Abstract
The effect of a previous hydrostatic high-pressure (HHP) treatment on hydrolysis, breakdown and aggregation events in chemical constituents of chilled farmed coho salmon (Oncorhynchus kisutch) was studied. Three different HHP conditions were applied (135 MPa-30 s; 170 MPa-30 s; 200 MPa-30 s; treatments T-1, T-2 and T-3, respectively) and compared to untreated fish for a 20-day chilled storage. Nucleotide degradation was important during the chilled storage in all kinds of samples; however, the K value did not afford differences related to previous pressure applied. HHP treatment led to an increased free fatty acid (FFA) formation (day 0 values); on the contrary, an inhibitory effect on FFA formation could be observed at the end of the storage (15–20 days) in T-3-treated fish as a result of microbial activity inhibition. A marked decrease in sarcoplasmic protein content was evident in samples corresponding to T-2 and T-3 treatments; the SDS–PAGE analysis of such protein fraction showed a partial loss of a band corresponding to 29 kDa. This band was excised, digested with trypsin, analysed by tandem mass spectrometry and identified as phosphoglycerate mutase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine foods have attracted great attention as a source of high amounts of important components (namely nutritional and digestible proteins, lipid-soluble vitamins, microelements and ω3-polyunsaturated fatty acids) on human health and nutrition [1, 2]. However, most of these constituents deteriorate rapidly post-mortem due to the effects of different damage pathways, these leading to a decreasing commercial value of fish products [3, 4].
During chilled storage, degradation processes are mostly carried out in the initial stage by endogenous muscle enzymes and later by microbial enzymes [5]. Thus, biochemical processes such as hydrolysis, aggregation and breakdown of the different chemical constituents are known to take place during traditional flake ice chilling [6]. With the aim of reducing quality losses in chilled fish, different advanced methods such as refrigerated sea water [7], slurry ice [8] and chemical addition [9] have been employed.
As a result of an increasing consumer’s demand for high-quality fresh products, fish technologists and the fish trade have developed different complementary processing systems. Among them, hydrostatic high-pressure (HHP) technology has proved to enlarge the shelf life time, while inactivating microbial development and deteriorative endogenous enzymes [10–12]. However, HHP has been reported to damage membranes, denature proteins, oxidise the lipid fraction, provoke browning development and cause constituents breakdown and aggregation [4, 10, 13]. Although covalent bonds are not broken by HHP employment, weak energy bonds like hydrogen and hydrophobic bonds can be irreversibly modified, thus leading to important consequences for the secondary, tertiary and quaternary structures in proteins [14, 15].
In recent years, the fishing sector is paying great attention to aquaculture development as a source of marine food products. Among cultivated fish, coho salmon (Oncorhynchus kisutch), also called silver salmon, has deserved a great interest because of its increasing production in countries like Chile, Japan and Canada [16]. Previous research related to the chilling storage of this species accounts for the development of different spoilage pathways and quality loss [17, 18]. The present work focuses on different hydrolysis, breakdown and aggregation events concerning chemical constituents that are known to be related to quality loss in chilled farmed coho salmon. The effect of a previous HHP treatment on such chemical constituent changes is studied.
Materials and methods
Raw fish, processing and sampling
Coho salmon specimens (50–52 cm length; 2.8–3.0 kg weight) were obtained from an aquaculture facility (Aquachile, S. A., Puerto Montt, X Región, Chile). Individuals were killed in the plant by a sharp blow to the head, the gills cut, bled in a water–ice mixture, beheaded, gutted and transported to the laboratory during 24 h under slurry ice condition (40% ice and 60% water; −1.0 °C) at a 1:1 fish to ice ratio. Then, the fish was filleted, cut into pieces (weight range: 125–150 g) and placed in flexible polyethylene bags.
The high-pressure equipment employed consisted of a cylindrical loading container provided with a 2-L pilot unit (Avure Technologies Incorporated, Kent, WA, USA). Three different HHP conditions (135 MPa for 30 s, 170 MPa for 30 s and 200 MPa for 30 s; treatments T-1, T-2 and T-3, respectively) were applied at room temperature (15 ± 2 °C) to fish and compared to untreated fish (control, treatment C). In all cases, water was employed as the pressurising medium, working at a 17 MPa/s ramp rate; decompression time was less than 5 s. Fish was then kept under chilling conditions (traditional flake ice) in a refrigerated room (4 °C). Sampling was carried out on salmon white muscle at days 0, 6, 10, 15 and 20 of chilled storage. For all kinds of samples, three different batches (n = 3) were considered and analysed separately to achieve the statistical analysis.
A different response to HHP treatment has been reported to occur in marine products according to different factors such as species nature, chemical composition and size [4, 11]. Accordingly, a preliminary study was undertaken before choosing the HHP treatment range to be applied in the present experiment. As a result, T-1, T-2 and T-3 conditions were chosen for the present research as being related to the best visual appearance (colour, gaping, elasticity and firmness) obtained and agreed to the optimised conditions previously recommended for farmed turbot (Scophthalmus maximus) fillets [19] and to the best conditions for colour retention in Atlantic salmon (Salmo salar) fillets [12]. Convenience of present HHP conditions was already proved in a study carried out in parallel to the present one [20]; in it, a marked microbial activity inhibition was observed as a result of T-1, T-2 and T-3 conditions employment, while a short lipid oxidation development was produced, although no losses in polyunsaturated fatty acid and endogenous antioxidant contents were detected.
Proximate composition
Moisture content was determined by the difference between the weight of fresh homogenised muscle (1–2 g) and the weight recorded after 4 h at 105 °C, according to the AOAC method [21]. Results were expressed as g water/100 g muscle.
Lipids were extracted by the Bligh and Dyer [22] method, by employing a single-phase solubilisation of the lipids using a chloroform–methanol (1:1) mixture. Quantification results were expressed as g lipid/100 g muscle.
Protein content was measured by the Kjeldahl method [21], employing the 6.25 conversion factor. Results were calculated as g total protein/100 g muscle.
Ash content was measured according to the AOAC [21] method by heating at 550 °C. Results were calculated as g/100 g muscle.
Lipid and nucleotide hydrolysis assessment
Free fatty acid (FFA) content was determined in the lipid extract of the fish muscle by the Lowry and Tinsley [23] method based on complex formation with cupric acetate–pyridine followed by spectrophotometric (715 nm) assessment (Beckman Coulter DU 640, London, UK). Results were expressed as mg FFA/100 g lipids.
Nucleotide degradation analysis was carried out starting from 6% perchloric acid extracts from the fish muscle according to previous research [24]. Analysis was performed by HPLC, using a Beckman device provided with the programmable solvent module 126 and the scanning detector module 167 connected to the System Gold software, version 8.1 (Beckman Coulter). Separations were achieved on a reverse-phase Spherisorb ODS-2 C18 250 × 4.60 mm column (Waters, Milford, MA, USA), with an internal particle diameter of 5 μm. Standard curves for adenosine 5′-triphosphate (ATP) and each compound involved in its degradation pathway, adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), inosine 5′-monophosphate (IMP), inosine (INO) and hypoxanthine (Hx), were constructed in the 0–1 mM range. Results obtained for each degradation compound were calculated as mmol/kg muscle. The K value was calculated according to the following concentration ratio:
Protein analysis
Two different protein extraction procedures were considered in this work.
Sarcoplasmic protein extracts were prepared in a low ionic strength extraction buffer A (10 mM Tris–HCl, pH 7.2, +50 mM pentamethyl sulphonic acid). Samples of 500 mg fish muscle were homogenised for 60 s in 4 mL of buffer solution, according to Piñeiro et al. [25]. Then, extracts were centrifuged at 12,500 rpm for 15 min, in a JA20.1 rotor (J221-M centrifuge, Beckman Coulter) at 4 °C, and the supernatants were recovered.
Preparation of SDS-soluble protein extracts was carried out in extraction buffer B (2% wt/vol SDS/0.1 DTT/60 mM Tris–HCl, pH 7.5) using the same conditions mentioned for the sarcoplasmic protein extraction [26] except that before the centrifugation at 4 °C, the SDS extracts were boiled at 100 °C for 2 min and homogenised for 30 s. All extracts were maintained at −80 °C until analysis.
The protein concentration in extracts prepared in buffer A was determined by means of the protein microassay method (Bio-Rad Laboratories Inc., Hercules, CA, USA), while extracts prepared in buffer B were subjected to the PlusOne 2-D Quant kit (Amersham Biosciences, Uppsala, Sweden). In both cases, a standard curve constructed for bovine serum albumin was used as reference, and results were expressed as g/100 g muscle.
Electrophoretic analysis was carried out by means of horizontal SDS–PAGE gels. Samples were mixed with sample buffer according to the procedure of Laemmli [26]. Because of their higher resolution and reproducibility, precast polyacrylamide 245 × 110 × 1 mm commercial gels (Excel-Gel SDS Homogeneous 15%, Amersham Biosciences) for horizontal electrophoresis were selected, according to previous research [24]. A low molecular weight protein standard (97–14 kDa) from Amersham Biosciences was employed as reference. This protein standard mixture was comprised of the following proteins (MW values are indicated in brackets): phosphorylase b (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20 kDa) and alpha-lactalbumin (14 kDa). The mobilities of protein bands were determined using the Whole Band Analyser Software (BioImage Systems Corporation, MI, USA), and the corresponding MWs were calculated by comparison with the size of the protein standards. Both kinds of protein extracts were studied by means of electrophoretic patterns.
Mass spectrometry analysis
According to the electrophoretic analysis of the sarcoplasmic fraction, the band of interest (29 kDa) was excised from the gel and subjected to in-gel digestion with trypsin (Roche Diagnostics GmbH, Mannheim, Germany), performed overnight at 37 °C as described elsewhere [27]. The final digestion solution was dried under vacuum and resuspended in 5% acetic acid.
Peptide digests were analysed online by HPLC electrospray ionisation ion trap tandem mass spectrometry (MS/MS), using a liquid chromatography system model SpectraSystem P4000 (Thermo-Finnigan, San Jose, CA, USA) coupled with an ion trap mass spectrometer model LCQ Deca XP Plus (Thermo-Finnigan) according to Ortea et al. [28]. Briefly, peptides were separated on a BioBasic-18 reverse-phase column (ThermoHypersil-Keystone, Bellefonte, PA, USA) using 0.5% acetic acid in water and 0.5% acetic acid in acetonitrile as mobile phases A and B, respectively, with a 90-min linear gradient from 5 to 60% B. Peptides were detected using survey scans from 300 to 1300 amu (3 μ scans), followed by a data-dependent zoom-scan (5 μ scans) and MS/MS scan (5 μ scans) using a normalised collision energy of 35%.
Uninterpreted MS/MS spectra were searched against the nr.fasta database (NCBI Resources, NIH, Bethseda, MD, USA) using SEQUEST (Bioworks 3.1 package, Thermo-Finnigan). The following constraints were used for the searches: tryptic cleavage after Arg and Lys, up to two missed cleavage sites, and tolerances ±1.5 Da for precursor ions and ±0.8 Da for MS/MS fragment ions. The variable modifications allowed were methionine oxidation, carbamidomethylation of Cys and acetylation of the N-terminus. In order to confirm the SEQUEST matches, de novo sequencing was performed by manual interpretation of the ion series in the spectra, and de novo-inferred sequences were searched against the NCBInr protein sequence databases using the BLAST tool (http://www.ncbi.nlm.nih.gov).
Statistical analysis
Data (n = 3) obtained from the different chemical analyses were subjected to the ANOVA method (p < 0.05) to explore differences by two different ways: high-pressure effect and chilling storage effect (Statsoft, Statistica, version 6.0, 2001); comparison of means was performed using a least-squares difference (LSD) method. Correlation analysis among parameters (chilling time and chemical constituent changes) was also carried out. In them, linear fittings are expressed; otherwise, the kind of fitting is mentioned.
Results and discussion
Proximate composition
Moisture and lipid contents of starting coho salmon white muscle (72.40 ± 0.97 and 4.74 ± 1.27 g/100 g muscle, respectively; Tables 1 and 2) agreed to previous research on the same farmed species [17] and can be considered in the expected value ranges of a fatty fish species [1].
When no chilled storage is encountered (time 0; Table 1), no differences are observed for the moisture content among the different kinds of samples so that an effect of HHP treatment cannot be concluded at this time. Later on (days 6 and 15), some differences can be observed, although a general pattern cannot be concluded as a result of pressure applied. However, a marked effect of chilling time was observed; thus, all kinds of samples showed a moisture content decrease by increasing the chilling time.
One of the most important effects reported for HHP treatment is to provoke changes in hydrophobic and electrostatic interaction, so that the water-holding capacity of proteins may be altered [10, 13]. In this sense, water content may undergo important changes, these including water release and accordingly, moisture loss in the fish material as has been obtained in the present work during the chilled storage.
Concerning the lipid content (Table 2), no differences could be observed as a result of the HHP treatment. Meantime, some differences were observed with chilling time in all kinds of samples. Although a general pattern could not be concluded, higher mean values were attained by increasing the chilling time in most cases. Comparison of such tendency with moisture results (Table 1) would agree with a known inverse ratio between water and lipid constituent contents [1].
Present results agree with Ohshima et al. [29] who did not find differences in moisture and lipid contents in cod and mackerel flesh after HHP treatment (200–600 MPa for 15 and 30 min). Contrary to the present research, Ramírez-Suárez and Morrissey [30] found a moisture content decrease and a lipid content increase as a result of HHP (275 and 310 MPa for 2, 4 and 6 min) treatment in albacore-minced muscle; then, no differences in both constituent contents were observed during further storage of fish muscle at 4 °C and at −20 °C.
Starting protein and ash contents in the present research were 20.3 ± 0.4 and 1.0 ± 0.1 g/100 g muscle, respectively, according to expected values for marine fish species [1]. Values obtained for both constituents remained quite constant throughout the experiment, so that no differences (p > 0.05) could be outlined as a result of previous HHP treatment, neither as a result of the chilling storage.
Nucleotide and lipid hydrolysis
During post-mortem fish storage, muscle nucleotides are known to degrade in a series of stages as a result of endogenous biochemical changes; the level of major adenine nucleotides and their related compounds (K value assessment) has been utilised extensively as an index of freshness of fish muscle [5].
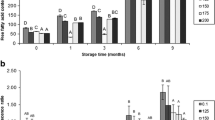
Figure 1 shows the evolution of the K value in this study. Nucleotide degradation was relevant throughout the chilled storage of all kinds of samples, especially at day 6; accordingly, a good correlation value was obtained between the K value and the storage time (r 2 = 0.93–0.97, logarithmic fitting). A sharp increase has also been reported during the first days of storage in other salmonid species such as Atlantic salmon (Salmo salar) [31] and rainbow trout (Oncorhynchus mykiss) [32].
Evolution of the K value (%)* in chilled salmon muscle previously treated under different conditions**. * Mean values of three (n = 3) independent determinations are expressed. Standard deviations are denoted by bars. ** Treatment abbreviations: Control (C), 135 MPa for 30 s (T-1), 170 MPa for 30 s (T-2) and 200 MPa for 30 s (T-3)
At time 0, no differences could be assessed as a result of HHP treatment; later on, some differences could be depicted among the different fish samples, although a differential pattern could not be established concerning the effect of pressure applied on nucleotide degradation throughout the chilled storage.
Contrary to the present results, previous research has shown a K value decrease during storage as a result of previous HHP treatment. Thus, when carp muscle was treated at various pressure values (200, 350 and 500 MPa for 30 min) and subsequently stored at 5 °C, suppression of the IMP decomposition was observed when employing 350 and 500 MPa values [33]; in such cases, a decreased K value was obtained and an increasing umami taste development was evident, this being the result of an increased IMP presence [10]. A lower K value was also found in pressurised fresh tilapia fillets (50–300 MPa for 2–12 h) when compared with non-pressurised ones [34]; as a result, a decreasing K value was found by increasing the pressure applied. In both cases [33, 34], results were explained as a result of protein denaturation and deactivation of enzymes involved in the degradation of ATP and related compounds (namely dephosphorylases) during HHP treatment. It could be argued that such deactivation failed to occur in the present study probably as a consequence of employing weaker pressure and holding time conditions.
FFA presence has shown an important role in fish muscle texture changes [35], in lipid oxidation enhancement [36] and as being strongly interrelated with off-odour development [37]. In the present study, FFA formation was relevant during the chilled storage for all kinds of samples (Table 3; g FFA/100 g muscle), so that good correlation values were obtained with time in all cases (r 2 = 0.93–0.97, quadratic fitting). When the lipid basis is considered (g FFA/100 g lipids), a relatively low value (0.77 ± 0.24) for FFA content is observed for the raw starting fish, according to previous research on this farmed species [17] and according to a fatty fish species value range [1]. As on muscle basis, an increasing FFA content with chilling time was also observed on lipid basis (2.6–4.0 g/100 g lipids at day 20) for the different kinds of samples.
Comparison among the different kinds of samples provided some differences. At day 0, higher mean values in fish treated under pressure conditions (T-1, T-2 and T-3 treatments) were obtained when compared to control fish. In this sense, an increasing FFA formation was observed in turbot fillets [19] and carp fillets [38] by increasing the pressure (from 100 till 200 MPa) conditions applied. In the present research, a lower mean value in control fish was maintained during the 6- to 10-day period, while a higher mean value was observed for fish treated under T-1 condition throughout the 10–20-day period; at the final stage (15- to 20-day period) of the experiment, the lowest values could be observed for fish from T-3 condition.
FFA have been reported to be mostly produced during the first stage of the chilling process (up to days 7–11, depending on several factors) as a result of endogenous enzyme (namely lipases and phospholipases) activity [3, 6]. Later on, microbial activity should gain importance, so that FFA formation is then mostly produced as a result of bacterial catabolic processes. According to this profile, present results on FFA formation in coho salmon show that some FFA formation was produced as a result of HHP treatment (day 0 values); in addition, FFA formation during the 6- to 10-day period would show no inactivation of lipases by means of the pressure-holding time conditions applied. Finally, partial inhibition by microbial activity was evident (days 15–20 values) in agreement to previous parallel research related to aerobe and psychrotroph group assessment [20].
According to the present results, He et al. [39] did not observe inhibition of lipase activity in refrigerated (4 °C) oysters, which were previously pressurised at 207–310 MPa for 1–2 min, and the same conclusion was attained by Gómez-Estaca et al. [40] when studying cold-smoked sardine storage (5 °C up to 21 days) previously treated under 300 MPa for 15 min. On the contrary, Ohshima et al. [29] found that enzymatic degradation of phospholipids in cod muscle was successfully inhibited during storage (−2 °C, 6 days) when a previous pressure of 400 MPa and over was applied for 15 and 30 min.
Protein changes
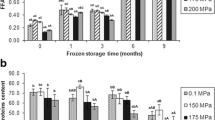
Both kinds of protein extracts were studied by means of electrophoretic pattern analysis. Figure 2 shows the evolution of SDS-soluble proteins. In general terms, no major change in the protein profiles was observed, so that HHP conditions applied and further chilled storage did not led to differences among the different kinds of fish samples.
Electrophoretic profiles* of SDS-soluble proteins of chilled salmon muscle previously treated under different conditions**. * Profiles corresponding to days 0, 10, 15 and 20 of chilled storage are indicated. A low molecular weight (LMW) standard of bovine serum albumin (97–14 kDa) is included in the central lane. ** Treatment abbreviations as expressed in Fig. 1
Such conclusions agree with previous research where SDS–PAGE analysis showed that HHP treatment of fish muscle caused neither degradation nor covalent cohesion of myofibrillar proteins [10]. Thus, mobilities of myosin heavy chain and actin from carp muscle were not altered (up to 500 MPa for 30 min) [33], and the same conclusion was obtained from Alaska Pollock surimi analysis when treated at 200–500 MPa for 10 min [41]. However, DSC analysis showed a full denaturation of turbot fillet myosin when submitted at 200 MPa for 15 and 30 min [19] and of cod muscle myosin processed at 100–200 MPa for 20 min [13].
Recent research concerning the employment of stronger HHP conditions than the ones applied in the present study has produced changes in myofibrillar electrophoretic profiles. Thus, in a previous research carried out on sea bass muscle [42], SDS–PAGE profiles of myofibrillar proteins were modified when applying a pressure of 300 MPa for 5 min, while no changes occurred at a lower pressure (100 MPa), according to the present research; in addition, no changes were obtained throughout a further refrigerated storage (4 °C during a 0- to 7-day period), whatever was the pressure applied. In the same way, SDS–PAGE electrophoresis of myofibrillar proteins of albacore tuna muscle indicated that HHP treatment (275 and 310 MPa for 2, 4 and 6 min) promoted the formation of high molecular weight polypeptides and reduction of myosin heavy chain band, most likely through disulphide bonding [30]; in this sense, the −SH group content in actomyosin decreased by increasing the pressure applied and pressure-holding time [43].
Electrophoretic analysis of the sarcoplasmic protein fraction is shown in Fig. 3. In it, the partial loss of a band placed at 29 kDa in fish samples corresponding to the two highest pressure treatments (T-2 and T-3) could be depicted. Such loss was evident at day 0 when only the effect of HHP treatment is encountered and was maintained throughout the chilled experiment in such fish samples. Additionally, fish corresponding to T-1 treatment showed this band loss at the end of the experiment.
Electrophoretic profiles* of sarcoplasmic proteins of chilled salmon muscle previously treated under different conditions**. * Profiles corresponding to days 0, 10, 15 and 20 of chilled storage are indicated. A low molecular weight (LMW) standard of bovine serum albumin (97–14 kDa) is included in the central lane. Arrows indicate the partially disappeared band (29 kDa). ** Treatment abbreviations as expressed in Fig. 1
Previous research has shown that the presence of certain sarcoplasmic bands decreased, or even disappeared from the SDS–PAGE gels without the expected appearance of low molecular weight bands. Thus, a specific loss of a major sarcoplasmic protein component with a molecular weight of 43 kDa was noted in cod and mackerel muscle as a result of applying an HHP treatment of 400 MPa or over for 15 and 30 min [29]. Further, the intensity of bands with molecular weights lower than 36 kDa decreased in carp fillets as a result of HHP treatment (100–200 MPa for 15 and 30 min) [38]. Consequently, it is suggested that rather than being degraded by HHP treatment, certain sarcoplasmic proteins become compacted and more covalently linked together, thereby becoming more resistant to extraction [14]. Thus, HHP would promote protein denaturation and their insolubilisation by altering the subtle equilibrium between the molecular interactions that stabilise the organised conformation of native proteins [10, 13]. Contrary to this conclusion, a 67-kDa sarcoplasmic protein band in cod muscle was found to be resistant to pressure treatment and increased in concentration after pressure treatment at 200–600 MPa [13].
Concerning the effect of a further storage to HHP treatment, contradictory results have been observed. Thus, Angsupanich and Ledward [13] found that storage at 4 °C for 7 days of cod muscle had no apparent effect on any electrophoretograms concerning the sarcoplasmic proteins, but Chéret et al. [42] found that sarcoplasmic protein profiles were modified according to the storage period (4 °C for 7 days).
Analysis of the sarcoplasmic protein content (Fig. 4) provided a marked decreasing tendency as long as the pressure applied increased. This detrimental effect on sarcoplasmic protein content could be observed at time 0, when no chilled storage is encountered, and also throughout the chilled storage in all kinds of samples, so that a good inverse correlation value was obtained between sarcoplasmic protein content and chilling time (r 2 = 0.90–0.92, quadratic fitting). In spite of the known relationship between protein denaturation and water-holding capacity [10, 13], correlation between moisture and sarcoplasmic protein contents was not satisfactory (r 2 = 0.78–0.88), although good values were obtained between sarcoplasmic protein and FFA contents (r 2 = 0.92–0.94).
Evolution of sarcoplasmic protein (SP) content (g/100 g muscle)* in chilled salmon muscle previously treated under different conditions**. * Mean values of three (n = 3) independent determinations are expressed. Standard deviations are denoted by bars. ** Treatment abbreviations as expressed in Fig. 1
This sarcoplasmic content decrease agrees to previous research [29]. In it, extracted sarcoplasmic protein content of cod muscle decreased slowly but regularly with the increase of pressure applied (from 0 to 600 MPa for 15 and 30 min), while extracted sarcoplasmic protein content of mackerel muscle decreased rapidly by applying a pressure value included in the 0–400 MPa range, causing no change in the use of a higher pressure. For both kinds of fish muscle (cod and mackerel), extending the pressure time from 15 to 30 min had no effect on the extractability of sarcoplasmic proteins.
Some additional research was carried out on the 29-kDa band, whose partial loss was observed in sarcoplasmic fraction SDS–PAGE gels of HHP-treated samples (Fig. 3). Thus, the band was excised from the gel, digested with trypsin, and the resulting peptides were subjected to MS analysis by means of an electrospray ion trap mass detector. Peptides were fragmented, and uninterpreted fragmentation spectra (MS/MS) or de novo-inferred sequences were searched against the protein sequence databases using the SEQUEST software or the BLAST tool, respectively. Several peptides were found that matched with the Atlantic salmon protein phosphoglycerate mutase, described in databases (accession numbers: 213515184 and 213515006). In Fig. 5, several fragmentation spectra as examples of the found phosphoglycerate mutase peptides are shown, together with the mass-to-charge ratio (m/z) of the precursor ions and the corresponding amino acidic sequences. Therefore, the band studied, identified by MS/MS as an isoform of the glycolytic enzyme phosphoglycerate mutase, seemed to be the most affected protein by the denaturation and insolubilisation caused by HHP treatment.
Previous reports by other authors have proposed the changes produced in certain electrophoretic bands as spoilage or freshness biomarkers [44, 45]. Partial increase or decrease content found in the electrophoretic profiles would be produced as a result of different damage processes (proteolysis, aggregation, denaturation, insolubilisation, etc.) happened in the myofibrillar and sarcoplasmic fractions and could be related to quality loss development. Present results show that phosphoglycerate mutase assessment could be a profitable tool in order to study the freshness loss of coho salmon during its chilled storage.
Final remarks
Present pressure-holding time condition range was chosen as corresponding to the best visual analysis (colour, gaping, elasticity and firmness) scores found in a preliminary test on salmon pieces. Additionally, such HHP conditions showed to be profitable to partially inhibit microbial development in a parallel study [20].
However, present results concerning hydrolytic, breakdown and aggregation events in chemical constituents showed that HHP itself led to an increased FFA formation and to a decrease in sarcoplasmic content (namely T-2 and T-3 conditions), while no effect on nucleotide degradation and myofibrillar electrophoretic profiles could be depicted. During the further chilled storage, previous HHP treatment failed to inhibit the nucleotide degradation and the FFA formation by means of endogenous enzymes; however, FFA formation by microbial activity development was partially inhibited, according to the parallel study results [20].
Concerning the sarcoplasmic protein fraction, a partial loss of an electrophoretic band corresponding to a molecular weight of 29 kDa was observed in fish treated under T-2 and T-3 conditions throughout the whole experiment; additionally, fish treated under T-1 condition showed the same result at the end of the experiment (day 20). Assessment of such band (phosphoglycerate mutase) could be considered a profitable tool in order to be employed as freshness biomarker in chilled coho salmon.
References
Piclet G (1987) Cah Nutr Diét 22:317–335
Simopoulos A (1997) Nutritional aspects of fish. In: Luten J, Börrensen T, Oehlenschläger J (eds) Seafood from producer to consumer, integrated approach to quality. Elsevier Science, London, pp 589–607
Pigott G, Tucker B (1990) Seafood: effects of technology on nutrition. Marcel Dekker Inc, New York, pp 66–84
Ashie I, Smith J, Simpson B (1996) Crit Rev Food Sci Nutr 36:87–121
Olafsdóttir G, Martinsdóttir E, Oehlenschläger J, Dalgaard P, Jensen B, Undeland I, Mackie I, Henehan G, Nielsen J, Nilsen H (1997) Trends Food Sci Technol 8:258–265
Whittle K, Hardy R, Hobbs G (1990) Chilled fish and fishery products. In: Gormley T (ed) Chilled foods: the state of the art. Elsevier Applied Science, New York, pp 87–116
Kraus L (1992) Refrigerated sea water treatment of herring and mackerel for human consumption. In: Burt J, Hardy R, Whittle K (eds) Pelagic fish. The resource and its exploitation. Fishing News Books, Aberdeen, pp 73–81
Piñeiro C, Barros-Velázquez J, Aubourg S (2004) Trends Food Sci Technol 15:575–582
Hwang K, Regenstein J (1995) J Aquat Food Prod Technol 4:19–30
Ohshima T, Ushio H, Koizumi C (1993) Trends Food Sci Technol 4:370–375
Murchie L, Cruz-Romero M, Kerry J, Linton M, Patterson M, Smiddy M, Kelly A (2005) Innov Food Sci Emerg Technol 6:257–270
Yagiz Y, Kristinsson H, Balaban M, Welt B, Ralat M, Marshall M (2009) Food Chem 116:828–835
Angsupanich K, Ledward D (1988) Food Chem 63:39–50
Ashie I, Simpson B (1996) Food Res Int 29:569–575
Hurtado J, Montero P, Borderías AJ (2001) J Food Sci 66:400–406
FAO Inform (2007) Fishery statistics. In food and agriculture organization of the United Nations. FAO, Rome (Italy), Yearbook 2005, vol 100/2, p 73
Aubourg S, Vinagre J, Rodríguez A, Losada V, Larraín MªA, Quitral V, Gómez J, Maier L, Wittig E (2005) Eur J Lipid Sci Technol 107:411–417
Aubourg S, Quitral V, Larraín MªA, Rodríguez A, Gómez J, Maier L, Vinagre J (2007) Food Chem 104:369–375
Chevalier D, Le Bail A, Ghoul M (2001) Food Res Int 34:425–429
Aubourg S, Tabilo-Munizaga G, Reyes J, Rodríguez A, Pérez-Won M (2010) Eur J Lipid Sci Technol (in press) (Ref: EJLT-2009-00173)
AOAC (1990) Official methods for analysis of the association of analytical chemistry (15th ed). In: Helrich K (ed) Association of Official Chemists, Inc., Arlington, pp 931–935
Bligh E, Dyer W (1959) Can J Biochem Physiol 37:911–917
Lowry R, Tinsley I (1976) J Am Oil Chem Soc 53:470–472
Aubourg S, Piñeiro C, Gallardo J, Barros-Velázquez J (2005) Food Chem 90:445–452
Piñeiro C, Barros-Velázquez J, Pérez-Martín R, Martínez I, Jacobsen T, Rehbein H, Kündiger R, Mendes R, Ettienne M, Jerome M, Craig A, Mackie I, Jessen F (1999) Electrophoresis 20:1425–1432
Lemmli U (1970) Nature 227:680–685
Jensen ON, Wilm M, Shevchenko A, Mann M (1999) Sample preparation methods for mass spectrometric peptide mapping directly from 2-DE gels. In: Link AJ (ed) 2-D proteome analysis protocols. Humana Press, Totowa, pp 513–530
Ortea I, Cañas B, Gallardo JM (2009) J Prot Res 8:5356–5362
Ohshima T, Nakagawa T, Koizumi C (1992) Effect of high pressure on the enzymatic degradation of phospholipids in fish muscle during storage. In: Bligh E (ed) Seafood science and technology. Fishing News Books, Oxford, pp 64–75
Ramírez-Suárez J, Morrisey M (2006) Innov Food Sci Emerg Technol 7:19–27
Erikson U, Beyer A, Sigholt T (1997) J Food Sci 62:43–47
Boyle J, Lindsay R, Stuiber D (1991) J Food Sci 56:1267–1270
Shoji T, Saeki H (1989) Use of high pressure on food. In: Hayashi R (ed) High pressure science for foods. San-ei Publications, Kyoto, pp 75–87
Ko W-C, Hsu K-C (2001) J Food Prot 64:94–98
Sikorski Z, Kolakowska A (1994) Changes in protein in frozen stored fish. In: Sikorski Z, Sun Pan B, Shahidi F (eds) Seafood proteins. Chapman and Hall, New York, pp 99–112
Mackie I (1993) Food Rev Intern 9:575–610
Refsgaard H, Brockhoff P, Jensen B (2000) J Agric Food Chem 48:1136–1140
Sequeira-Muñoz A, Chevalier D, Le Bail A, Ramaswamy H, Simpson J (2006) Innov Food Sci Emerg Technol 7:13–18
He H, Adams R, Farkas D, Morrisey MT (2002) J Food Sci 67:640–645
Gómez-Estaca J, Montero P, Jiménez B, Gómez-Guillén MC (2007) Food Chem 105:511–520
Shoji T, Saeki H, Wakameda A, Nakamura M, Nonaka M (1990) Nippon Suisan Gakkaishi 56:2069–2076
Chéret R, Hernández-Andrés A, Delbarre-Ladrat C, de Lamballerie M, Vérrez-Bagnis V (2006) Eur Food Res Technol 222:527–535
Hsu K-C, Hwang J-S, Yu C-C, Jao C-L (2007) Food Chem 103:560–564
Papa I, Álvarez C, Vérrez-Bagnis V, Fleurence J, Benyamin Y (1996) J Sci Food Agr 72:63–70
Morzel M, Vérrez-Bagnis V, Arendt E, Fleurence J (2000) J Agr Food Chem 48:239–244
Acknowledgments
The authors thank Mr. Marcos Trigo, Mrs. Teresa Roco and Mrs. Lorena Briones for their excellent technical assistance and AQUACHILE S. A. for kindly providing the coho salmon fish. This work was supported by the Universidad de Chile (Chile)-Consejo Superior de Investigaciones Científicas (Spain) program (Project 2006 CL 0034) and by the FONDECYT program (Chile; project number: 1080626).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortea, I., Rodríguez, A., Tabilo-Munizaga, G. et al. Effect of hydrostatic high-pressure treatment on proteins, lipids and nucleotides in chilled farmed salmon (Oncorhynchus kisutch) muscle. Eur Food Res Technol 230, 925–934 (2010). https://doi.org/10.1007/s00217-010-1239-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1239-1