Abstract
Large amounts of a waste known as tomato pomace and consisting mainly of the fruit peel and seeds are generated annually from the industrial processing of tomatoes. This material is rich in lycopene, a phytochemical with antioxidant and chemopreventive properties, and contains many valuable nutrients. In this study, we have investigated the possibility of using the whole waste to produce a lycopene-enriched seed oil. The oil was obtained by cold-pressing the seeds and was subsequently enriched in lycopene (up to 500 ppm) by incorporation of a tomato oleoresin derived from the peels. To increase lycopene recovery, the peels were pretreated with cell-wall-degrading enzymes and solvent extracted. This procedure allowed the production of about 25 kg/ton oleoresin with an average lycopene content of 6.8 wt.%. The compositional characteristics of the oil combined with the production of significant amounts of oleoresin strongly support the use of tomato pomace for producing lycopene-based functional products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 30 million tons of tomatoes is processed annually worldwide to produce canned tomatoes, ketchup, tomato juice, sauce and many other products (WPTC 2011). During tomato processing, a waste known as tomato pomace is generated which consists mainly of the fruit peels and seeds. These parts represent about 4 % by weight of processed tomatoes, which leads to an estimated world production of the waste of more than 1 million tons/year (Del Valle et al. 2006). Tomato pomace has no commercial value and is currently disposed of as a solid waste or used for animal feeding. However, a careful examination of this material reveals that it is a rich source of important nutrients and phytochemicals. In particular, tomato seeds contain an oil of high nutritional quality (Eller et al. 2010), and significant amounts of flavonoids (Bovy et al. 2007) and carotenoids (Khachik et al. 2002) are present in the peels. Among them, lycopene has attracted the greatest attention in recent years for its potential health benefits (Kong et al. 2010).
Lycopene (ψ,ψ-carotene) is an acyclic tetraterpenic hydrocarbon with 13 carbon–carbon double bonds, 11 of which are conjugated (Fig. 1). The high degree of conjugation makes this carotenoid one of the most potent natural antioxidants, with a singlet-oxygen quenching ability twice as high as that of β-carotene and ten times higher than that of α-tocopherol (Di Mascio et al. 1989). In addition, its activity is synergistically enhanced by the presence of other tomato phytochemicals including β-carotene, phytoene and phytofluene (Shixian et al. 2005). These properties are thought to be responsible for the apparent inverse association between the consumption of lycopene-rich foods and the incidence of cardiovascular disease and cancer (Kelkel et al. 2011; Riccioni et al. 2008; Seren et al. 2008).
During fruit ripening, lycopene accumulates in the outer skin layer, where carotenoid levels above five times higher than in the pulp are generally found (Sharma and LeMaguer 1996). While the peel fraction of tomato pomace is a particularly rich source of lycopene, attempts to recover it by either conventional (Strati and Oreopoulou 2011) or supercritical (Rozzi et al. 2002; Sabio et al. 2003) extraction have met only limited success. One reason is to be found in the compactness of the tomato peel tissue, which hinders solvent penetration and transport to the lycopene-containing chromoplasts (Hetzroni et al. 2011). In addition, in tomato peels, contrary to what is observed in the fruit pulp, lycopene is found almost exclusively in the bound form (Sharma and LeMaguer 1996; Shi et al. 2002), which results in the necessity to operate under harsher conditions to allow its recovery. Finally, once released from the protective plant matrix, lycopene becomes easily subject to degradation. Trans-to-cis isomerization is the first step of degradation, followed by oxidation, from which a variety of compounds belonging to the classes of apo-lycopenals and apo-lycopenones are formed (Caris-Veyrat et al. 2003; Xianquan et al. 2005).
Recently, we found that pretreating tomato peels by cell-wall-degrading enzymes can significantly reduce the extraction time and temperature and increase the efficiency of lycopene recovery (Lavecchia and Zuorro 2008; Zuorro et al. 2011). Similar results were obtained with tomato paste as the lycopene source (Zuorro and Lavecchia 2010), suggesting that the use of enzymes can be a simple and effective means for enhancing lycopene recovery from tomato plant tissue and allowing extraction to be performed under milder conditions.
In this study, we have investigated the possibility of using the enzyme-based approach to produce a lycopene-enriched seed oil from tomato pomace. The oil was extracted from the seed fraction of the waste and was subsequently enriched in lycopene by incorporation of a tomato oleoresin obtained from the enzymatically treated peels. The feasibility of this approach was tested by using the waste produced from a tomato processing company in Italy and a commercially available enzyme preparation for food applications.
Materials and Methods
Chemicals and Enzymes
Acetone, ethanol and hexane with purities greater than 99.7, 99.5 and 99 %, respectively, were purchased from Carlo Erba (Milano, Italy). Butylated hydroxytoluene (BHT) with a purity greater than 99 % was from Sigma-Aldrich (Milan, Italy). Natural lycopene standard (10 % by weight, from tomatoes) was obtained from LycoRed Natural Products Industries Ltd. (Beer-Sheva, Israel).
Peclyve LI, a multi-enzyme preparation from a selected strain of Aspergillus niger, was from Lyven S.A. (Colombelles, France). The product was in liquid form and was stored in the dark at 4 °C. The main enzymes in the preparation were polygalacturonase (EC 3.2.1.15) and pectin methylesterase (EC 3.1.1.11), with minor cellulase and hemicellulase activities. The declared activities, expressed per unit weight of the enzyme preparation, were 1,300 U mg−1, for polygalacturonase, and 400 U mg−1, for pectin methylesterase, where 1 U is defined as the amount of enzyme that liberates 1 μmol of reducing endgroups from polygalacturonic acid in 1 min at 30 °C and pH 4.2. To obtain the desired enzyme concentration in the pretreatment solution, appropriate amounts of the enzyme preparation were diluted with distilled water.
Tomato Processing Waste
Tomato waste was obtained from ASSO.PRO., a tomato processing company located in central Italy (Guglionesi, CB). The seeds were separated from the peels by sedimentation in water using a glass cylinder with a height of 550 mm and an inner diameter of 70 mm. Due to the lower specific gravity, tomato peels floated on the surface of water while the seeds settled to the bottom. After separation, the two fractions were recovered, rinsed thoroughly in water and left to dry in air for a few hours. Tomato seeds were dried at 50 °C for 12–15 h and stored at 4 °C together with the partially dehydrated peels. Moisture measurements were made using an electronic moisture analyser (MAC-50, Radwag Waagen GmbH, Germany).
Seed Oil Extraction
A continuous laboratory press extractor (Komet CA59G, IBG Monforts, Germany) was used for the extraction of oil from tomato seeds. The extractor had a nominal capacity of 3–5 kg seeds/h and was driven by a 1.1-kW variable-speed motor. In a typical test, 5 to 6 kg of dried tomato seeds was processed. The collected oil and the seed cake were stored at room temperature in the dark.
Lycopene Recovery from Tomato Peels
Lycopene extraction experiments were carried out in 100-mL stirred flasks placed in a thermostated water bath (± 0.1 °C). In a typical experiment, 0.5 g of partially dehydrated tomato peels was charged into the flasks together with the enzyme solution. The other experimental conditions (temperature, liquid-to-solid ratio, pretreatment and extraction times) were varied according to the factorial design described below. Lycopene recovery after pretreatment was made by adding 30 mL of hexane in each flask and keeping the system under agitation for the required time. Once the extraction was completed, stirring was stopped and the organic and aqueous phases were allowed to separate. A sample of the hexane supernatant was then taken, passed through a 0.45-μm filter and analysed for lycopene content.
In order to determine the influence of the main process variables on lycopene recovery, a two-level full-factorial design was used. The factors investigated were: temperature (T), pretreatment time (t P), extraction time (t E), liquid-to-solid ratio (R) and enzyme load (L) at two levels (Table 1). The levels of each factor were chosen to cover a range of values of practical interest. Then the test variables were coded to vary between −1 and +1 using the following equations:
Table 2 reports the experimental design, consisting of 25 runs. They were performed in a randomized order to minimize the effects of variability in the observed responses due to extraneous factors.
Preparation of Lycopene-Enriched Seed oil
Tomato oleoresin was produced in a glass reactor with a working volume of about 1 L provided with a thermostated water jacket and a mechanical stirrer (60-mm-diameter two-blade impeller operated at 300 rpm). The reactor was operated under conditions close to those found to be most effective in small-scale experiments. More specifically, 50 g of partially dehydrated tomato skins and 250 mL of the enzyme solution were first loaded into the reactor. The suspension was incubated at 25 °C for 4 h, after which time 400 mL of hexane was added and the system was kept under stirring for further 3 h. Then, the organic solvent was recovered from the reactor and evaporated in a rotary evaporator (Rotavapor R-215, BÜCHI Labortechnik AG, Switzerland). The resulting residue, the tomato oleoresin, was weighed and analysed for lycopene content. Finally, the lycopene-enriched oil samples were prepared by dissolving the appropriate amounts of oleoresin in the tomato seed oil.
Physicochemical Characterization of Materials
The physicochemical properties of the oil and the seed cake were determined according to the official methods of American Oil Chemists’ Society, International Organization for Standardization, Norme Grassi e Derivati (NGD) and Ente Nazionale Italiano di Unificazione. Tomato seed oil was characterized for acidity, peroxide value, tocopherols, fatty acid composition and sterol profile. The seed cake was analysed for total protein, crude fibre, ash content and amino acid profile. Total lycopene content in the peels was evaluated according to the procedure of Fish et al. (2002) using the mixture hexane–acetone–ethanol 50:25:25 (v/v) as extraction solvent and BHT (0.05 % w/v in acetone) as antioxidant.
Lycopene concentration in the enriched oil samples was determined by a double-beam UV–VIS spectrophotometer (Lambda 25, Perkin Elmer, USA). Figure 2 shows a typical absorption spectrum of the oil, with the three characteristic peaks of lycopene at around 456, 483 and 518 nm. To minimize interference from other carotenoids, measurements were made at 518 nm using a molar extinction coefficient of 1.303 × 105 M−1 cm−1. This value was determined from a calibration curve obtained by dissolving the lycopene standard in the oil.
Statistical Analysis
All analytical determinations were carried out in duplicate on three oil or oleoresin samples. The results were expressed as the mean ± standard deviation. The design of experiments and the statistical analysis of influential factors were performed using the Minitab® software package (version 15.1.0.0, Minitab Inc., State College, PA, USA).
Results and Discussion
Preliminary characterization of the waste as received showed that its moisture content was 83.6 wt.% and that it consisted of about 80 % peels and 20 % seeds. The moisture content of the single fractions was about 86 wt.% for the peels and 74 wt.% for the seeds.
Seed Oil Extraction
The oil content of tomato seeds was determined by the NGD method B4-1976 and found to be 30.8 % on a dry weight basis. About 53 % of the oil was recovered by pressing, which corresponds to a production of approximately 153 kg of oil/ton of dry seeds.
The oil content found in this study falls within the range of previous estimates of 21.8 % (Lazos et al. 1998) and 34.5 % (Giannelos et al. 2005), confirming that tomato seeds are a rich source of oil. The attempts made so far to obtain an edible oil from these seeds have predominantly focused on solvent extraction. For this purpose, both conventional organic solvents and supercritical fluids were used. Eller et al. (2010) showed that extraction rates between 20 and 23 % can be achieved using hot hexane and hot ethanol as solvents. The amounts of phytosterols extracted by the two solvents were essentially the same, but the extraction with hexane provided a greater amount of antioxidants. Similar results were obtained with petroleum ether as the solvent (Lazos et al. 1998). Although hexane is commonly used for the commercial production of seed oils, its use raises a number of problems. In particular, the presence of solvent residues in the final products is a matter of growing concern to consumers. In addition, a large proportion of polar compounds such as flavonoids and phenolic acids may remain unextracted, thereby reducing the nutritional value and the functional properties of the oil (Van Hoed 2010).
Application of supercritical fluid technology to the recovery of tomato seed oil indicated that high yields can be obtained by appropriate selection of operating conditions. For example, about 96 % of the oil was recovered with supercritical carbon dioxide at 50 °C and 30 MPa (Shen and Xu 2005). Another study conducted in the temperature range 40–70 °C and pressure 10.8–24.5 MPa showed that higher extraction efficiencies can be achieved by operating at lower temperatures and higher pressures (Roy et al. 1996). This was attributed to the positive effect of increased solvent density on the oil solubility in carbon dioxide. Similar results were provided by Eller et al. (2010), who also found that the phytosterol content of the extracted oil was higher than that obtained by conventional organic solvents, whereas the endogenous antioxidants were in lower amount. In addition to compositional changes caused by solvent-affinity effects, during the extraction process chemical changes may occur. For example, polyunsaturated fatty acids can undergo oxidative degradation, leading to the formation of hydroperoxides and other oxidation products (Demirbas 2010). Harsher extraction conditions can also promote the isomerization of lycopene and β-carotene and modify the functional characteristics and the stability of the oil (Spanos et al. 1992; Longo et al. 2012).
From the above considerations, it can be concluded that, compared to solvent or supercritical fluid extraction, mechanical pressing gives lower extraction yields but preserves the functional properties of the oil to a greater extent. Furthermore, this procedure has no detrimental effects on the environment and human health.
Tomato Seed Cake
Inspection of Table 3 shows that the seed cake, the by-product of oil extraction, was rich in protein (38.4 wt.%) and crude fibre (17 wt.%) and had a low ash content (1.9 wt.%). Determination of the amino acid composition of the protein after hydrolysis revealed that Glu, Asp and Arg were the major amino acids, while Met was the less abundant (Fig. 3). It can be seen that the seed cake contained all essential amino acids except Trp. These properties, and the fact that tomato seeds were not solvent extracted, suggest a possible use of the cake for food fortification or other nutritional applications. In this regard, some experimental studies have demonstrated that the incorporation of tomato seed meal in bread could improve loaf volume, texture and crumb quality (Carlson et al. 1981; Sogi et al. 2002; Majzoobi et al. 2011). Other studies have highlighted its suitability for enriching cereal products or low-lysine foods (Ekthamasut 2006; Altan et al. 2008). Accordingly, it can be stated that tomato seed cake could be utilized as a functional component rather than simply composted or used for animal feeding.
Enzyme-Assisted Extraction of Lycopene from Tomato Peels
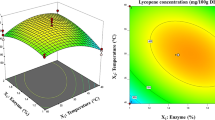
The lycopene content of tomato peels was 2.88 ± 0.23 mg/g dw. Lycopene extraction yields (y) were expressed as the percentage of the amount of lycopene extracted to the total amount of lycopene in the peels. As is evident from Table 2, the measured yields were between 27.6 and 82.3 % and the maximum was achieved under the following conditions: temperature = 25 °C, pretreatment time = 4 h, extraction time = 3 h, liquid-to-solid ratio = 20 mL/g and enzyme load = 0.25 g/g.
To evaluate the contribution of the five main factors and their interactions to the extraction efficiency, we used the following polynomial equation:
where a i are the coefficients associated with the main effects; a ij , a ijk and a ijkl are those related to the binary, ternary and quaternary interactions; a 12345 is the fifth-order interaction coefficient and the x’s are the coded variables. The model described by the above equation contains 32 unknown coefficients, representing the contribution of each factor, alone or in combination with the others, to y. It should be noted that because the independent variables were made dimensionless and normalized between −1 and +1, all of the coefficients can be compared directly with one another. Moreover, a positive (negative) value for a coefficient is indicative of a direct (inverse) association between the term containing that coefficient and the dependent variable.
The 32 coefficients were determined from the data in Table 2, leading to the results reported in Table 4. The standard deviation of the experimental response was estimated from the central point of the factorial design (x 1 = x 2 = x 3 = x 4 = x 5 = 0), which was replicated six times. Then, the Student’s t value for each coefficient at the 95 % confidence level was calculated. As indicated in Table 4, eight out of the 32 coefficients were statistically significant. In addition to the intercept, a 0, they included the five coefficients associated with the main effects (T, t P, t E, R, L) and those related to the binary interactions T × t P and T × R. From the Pareto chart in Fig. 4, it can also be seen that the interaction effects were smaller than those for the main factors and that both positive and negative associations were present. The negative sign of the temperature coefficient could be the result of increased degradation of lycopene at higher extraction temperatures (Xianquan et al. 2005), while that of the liquid-to-solid ratio term could be explained by considering that the larger the volume of the enzyme solution, the higher the mass-transfer resistance for the lycopene molecules diffusing into the solvent. It follows that lycopene recovery should be carried out at the lowest possible temperature and liquid-to-solid ratio.
For practical purposes, a simplified expression can be derived for the yield of lycopene extraction from tomato peels by removing the non-significant terms from the complete polynomial model. Under these conditions, Eq. 2 reduces to:
The eight coefficients in Eq. 3 were estimated by least-squares regression using the data in Table 2. A fairly good agreement was found between the experimental and calculated yields (Fig. 5), with an average error of about 7 %.
Comparison between experimental (y exp) and calculated (y calc) lycopene extraction yields obtained by using Eq. 3. The dashed lines delimit the ±15 % deviation band
To test for normality of residuals, a normal-probability plot was constructed by plotting the model residuals:
against the corresponding normal-order statistics medians:
where F represents the standard normal cumulative distribution function. Data plotted in this way should lie on a straight line, with the intercept and slope being, respectively, equal to the location and scale parameters of the normal distribution (Myers and Montgomery 1995). The results shown in Fig. 6 indicate that a linear plot is obtained (R 2 = 0.965), with only small deviations in the lower and upper parts of the diagram. As a result, the simplified model described by Eq. 3 can be considered statistically significant and used for correlating the experimental data or for describing the influence of process conditions on the yield of lycopene extraction.
Preparation and Properties of Lycopene-Enriched Seed Oil
The production rate of tomato oleoresin in the batch stirred reactor was 24 ± 1.3 g/kg of dry peels, and its lycopene content was 6.8 ± 0.5 wt.%. The oleoresin was incorporated into the oil in amounts appropriate to achieve the desired lycopene levels. The resulting product had a pleasant tomato fruit-like odour and a colour ranging from bright orange to deep red, depending on the lycopene content (Fig. 7). Its properties are presented in Tables 5, 6 and 7.
Free acidity (FA), expressed as oleic acid, was 1.6 wt.% and the peroxide value (PV) was 2 mEq of active oxygen per kg of oil (Table 5). FA and PV are important quality parameters for edible oils. FA is a measure of the extent to which hydrolysis has liberated fatty acids from their ester linkage with the parent triglyceride molecule, while PV is an indicator of oxidative stability, being related to the amount of peroxides present in the oil. Peroxides are unstable compounds that tend to degrade into secondary oxidation products such as aldehydes, ketones and conjugated dienes (Köckritz and Martin 2008). Since oxidation can cause the development of off-flavours and negatively affect the nutritional properties of the oil, the lower the PV, the better the oil quality and the higher its shelf life (Leclercq et al. 2007). In most cases, oils become rancid when PV reaches a value between 20 and 40 mEq O2/kg. For virgin or cold-pressed oils, guidelines from the Codex Alimentarius Commission recommend a maximum level of 15 mEq O2/kg (Codex Alimentarius Commission 2005). However, the rate of oil deterioration also depends on additional factors such as the fatty acid composition of the oil and the presence of antioxidants or other minor components (Chen et al. 2011).
Over 78 % of the total fatty acids were unsaturated (Table 6). Linoleic (C18:2) and oleic (C18:1) acids were the major unsaturated components, followed by linolenic (C18:3) and palmitoleic (C16:1) acids. The predominant saturated fatty acids were palmitic (16:0) and stearic (C18:0) acids. Thus, tomato oil falls in the linoleic–oleic acid oils category and is similar, in terms of fatty acid composition, to sunflower and soybean oils (Ryan et al. 2008).
The total tocopherol content was about 1,300 mg/kg, a value that is among the highest for seed oils (Codex Alimentarius Commission 2005). As shown in Table 5, γ-tocopherol was the most abundant homologue, accounting for over 97 % of total tocopherols, followed by α- and δ-tocopherol. The importance of tocopherols in vegetable oils is primarily related to their antioxidant activity (Choe and Min 2009). At room and moderate temperatures, tocopherols can interrupt the free-radical chain reactions responsible for oxidation by donating hydrogen from their phenolic group. In the case of γ-tocopherol, this mechanism leads to the formation of two dimers, γ-tocopherol biphenyl-dimer and γ-tocopherol ether-dimer, which are still effective as antioxidants. In contrast, α-tocopherol degrades into products exhibiting low or no antioxidant activity (Kochhar 2000). It can, thus, be concluded that the predominance of γ-tocopherol over the α-form makes an oil more resistant to oxidative degradation and increases its shelf life.
The total sterol content was about 4,000 mg/kg (Table 7). The major sterols were β-sitosterol (58.4 %) and stigmasterol (11.3 %) while those found in smaller amounts were Δ7-stigmastenol and Δ7-avenasterol (both at 0.2 %). Phytosterols are widely recognized as important components of healthy diets. β-Sitosterol, in particular, has been the subject of increasing interest in recent years because of its presumed ability to provide cardiovascular protection (Loizou et al. 2010) and suppress the progression of certain types of cancer (Park et al. 2007; Zhao et al. 2009).
The lycopene content of the enriched oil ranged from 50 to 500 mg/kg and was obtained by adding from 0.73 to 7.3 g of oleoresin/kg of oil. This range of values was chosen in view of possible applications in the pharmaceutical, nutraceutical and cosmetic fields. As is known, humans do not synthesize lycopene and depend entirely on dietary sources for an adequate supply of this nutrient (Rao and Ali 2007). Increasing evidence suggests that a lycopene-rich diet can prevent or reduce the risk of cardiovascular disease and some cancers. Although more than one mechanism might be responsible for such effects, the observed health benefits are mainly attributed to its strong antioxidant activity (Omoni and Aluko 2005). This is consistent with the widely accepted view that increased oxidative stress is a key event in the development of atherosclerosis as well as carcinogenesis and aging.
A daily intake of 5–7 mg lycopene is considered sufficient to combat oxidative stress and prevent some chronic diseases (Rao and Shen 2002), while higher levels are required for individuals with cancer (Kucuk et al. 2001). Evidence also indicates that dissolving lycopene in fats such as vegetable oils allows a significant increase in bioavailability and hence in efficacy (Sies and Stahl 1998; van het Hof et al. 2000; Fielding et al. 2005). If the lycopene-enriched tomato seed oil were to be used as a source of lycopene, 10 to 14 g of the product with a lycopene content of 500 mg/kg would be sufficient to provide the recommended daily intake for this carotenoid. This amount could, of course, be reduced in the presence of additional dietary sources of lycopene (Rao et al. 2006).
Another interesting application for the enriched oil or the tomato oleoresin would be in the area of nutricosmetics or cosmeceuticals. In particular, they could be used, alone or in combination with sunscreen products, for skin photoprotection. As highlighted by many investigations, skin aging and most types of skin cancers are caused by prolonged or excessive exposure to UV radiation (Andreassi 2011; Rittié and Fisher 2002). Since UV irradiation is associated with increased ROS production, the topical application of antioxidants is considered an effective measure to reduce UV-related skin damage (González et al. 2008). Evidence has also been provided that photoprotection can be further enhanced by systemic administration of carotenoids, particularly lycopene (Anunciato and da Rocha Filho 2012). For example, a significant decrease in sensitivity towards UV-induced erythema was observed in volunteers after 10 to 12 weeks’ intake of lycopene (Stahl et al. 2006). In another study performed on human dermal fibroblasts exposed to UV light, the photoprotective potential of lycopene in combination with vitamin C and/or E was clearly assessed (Offord et al. 2002).
It seems therefore reasonable to say that the lycopene-enriched oil and the oleoresin derived from tomato pomace may have important applications in the functional food sector as well as in the production of cosmetic or cosmeceutical products.
Examples of Application of the Technology
A layout of the proposed treatment system is illustrated in Fig. 8. Tomato pomace is first separated into the peel and seed fractions. Each fraction is partially dehydrated to the appropriate moisture content. The seeds are pressed to yield the vegetable oil and the seed cake, while the peels are subjected to enzymatic treatment. The enzyme-treated material is then contacted with the solvent to recover lycopene and the other compounds released from the peel tissue. This operation results in the production of the exhausted plant material and a two-phase water/organic solvent system. The aqueous enzyme solution is separated from the solvent and recycled to the treatment step. The solvent is vacuum-evaporated and reused as extraction agent. Solvent evaporation leads to the formation of tomato oleoresin, which is finally incorporated into the oil to obtain the lycopene-enriched oil product with the desired degree of enrichment.
Some simulations were performed to estimate the amount of enriched oil obtainable by applying the above-described technology to tomato processing waste. In these calculations, three different amounts of tomato pomace were considered: 1,000, 5,000 and 10,000 tons/year, with the following seed-to-peel weight ratios: 10 and 50 %. The latter range of values accounts for possible changes in the waste composition resulting from the different tomato processing operations (e.g. production of canned tomatoes, tomato paste or juice).
The moisture content of tomato peels and seeds was set to 85 and 75 wt.%, respectively. The seed oil recovery was assumed to be 15 % on a dry weight basis, that of the oleoresin was taken as 20 kg/ton of dry peels and its lycopene content as 6.5 wt.%. Finally, two levels of lycopene enrichment were considered: 100 and 500 ppm. The results are summarized in Table 8. As can be seen, the amount of enriched oil that can be obtained from tomato pomace varies from about 4 to 190 tons/year, depending on the amount of waste treated and its seed content. Another interesting point is the high amount of oleoresin (from 2.7 to 27 tons/year) produced from the peel fraction of the waste. It should be noted that only a small portion of this amount (from approximately 0.4 to 10 %) is required for obtaining the oil with a lycopene level of 100 to 500 ppm. Considering the high selling price of lycopene oleoresin from tomatoes, it can therefore be expected that its production in combination with the enriched oil can further increase the economic value of the initiative.
Conclusions
The accumulation, handling and disposal of tomato pomace is a major problem faced by the tomato processing industry. For this reason, the possibility of transforming it into value-added products could not only be an economic opportunity for the agri-food sector but also a viable solution to the disposal problem. The results of the present investigation demonstrate that tomato pomace can be effectively used for the production of a lycopene-enriched seed oil and a tomato oleoresin. The oil content of the seeds is high enough to justify its recovery by pressing, thus avoiding the use of solvents and retaining a higher proportion of polar compounds such as flavonoids and phenolic acids. The compositional characteristics of the oil and the possibility of enhancing its functional properties by incorporation of the oleoresin obtained from the peel fraction of the waste make this product suitable for a wide range of applications in the food, nutraceutical and cosmetic sectors. The approach followed in this study can therefore be suggested as a possible strategy for the sustainable and profitable management of tomato pomace.
References
Altan, A., McCarthy, K. L., & Maskana, M. (2008). Evaluation of snack foods from barley–tomato pomace blends by extrusion processing. Journal of Food Engineering, 84, 231–242.
Andreassi, L. (2011). UV exposure as a risk factor for skin cancer. Expert Review of Dermatology, 6, 445–454.
Anunciato, T. P., & da Rocha Filho, P. A. (2012). Carotenoids and polyphenols in nutricosmetics, nutraceuticals and cosmeceuticals. Journal of Cosmetic Dermatology, 11, 51–54.
Bovy, A., Schijlen, E., & Hall, R. D. (2007). Metabolic engineering of flavonoids in tomato (Solanum lycopersicum): the potential for metabolomics. Metabolomics, 3, 399–412.
Caris-Veyrat, C. C., Schmid, A., Carail, M., & Böhm, V. (2003). Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. Journal of Agricultural and Food Chemistry, 51, 7318–7325.
Carlson, B. L., Knorr, D., & Watkins, T. R. (1981). Influence of tomato seed addition on the quality of the wheat flour breads. Journal of Food Science, 46, 1029–1031.
Chen, B., McClements, D. J., & Decker, E. A. (2011). Minor components in food oils: a critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Critical Reviews in Food Science and Nutrition, 51, 901–916.
Choe, E., & Min, D. B. (2009). Mechanisms of antioxidants in the oxidation of foods. Comprehensive Reviews in Food Science and Food Safety, 8, 345–358.
Codex Alimentarius Commission. (2005). CODEX standard for named vegetable oils, CODEX STAN 210. Rome: Food and Agriculture Organisation of the United Nations.
Del Valle, M., Cámara, M., & Torija, M. E. (2006). Chemical characterization of tomato pomace. Journal of the Science of Food and Agriculture, 86, 1232–1236.
Demirbas, A. (2010). Oil, micronutrient and heavy metal contents of tomatoes. Food Chemistry, 118, 504–507.
Di Mascio, P., Kaiser, S., & Sies, H. (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Archives of Biochemistry and Biophysics, 274, 532–538.
Ekthamasut, K. (2006). Effect of tomato seed meal on wheat pasting properties and alkaline noodle qualities. Australian Journal of Technology, 9, 147–152.
Eller, F. J., Moser, J. K., Kenar, J. A., & Taylor, S. L. (2010). Extraction and analysis of tomato seed oil. Journal of the American Oil Chemists' Society, 87, 755–762.
Fielding, J. M., Rowley, K. G., Cooper, P., & O’Dea, K. (2005). Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pacific Journal of Clinical Nutrition, 14, 131–136.
Fish, W. W., Perkins-Veazie, P., & Collins, J. K. (2002). A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. Journal of Food Composition and Analysis, 15, 309–317.
Giannelos, P. N., Sxizas, S., Lois, E., Zannikos, F., & Anastopoulos, G. (2005). Physical, chemical and fuel related properties of tomato seed oil for evaluating its direct use in diesel engines. Industrial Crops and Products, 22, 193–199.
González, S., Fernandez-Lorente, M., & Gilaberte-Calzada, Y. (2008). The latest on skin photoprotection. Clinics in Dermatology, 26, 614–616.
Hetzroni, A., Vana, A., & Mizrach, A. (2011). Biomechanical characteristics of tomato fruit peels. Postharvest Biology and Technology, 59, 80–84.
Kelkel, M., Schumacher, M., Dicato, M., & Diederich, M. (2011). Antioxidant and anti-proliferative properties of lycopene. Free Radical Research, 45, 925–940.
Khachik, F., Carvalho, L., Bernstein, P. S., Muir, G. J., Zhao, D. Y., & Katz, N. B. (2002). Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Experimental Biology and Medicine, 227, 845–851.
Kochhar, S. P. (2000). Stabilisation of frying oils with natural antioxidative components. European Journal of Lipid Science and Technology, 102, 552–559.
Köckritz, A., & Martin, A. (2008). Oxidation of unsaturated fatty acid derivatives and vegetable oils. European Journal of Lipid Science and Technology, 110, 812–824.
Kong, K. W., Khoo, H. E., Prasad, K. N., Ismail, A., Tan, C. P., & Rajab, N. F. (2010). Revealing the power of the natural red pigment lycopene. Molecules, 15, 959–987.
Kucuk, O., Sarkar, F. H., Sakr, W., Djuric, Z., Pollak, M. N., Khachik, F., et al. (2001). Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiology, Biomarkers & Prevention, 10, 861–868.
Lavecchia, R., & Zuorro, A. (2008). Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. European Food Research and Technology, 228, 153–158.
Lazos, E. S., Tsaknis, J., & Lalas, S. (1998). Characteristics and composition of tomato seed oil. Grasas y Aceites, 49, 440–445.
Leclercq, S., Reineccius, G. A., & Milo, C. (2007). Effect of type of oil and addition of δ-tocopherol on model flavor compound stability during storage. Journal of Agricultural and Food Chemistry, 55, 9189–9194.
Loizou, S., Lekakis, I., Chrousos, G. P., & Moutsatsou, P. (2010). Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Molecular Nutrition & Food Research, 54, 551–558.
Longo, C., Leo, L., & Leone, A. (2012). Carotenoids, fatty acid composition and heat stability of supercritical carbon dioxide-extracted-oleoresins. International Journal of Molecular Sciences, 13, 4233–4254.
Majzoobi, M., Ghavi, F. S., Farahnaky, A., Jamalian, J., & Mesbahi, G. (2011). Effect of tomato pomace powder on the physicochemical properties of flat bread (Barbari bread). Journal of Food Processing and Preservation, 35, 247–256.
Myers, R. H., & Montgomery, D. C. (1995). Response surface methodology. Process and product optimization using designed experiments. New York: Wiley.
Offord, E. A., Gautier, J. C., Avanti, O., Scaletta, C., Runge, F., Krämer, K., et al. (2002). Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radical Biology & Medicine, 32, 1293–1303.
Omoni, A. O., & Aluko, R. E. (2005). The anti-carcinogenic and anti-atherogenic effects of lycopene: a review. Trends in Food Science and Technology, 16, 344–350.
Park, C., Moon, D., Rhu, C. H., Choi, B. T., Lee, W. H., Kim, G. Y., et al. (2007). β-Sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biological & Pharmaceutical Bulletin, 30, 1317–1323.
Rao, A. V., & Ali, A. (2007). Biologically active phytochemicals in human health: lycopene. International Journal of Food Properties, 10, 279–288.
Rao, A. V., & Shen, H. (2002). Effect of low dose lycopene intake on lycopene bioavailability and oxidative stress. Nutrition Research, 22, 1125–1131.
Rao, A. V., Raym, M. R., & Rao, L. G. (2006). Lycopene. Advances in Food and Nutrition Research, 51, 99–164.
Riccioni, G., Mancini, B., Di Ilio, E., Bucciarelli, T., & D’Orazio, N. (2008). Protective effect of lycopene in cardiovascular disease. European Review for Medical and Pharmacological Sciences, 12, 183–190.
Rittié, L., & Fisher, G. J. (2002). UV-light-induced signal cascades and skin aging. Ageing Research Reviews, 1, 705–720.
Roy, B. C., Goto, M., & Hirose, T. (1996). Temperature and pressure effects on supercritical CO2 extraction of tomato seed oil. International Journal of Food Science and Technology, 31, 137–141.
Rozzi, N. L., Singh, R. K., Vierling, R. A., & Watkins, B. A. (2002). Supercritical fluid extraction of lycopene from tomato processing by-products. Journal of Agricultural and Food Chemistry, 50, 2638–2643.
Ryan, L., Mestrallet, M. G., Nepote, V., Conci, S., & Grosso, N. R. (2008). Composition, stability and acceptability of different vegetable oils used for frying peanuts. International Journal of Food Science and Technology, 43, 193–199.
Sabio, E., Lozano, M., Montero de Espinosa, V., Mendes, R. L., Pereira, A. P., Palavra, A. F., et al. (2003). Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Industrial and Engineering Chemistry Research, 42, 6641–6646.
Seren, S., Lieberman, R., Bayraktar, U. D., Heath, E., Sahin, K., Andic, F., et al. (2008). Lycopene in cancer prevention and treatment. American Journal of Therapeutics, 15, 66–81.
Sharma, S. K., & LeMaguer, M. (1996). Lycopene in tomatoes and tomato pulp fractions. Italian Journal of Food Science, 2, 107–113.
Shen, X., & Xu, S. (2005). Supercritical CO2 extraction of tomato seed oil. Journal of Food Technology, 3, 226–231.
Shi, J., LeMaguer, M., Mazza, G. (2002). Lycopene from tomatoes. In Functional foods (pp. 135–167). Boca Raton: CRC.
Shixian, Q., Dai, Y., Kakuda, Y., Shi, J., Mittal, G., Yeung, D., et al. (2005). Synergistic anti-oxidative effects of lycopene with other bioactive compounds. Food Reviews International, 21, 295–311.
Sies, H., & Stahl, W. (1998). Lycopene: antioxidant and biological effects and its bioavailability in the human. Proceedings of the Society for Experimental Biology and Medicine, 218, 121–124.
Sogi, D. S., Sidhu, J. S., Arora, M. S., Garg, S. K., & Bawa, A. S. (2002). Effect of tomato seed meal supplementation on dough and bread characteristics of wheat (PBW-343) flour. International Journal of Food Properties, 5, 563–571.
Spanos, G. A., Chen, H., & Schwartz, S. J. (1992). Supercritical CO2 extraction of β-carotene from sweet potatoes. Journal of Food Science, 38, 817–820.
Stahl, W., Heinrich, U., Aust, O., Tronnier, H., & Sies, H. (2006). Lycopene-rich products and dietary photoprotection. Photochemical & Photobiological Sciences, 5, 238–242.
Strati, I. F., & Oreopoulou, V. (2011). Effect of extraction parameters on the carotenoid recovery from tomato waste. International Journal of Food Science and Technology, 46, 23–29.
van het Hof, K. H., West, C. E., Weststrate, J. A., & Hautvast, J. G. A. J. (2000). Dietary factors that affect the bioavailability of carotenoids. Journal of Nutrition, 130, 503–506.
Van Hoed, V. (2010). Phenolic compounds in seed oil. Lipid Technology, 22, 247–249.
WPTC (2011). World production estimate as of 21 October 2011, Release # 38. http://www.wptc.to/releases/releases38.pdf. Accessed 18 June 2012.
Xianquan, S., Shi, J., Kakuda, Y., & Yueming, J. (2005). Stability of lycopene during food processing and storage. Journal of Medicinal Food, 8, 413–422.
Zhao, Y., Chang, S. K., Qu, G., Li, T., & Cui, H. (2009). Beta-sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. Journal of Agricultural and Food Chemistry, 24, 5211–5218.
Zuorro, A., & Lavecchia, R. (2010). Mild enzymatic method for the extraction of lycopene from tomato paste. Biotechnology and Biotechnological Equipment, 24, 1854–1857.
Zuorro, A., Fidaleo, M., & Lavecchia, R. (2011). Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme and Microbial Technology, 49, 567–573.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuorro, A., Lavecchia, R., Medici, F. et al. Enzyme-Assisted Production of Tomato Seed Oil Enriched with Lycopene from Tomato Pomace. Food Bioprocess Technol 6, 3499–3509 (2013). https://doi.org/10.1007/s11947-012-1003-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-1003-6