness with an enzyme sensor system. Journal of Agricultural and Food Chemistry, 32(2), 314–319.
Ke, P. J., Nash, D. M., & Ackman, R. G. (1976). Quality preservation in frozen mackerel. Canadian Institute Food Science and Technology Journal, 9, 135–138.
Meilgaard, M. C., Carr, B. T., & Civille, G. V. (1999). Sensory evaluation techniques. Boca Raton: CRC Press.
Mendes, R., Cardoso, C., & Pestana, C. (2009). Measurement of malondialdehyde in fish: a comparison study between HPLC methods and the traditional spectrophotometric test. Food Chemistry, 112(4), 1038–1045.
Mendes, R., & Gonçalves, A. (2008a). Effect of soluble CO2 stabilisation and vacuum packaging in the shelf life of farmed sea bream and sea bass fillets. International Journal of Food Science and Technology, 43(9), 1678–1687.
Mendes, R., & Gonçalves, A. (2008b). Effect of soluble CO2 stabilization on the quality of fillets from farmed gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax). Journal of Aquatic Food Product Technology, 17(4), 342–366.
Mendes, R., Quinta, R., & Nunes, M. L. (2001). Changes in baseline levels of nucleotides during ice storage of fish and crustaceans from the Portuguese coast. European Food Research and Technology, 212(2), 141–146.
Nielsen, M. K., & Nielsen, H. H. (2006). Seafood enzymes. In Y. H. Hui, W. Nip, L. M. L. Nollet, G. Paliyath, & B. K. Simpson (Eds.), Food biochemistry and food processing (pp. 379–400). Ames: Blackwell.
Oehlenschläger, J., & Rehbein, H. (2009). Basic facts and figures. In H. Rehbein & J. Oehlenschläger (Eds.), Fishery products: quality, safety and authenticity (pp. 1–18). Oxford: Wiley-Blackwell.
Ortea, I., Rodríguez, A., Tabilo-Munizaga, G., Pérez-Won, M., & Aubourg, S. P. (2010). Effect of hydrostatic high-pressure treatment on proteins, lipids and nucleotides in chilled farmed salmon (Oncorhynchus kisutch) muscle. European Food Research and Technology, 230(6), 925–934.
Özden, Ö., Inugur, M., & Erkan, N. (2007). Effect of different dose gamma radiation and refrigeration on the chemical and sensory properties and microbiological status of aqua cultured sea bass (Dicentrarchus labrax). Radiation Physics and Chemistry, 76(7), 1169–1178.
Patterson, M. F., Linton, M., & Doona, C. J. (2007). Introduction to high pressure processing of foods. In C. J. Doona & F. E. Feeherry (Eds.), High pressure processing of foods (pp. 1–14). Ames: Blackwell and Institute of Food Technologists.
Ruiz-Capillas, C., & Moral, A. (2005). Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chemistry, 89(3), 347–354.
Ryder, J. M. (1985). Determination of adenosine triphosphate and its breakdown products in fish muscle by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry, 33(4), 678–680.
Sahin, S., & Sumnu, S. G. (2006). Physical properties of food. New York: Springer.
Schubring, R. (2009). Colour measurement. In H. Rehbein & J. Oehlenschläger (Eds.), Fishery products: quality, safety and authenticity (pp. 127–172). Oxford: Wiley-Blackwell.
Seljeskog, E., Hervig, T., & Mansoor, M. A. (2006). A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clinical Biochemistry, 39(9), 947–954.
Sotelo, C. G., & Rehbein, H. (2000). TMAO-degrading enzymes. In N. F. Haard & B. K. Simpson (Eds.), Seafood enzymes - utilization and influence on postharvest seafood quality (pp. 167–190). Basel: Marcel Dekker.
Taliadourou, D., Papadopoulos, V., Domvridou, E., Savvaidis, I. N., & Kontominas, M. G. (2003). Microbiological, chemical and sensory changes of whole and filleted Mediterranean aquacultured sea bass (Dicentrarchus labrax) stored in ice. Journal of the Science of Food and Agriculture, 83(13), 1373–1379.
Teixeira, B., Fidalgo, L., Mendes, R., Costa, G., Cordeiro, C., Marques, A., et al. (2013). Changes of enzymes activity and protein profiles caused by high pressure processing in sea bass (Dicentrarchus labrax) fillets. Journal of Agricultural and Food Chemistry, 61(11), 2851–2860.
Yagiz, Y., Kristinsson, H. G., Balaban, M. O., Welt, B. A., Ralat, M., & Marshall, M. R. (2009). Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chemistry, 116(4), 828–835.
Acknowledgments
Bárbara Teixeira and António Marques acknowledge the Portuguese Foundation for Science and Technology (FCT) and the European Social Fund (FSE) for supporting a PhD grant (Ref. SFRH/BD/44254/2008) and a Research contract (Programa Ciência 2008), respectively. The authors would like to thank Research Unit 62/94 QOPNA (project PEst-C/QUI/UI0062/2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira, B., Marques, A., Mendes, R. et al. Effects of High-Pressure Processing on the Quality of Sea Bass (Dicentrarchus labrax) Fillets During Refrigerated Storage. Food Bioprocess Technol 7, 1333–1343 (2014). https://doi.org/10.1007/s11947-013-1170-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1170-0