Abstract
The present study focused on the full valorization of the tomato by-product, also known as tomato pomace consisting mainly of tomato peels and tomato seeds, by recovering natural antioxidants and edible oil, and subsequently reutilizing the leftover solid residues for the production of low-cost biosorbent. The tomato peel extract recovered using ethanol as food-grade solvent contained high phenol and flavonoid contents (199.35 ± 0.35-mg gallic acid equivalents (GAE)/g and 102.10 ± 0.03-mg quercetin equivalent (QE)/g, respectively). Even its lower content of lycopene (3.67 ± 0.04 mg/100 g), tomato peel extract showed potent antioxidant activity and can be therefore used as natural antioxidants either for food or cosmetic applications. High nutritional quality edible oil (17.15%) was extracted from tomato seeds and showed richness in unsaturated fatty acids (74.62%), with linoleic acid being the most abundant polyunsaturated fatty acid (49.70%). After recovery of these valuable compounds, the extraction solid leftovers were used to produce low-cost biosorbent tested for dye removal. Results showed that the highest biosorption yields were increasingly attributed to the acidic, direct, anthraquinone, then reactive dyes. Overall, the obtained results strongly support the complete utilization of tomato pomace for the recovery of valuable compounds and the sequential production of low-cost biosorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, the problem of food wastes is getting considerable interest since they present an incessant threat to the environment and a serious operational problem for the food industries when suitable management strategies are lacking (Goula and Lazarides 2015). However, many food by-products could serve as source of potentially valuable compounds to be processed inside the food chain as functional additives in different products (Augustin et al. 2020; Azabou et al. 2017; Azabou et al. 2016; Grassino et al. 2019; Kumar et al. 2018; Szabo et al. 2019). Thus, the effective reprocessing of these by-products by adopting the circular economy approach could lead to the notion of zero waste and reduce their environmental impact.
Tunisia is ranked between the top ten global manufacturers of processed tomato products with annual processing capacity of almost 650,000 to 950,000 t of fresh tomatoes, which results in almost 20,000 to 30,000 t of solid waste material called “tomato pomace.” This by-product consists mainly of peels and seeds, is unsuitable for direct human consumption and mostly disposed of as a solid waste (Heguy et al. 2015), or is partially used as a food ingredient in animal feed (Arco-Perez et al. 2017; Biondi et al. 2020). However, the analysis of tomato pomace composition shows that it could constitute an alternative source for the recovery of high value-added compounds. As recently reviewed by Lu et al. (2019), a great variety of biologically active substances are present in both tomato peels and tomato seeds. In fact, tomato peels are rich in dietary fiber (62.76–88.53 g/100 g), lycopene (288 mg/100 g), and phenolic compounds (157.8-mg gallic acid equivalents (GAE)/100 g), whereas tomato seeds are mainly composed of oil (17.8–24.5 g/100 g) and protein (23.6–40.9 g/100 g). Recent strategies were proposed for the recycling and the valorization of tomato pomace by the extraction of high value-added compounds such as phenolic compounds and lycopene (Abid et al. 2017; Bakić et al. 2019; Grassino et al. 2019; Perea-Dominguez et al. 2018; Szabo et al. 2018), pectin (Grassino et al. 2016), and fatty acids (Grassino et al. 2019; Szabo et al. 2019). The possibility of using these valuable compounds for innovative functional food products has become lately of great interest. They were successfully incorporated in meat products (Savadkoohi et al. 2014), wheat flour-based foods (Isik and Topkaya 2016), and fats (Abid et al. 2017; Kehili et al. 2018). Besides to the abovementioned valorization routes, tomato by-products were used as material to produce biosorbents for bioremediation applications (Najafi et al. 2016; Yargic et al. 2015), biodiesel (Giuffrè and Capocasale 2016), and biogas (Allison and Simmons 2017; Li et al. 2016).
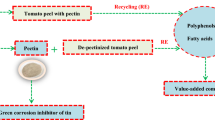
Regardless of the type of the targeted bioactive compound to recover from tomato pomace, an extraction solid residue is usually generated. In compliance with the circular economy concepts, this residue must be further valorized. Allison and Simmons (2017) as well as Scaglia et al. (2020) have recently reported the valorization of the extraction solid residue remained after lycopene extraction from tomato pomace into methane by using the anaerobic digestion process. During the present study, a set of approaches was investigated for the full valorization of tomato pomace (Fig. 1). Polyphenols and lycopene were recovered from tomato peels, while edible oil was extracted from tomato seeds. After this step, the extraction solid leftovers from tomato peels and tomato seeds were mixed and used to produce low-cost biosorbent for further application in dye removal. To the best of our knowledge, the sequential utilization of these extraction solid residues as biosorbent has not been before attempted.
Material and methods
Tomato by-product preparation
Tomato pomace was collected from a local tomato paste factory (SICAM, Mdjez Elbeb). It was open air dried at 45 °C ± 3 °C for 3 days. The dried tomato pomace was then sieved to prepare two different fractions: tomato peels (40%) and tomato seeds (60%). These by-products (tomato pomace, tomato peels, and tomato seeds) were ground into fine powder using a laboratory mill (Grindomix Retsch, Fisher Scientific, Waltham, MA, USA) before being transferred into hermetic storage glass containers and stored at − 20 °C until use.
Chemicals
Folin–Ciocalteu’s reagent, sodium carbonate anhydrous, gallic acid, sodium hydroxide, aluminum chloride anhydrous, 2,2-diphenyl-1-picrylhydrazyl (DPPH), β-carotene, linoleic acid, trichloroacetic acid (TCA), ferric chloride anhydrous, ascorbic acid, quercetin, and dyes were purchased from Sigma-Aldrich (Germany). Potassium ferrocyanide and potassium acetate were obtained from Merck (Darmstadt, Germany), whereas HPLC-grade ethanol, chloroform, and n-hexane were supplied by Sigma Chemical Co. (USA). All used chemicals were of analytical grade (purity > 95%) and were used as received without any further purification.
Physicochemical analysis of tomato pomace, tomato peels, and tomato seeds
The proximate composition of tomato pomace, tomato peels, and tomato seeds was undertaken by analyzing moisture, protein, lipid, and ash contents according to the AOAC official methods (AOAC 2000). Iron, zinc, copper, sodium, calcium, potassium, and magnesium were determined using an atomic absorption spectrophotometer (Hitachi Z6100, Tokyo, Japan) according to Luten et al. (1996). Total, soluble, and insoluble dietary fibers were determined with the enzymatic and gravimetric methods described by Prosky et al. (1988).
Extraction procedure of bioactive compounds from tomato peels
Powder of tomato peels was extracted using the shaking method with absolute ethanol (purity ≥ 99.8%) in dark flasks at a sample to solvent ratio of 1:60 (w/v), at 150 rpm and 25 °C for an extraction time of 6 h. Afterwards, the extracts were filtered using Whatman No. 1 filter paper (Whatman, Fisher Scientific, Schwerte, Germany). Ethanol was removed in a vacuum rotary evaporator at 40 °C, and the extract was kept in a refrigerator at 4 °C until further analyses.
Quantification of bioactive compounds in tomato peel extract
The lycopene content of tomato peel extract was measured at 472 nm using a UV/vis spectrophotometer (UV mini-1240, Shimadzu, Kyoto, Japan) according to Strati and Oreopoulou (2011a). The lycopene concentration C (mg/L) was calculated as:
where A is the absorbance of the extract at λmax specified for each solvent and E is the absorption coefficient (absorbance at a given wavelength of a 1% solution in a spectrophotometer cuvette with a 1-cm light path) of lycopene in the respective solvent (Strati and Oreopoulou 2011b). The extraction yield was calculated as:
where C is the lycopene concentration in the solvent, V is the liquid volume, and M is the amount of tomato peel powder.
The total phenol content (TPC) of tomato peel extract was determined according to Cicco et al. (2009). TP ethanolic extract (20 μL) was mixed with 100 μL of Folin–Ciocalteu’s reagent. Afterwards, the mixture was shaken and then incubated for 2 min at 25 °C. A total of 800 μL of sodium carbonate solution (5%) was added, and the mixture was shaken once again for 1 min, incubated in the dark for 20 min at 40 °C, and then immediately cooled in ice bath. The absorbance of the resulting color was measured at 760 nm against a distilled water/sodium carbonate/Folin–Ciocalteu’s reagent blank. Gallic acid (GA) was used as the standard for the calibration curve. TPC was expressed as mg GAE per gram of extract.
The total flavonoid content (TFC) of tomato peel extract was determined using the method described by Chang et al. (2002). Briefly, 500 μL of tomato peel extract was mixed with 1.5 mL of ethanol, 100 μL of potassium acetate (1 M), 100 μL of AlCl3 (10%, w/v), and 2.8 mL of distilled water. The mixture was well mixed and then incubated for 30 min at 25 °C. The absorbance of the mixture was then measured at 415 nm. TFC was expressed as mg quercetin equivalent (QE) per gram of extract.
Antioxidant assays
Different methods have been tested to assess the antioxidant potential of the ethanolic extract prepared from tomato peels.
DPPH radical scavenging activity
The antiradical ability of tomato peel extract was evaluated according to Bersuder et al. (1998). Tomato peel extract (500 μL) was mixed with 375 μL of ethanol and 125 μL of an ethanolic DPPH solution (0.02%). The mixture was stirred vigorously and then placed for 1 h at 25 °C in the obscurity. The decrease in absorbance was measured at 517 nm using a UV-vis spectrophotometer. The radical scavenging activity (RSA) was calculated as a percentage of DPPH∙ inhibition as follow:
where Acontrol is the absorbance at 517 nm of 875 μL of ethanol mixed with 125 μL of ethanolic DPPH solution, ADPPH∙ (sample) is the absorbance at 517 nm of 500 μL of tomato peel extract mixed with 375 μL of ethanol and 125 μL of ethanolic DPPH∙ solution, and ADPPH∙ (blank) is the absorbance at 517 nm of 500 μL of tomato peel extract mixed with 500 μL of ethanol.
Reducing power assay
The Fe-reducing power which measures the change of Fe3+ to Fe2+ was determined according to Yildirim et al. (2001). Tomato peel extract (1 mL) was mixed with 1.25 mL of phosphate buffer (0.2 M; pH 6.6) and 1.25 mL of potassium ferrocyanide [K3Fe(CN)6] (10 g/L). After incubation for 30 min at 50 °C, 1.25 mL of TCA (10%) was added. A total of 1.25 mL of the obtained mixture was added to 1.25 mL of distilled water and 0.25 mL of FeCl3 (1 g/L). After 10 min, the absorbance at 700 nm was measured. A higher absorbance indicates a better Fe-reducing power and thus a better reducing power of the tomato peel extract.
β-carotene bleaching assay
The antioxidant activity of tomato peel extract was also evaluated by using the β-carotene linoleate model system according to Koleva et al. (2002). β-carotene solution and linoleic acid were prepared with Tween 80 and chloroform (≥ 99.5%). The solvent was then evaporated, and the residue thus obtained was dissolved in 100 mL of bi-distilled water. A total of 500 μL of different dilutions of tomato peel extract was added to 2.5 mL of the prepared solution. The mixtures were then incubated for 2 h at 50 °C, and the absorbance was measured at 470 nm before and after incubation. Controls and blanks were prepared for each dilution of tomato peel extract by using the same procedure, but the control contained 500 μL of bi-distilled water instead of the tomato peel extract and the blanks were β-carotene-free. The zero sample was prepared with 500 μL of ethanol and 2.5 mL of the solution without β-carotene. The antioxidant activity was measured using the following equation:
where A0sample, A0b, and A0c are absorbances of the sample, blank, and control, respectively, at 470 nm before incubation, and A120 sample, A120b, and A120c are those of the sample, blank, and control, respectively after incubation for 120 min at 50 °C.
Extraction and analysis of oil from tomato seeds
Tomato seeds (25 g) were placed in a 1-L dark flask and homogenized with 250 mL of n-hexane (purity ≥ 99.0%). After mixing for 4 h in a shaker at a rate of 180 rpm/min, the mixture was centrifuged for 15 min at 1000g at room temperature (20 °C). The supernatant was then filtered through a Whatman No. 2 filter paper (Whatman, Fisher Scientific, Schwerte, Germany). The extraction procedure was repeated twice, and the collected solvent was removed using a rotary evaporator at 40 °C. The obtained seed oil was finally drained under a stream of nitrogen and then stored in a freezer (− 20 °C) for subsequent physicochemical analyses (Cheikh-Rouhou et al. 2007). Peroxide, iodine, saponification, and acidity values were determined according to the AOCS official methods (1997). Determination of refractive index (at 40 °C) was determined with a refractometer (type Abbe optic system, Germany). K232 and K270 extinction coefficients were calculated from absorbances at 232 and 270 nm, respectively, with a UV/vis spectrophotometer (UV mini-1240, Shimadzu, Kyoto, Japan), using a 1% solution of oil in cyclohexane and a path length of 1 cm. Fatty acid (FA) composition of tomato seed oil was determined using gas chromatography after conversion of FA to fatty methyl esters (FAMEs) according to the AOAC method 965.33 ( 2000). Analyses of FAMEs were carried out using a Hewlett Packard 5890 Series II gas chromatograph (Hewlett Packard Co., Amsterdam, Netherlands) equipped with a hydrogen flame ionization detector (Shimadzu, Japan) and a capillary column (3 m × 0.25 mm, Agilent, Machelen, Belgium). The column temperature was programmed from 180 to 240 °C at 5 °C/min, and the injector and detector temperatures were set at 250 °C. Identification and quantification of FAMEs were accomplished by comparing the retention times of peaks with those of pure standards purchased from Sigma and analyzed under the same conditions. The results were expressed as a percentage of individual FA in the lipid fraction.
Biosorption study
Preparation and characterization of the biosorbent
The extraction solid residues that remained after the extraction of bioactive compounds and oil respectively from tomato peels and tomato seeds were mixed and used to produce biosorbent. In order to increase their surface area, these solid residues were pretreated (activated) by carbonization at 500 °C for 1 h. The thermally treated solid residues were then immersed in a sodium hydroxide aqueous solution (0.5 N) at the solid-liquid ratio of 1/50 (w/v). The biosorbent was finally washed with distilled water until the pH of water was neutral. The obtained biosorbent has a particle size ranged from 13 to 445 μm.
FT-IR spectroscopy was used to identify the chemical groups in the biosorbent. The infrared spectra of biosorbent were recorded with a light source in the middle infrared range (650–4000 cm−1) in a NICOLET spectrometer (Cary 630 Agilent, Germany). The particle morphology of the biosorbent was examined by using a scanning electron microscope JEOL (JFC-1100E, USA) with an accelerating voltage of 15 kV.
Adsorbates
Several dyes, purchased from Sigma-Aldrich (Germany), were used in the present study. They are belonging to different families namely; reactive black 5 (di-azoic), acid orange 51 (acidic), direct red 75, direct blue 86 (direct), and remazol brilliant blue R (anthraquinone). Dyes stock solutions were prepared by dissolving 5 g/L of each dye in distilled water. The working concentrations were obtained by diluting the stock solutions, and dye concentrations were measured at the λmax 597, 592, 520, 446, and 614 nm respectively corresponding to reactive black 5, remazol brilliant blue R, direct red 75, acid orange 51, and direct blue 86.
Biosorption experiments
Biosorption assays were conducted in 50-mL flasks at a fixed biosorbent concentration (1 g/L), dye concentrations (50 and 100 mg/L), pH (3.0), temperature (30 °C), incubation time (1 h), and speed stirring (150 rpm). The solution pH was adjusted using a 0.1-M HCl solution. The concentration of the remaining dyes in the adsorption medium was determined, after incubation, by measuring the optical density at the appropriate λmax of each dye, using an UV/vis spectrophotometer (UV mini-1240, Shimadzu, Kyoto, Japan).
Biosorption yields corresponding to the different used dyes were calculated using the following equations:
where:
C0 (mg/L): initial dye concentration
Ceq (mg/L): dye concentration at equilibrium
qeq (mg/g): quantity of biosorbed dye per unit mass of biosorbent at equilibrium
r (g/L): mass of biosorbent per liter of aqueous solution.
Statistical analysis
All analytical determinations and measurements were performed at least in triplicate. Values were expressed as the mean ± standard deviation (n = 3). Analysis of variance was conducted, and differences between variables were tested for significance by one-way analysis of variance using the SPSS software, 17.0 (Professional edition, SPSS Inc., Hong Kong, China). A difference was considered statistically significant when P < 0.05.
Results and discussion
Chemical composition of tomato pomace, tomato peels, and tomato seeds
Proximate composition, dietary fiber, and mineral content of tomato pomace, tomato peels, and tomato seeds are summarized in Table 1. All these by-products were characterized by the predominance of carbohydrates followed by proteins and lipids. Crude proteins in tomato seeds (25.50 ± 0.53%) were almost twice of that in tomato peels (14.47 ± 0.50%). Moreover, tomato seeds were particularly rich in fat (17.15%) compared with tomato peels, which depicted a very low fat content (1.77%). Both oil and protein average contents in tomato seeds are in the interval of those reported by Lu et al. (2019) that varied from 17.8 to 24.5% and from 23.6 to 40.9%, respectively. The mineral composition of tomato peels and tomato seeds showed that the major elements were K, Ca, Na, Fe, and Mg, while Zn was noticeably present in tomato seeds. These results agree with those of Elbadrawy and Sello (2016) and Navarro-González et al. (2011) who also suggested that tomato by-products could be used as protective agent for cardiovascular diseases, taking into account the low Na/K ratio. On the other hand, both tomato peels and seeds contained most of the antioxidant minerals especially Ca, Mg, Zn, Fe, and Cu (Navarro-González et al. 2011). Regarding the total dietary fiber content, results showed that crude fiber content was much higher in tomato peels than in tomato seeds. The present values are lower than those reported by Navarro-González et al. (2011), which could be due to the genetic diversity and climatic variations of the tomato fruits. The ratio of insoluble dietary fibers/soluble dietary fibers was around 8/1 and 4/1 for tomato peels and tomato seeds, respectively. In fact, these compartments of vegetables are usually rich in cellulose, hemicellulose, and lignin that make the insoluble dietary fibers. Unlike tomato seeds, the TPC was higher in tomato peels (46.83 ± 0.73-mg GAE/g against 27 ± 0.03-mg GAE/g, respectively). These values were much higher than those reported by Navarro-González et al. (2011), but lower than TPC stated by Bakić et al. (2019) in tomato peels (53.12-mg GAE/g). On the other hand, Toor and Savage (2005) have already shown that peels of some tomato fruits contain significantly higher levels of polyphenols, flavonoids, and lycopene, and thus a higher antioxidant activity than pulp and seed fractions.
Extraction of bioactive compounds from tomato peels
Tomato peels were investigated as potential source of natural antioxidants such as polyphenols and lycopene. These components were simultaneously extracted after the maceration of tomato peels with 99.8% ethanol as an eco-friendly conventional solvent extraction technique. Ethanol was chosen to extract antioxidant compounds since it is a non-toxic, food-grade, cheap, and environmentally friendly solvent (Azabou et al. 2020; Butsat and Siriamornpun 2016). Ethanolic extract recovered from tomato peels was red colored revealing the presence of the red-colored pigment lycopene. The total phenol content and total flavonoid content in tomato peel extract were about 199.35 ± 0.25-mg GAE/g and 102.10 ± 0.03-mg QE/g of extract, respectively (Table 2). Further studies should be performed to enhance the extraction of polyphenols from tomato peels, using newer extraction procedures rather than the traditional methods like maceration. In fact, Bakić et al. (2019) have obtained remarkable amounts of polyphenols from tomato peel waste with minimal time expenditure by applying the microwave-assisted extraction as an emerging technique. In the same vein, Coelho et al. (2019) have reported that ohmic heating can significantly increase flavonoid (naringenin, rutin, and kaempferol) extraction from tomato by-products. Juric et al. (2019) showed that treatment of tomato peels by high-pressure homogenization increased the release of intracellular compounds including polyphenols.
Regarding the lycopene content in the tomato peel extract, its value was in the range of 3.67 ± 0.04 mg/100 g. Several attempts have been performed for the lycopene extraction from tomato pomace and tomato peels, using either conventional or non-conventional extraction techniques. In summary, the lycopene extraction was enhanced by using non-polar solvents such as hexane (Lu et al. 2019) and olive oil (Kehili et al. 2019), which are suitable to extract lipophilic compounds. However, due to the defects of conventional solvent methods, innovative approaches were suggested with promising perspectives, such as ultrasound-, pulsed electric fields-, and enzyme assisted methods as well as supercritical carbon dioxide extraction (Allison and Simmons 2017; Lu et al. 2019; Pataro et al. 2020; Scaglia et al. 2020).
Tomato peel extract was examined for its antioxidant potential, owing to the high levels of bioactive compounds, mainly polyphenols. Results from Table 3 showed that tomato peel extract showed potent antioxidant activity particularly in terms of the inhibition of the β-carotene bleaching, which reached 80.64% at 400 μg/mL of extract. Moreover, it should be noted that tomato peel extract showed a dose-response behavior in all the tested antioxidant assays. Therefore, tomato peels showed a promising source for natural food additives to replace synthetic antioxidants, avoiding the lipid oxidation and extending the food shelf life.
Characterization of tomato seed oil
The oil content of tomato seeds was about 17.15%. It has a strong odor recalling the odor of sun-dried tomatoes and a yellow orange color as shown in Fig. 1. Basing on the data presented in Table 4, tomato seed oil presents worthy qualities conformingly to the Codex standard for vegetable oils (CAC 1999). In fact, its refractive index determined at 40 °C is 1.4655, which is comparable with soy bean oil and olive oil (Cheikh-Rouhou et al. 2007). Its higher iodine value expresses its particular nutritional quality, owing to the high unsaturated fatty acid content as shown in Table 5. The acid value of 12.8% indicates that the proportion of the free fatty acid content was in the interval range of those in the edible oils soybean oil, mustard oil, and palm oil (CAC 1999). In fact, at free fatty acid percentage below 1.5%, oil could be supposed as suitable for edible purposes. Tomato seed oil showed peroxide value of 1.74-meq O2/kg and specific extinction coefficients of 2.357 and 0.605 as calculated from absorbance at 232 and 270 nm, respectively.
Table 5 shows the FA composition of tomato seed oil that involves particularly unsaturated fatty acids (74.62%). In fact, oleic acid occurs in the greatest amounts among monounsaturated fatty acids (23.64%) and linoleic acid as the prominent polyunsaturated fatty acid (49.70%). This result is in good agreement with that reported by Yilmaz et al. (2015). Therefore, tomato seed oil could be considered as an up-coming edible oil due to its high nutritional quality, owing to its high polyunsaturated fatty acid content as recently reviewed by Lu et al. (2019). In fact, linoleic acid may have favorable nutritional implications and beneficial physiological effects in the prevention of coronary heart disease and cancer. Thus, tomato seed oil could be very appealing for food and pharmaceutical industrial applications.
Biosorbent production from the extraction solid residues
Agro-industrial wastes may have a potential as inexpensive sorbents. They could be assumed as “low cost” since they derived from by-products which are easily available in large quantities, renewable, non-toxic, and generally require little processing, and some of them can be regenerated and reused (Yargic et al. 2015). In this section, the extraction solid residues that remained after the recovery of bioactive compounds and edible oil respectively from tomato peels and tomato seeds were mixed to produce low-cost biosorbent by activation using a cascade of thermal and chemical treatments.
Biosorbent characterization
The FT-IR spectroscopy performed in the range of 650–4000 cm−1 was used to identify functional groups at the biosorbent surface that may be responsible for dye binding. Figure 2 showed a large band at 3226 cm−1, which may be assigned to the O–H stretching vibration of carboxylic acids. The sharp bands at 2920 cm−1 and 2851 cm−1 are attributed to the C–H stretching. The peak at 1707 cm−1 mainly refers to the characteristics of the C=O stretches of the carbonyls such as those of ketones and esters. Peak at 1517 cm−1 could be explained by the presence of amide I and amide II stretching, corresponding to the proteins on the biosorbent surface. The band at 1161 cm−1 is attributed to the C–O stretching vibration of esters. The important band at 1099 cm−1 is due to the C–O stretching of the aliphatic ether. Peaks in the wavenumber region below 800 cm−1 can be attributed to the presence of N bioligands. These spectra indicate that the studied biosorbent contains a wide variety of functional groups such as hydroxyl, carboxyl, amides, ethers, ketones, and esters that play important role for binding dyes through different mechanisms (Barka et al. 2013a, b; Peláez-Cid et al. 2013). Figure 3 shows the SEM micrographs conducted to examine the physical morphologies and surface properties of the biosorbent. Results demonstrated that the biosorbent surface retained the famous structural characteristics of the activated carbon, thus is mostly irregular in shape and porous with irregular and broken edges. The whole surface porosity and roughness increase due to the presence of pores, and reliefs confer to the biosorbent to interact with different dye molecules.
Performance of biosorbent for dye removal
Differences in biosorption yields were observed when varying the dye family at the same batch conditions. The highest biosorption yields were increasingly attributed to the acidic, direct, anthraquinone, then reactive dyes (Fig. 4a). The biosorption percentages of acid orange 51 (AO51), direct blue 86 (DB86), direct red 75 (DR75), remazol brilliant blue R (RBBR), and reactive black 5 (RB5) were respectively of 90.11%, 63.19%, 47.19%, 24.28%, and 13.13%. Quantity of dye adsorbed per unit mass of biosorbent at equilibrium qeq corresponding to AO51, DB86, DR75, RBBR, and RB5 at initial dye concentration of 100 mg/L varied from 66.5 to 12.18 mg/g (Fig. 4b). The obtained results were found to be comparable with those of many other low-cost biosorbent in literature such as dried prickly pear cactus (Opuntia ficus indica) cladodes powder and fruit wastes, orange peel, modified zeolite, and high-lime fly ash (Barka et al. 2013b; Gupta and Suhas 2009; Peláez-Cid et al. 2013). Batch experiments confirmed the previous findings of Gupta and Suhas (2009), relating the biosorption efficiency to the structure of dye molecules. In the same vein, Yargic et al. (2015) reported that tomato waste could be used as an alternative and low-cost biosorbent for the removal of copper(II) ion from aqueous solutions, when suitable conditions were performed. Therefore, in-depth studies of the biosorption parameters including biosorbent particle size and batch conditions (pH, temperature, dye concentration, biosorbent concentration, etc.) are needed in order to design and carry out a pilot plant-scale study and to check the industrial-level feasibility.
Conclusion
The present study aimed at developing a sustainable path towards a zero-waste exploitation of tomato pomace, showing that it could represent a potential feedstock for the extraction of valuable compounds and the sequential production of low-cost biosorbent. In fact, significant amounts of natural antioxidants like polyphenols and lycopene were simultaneously extracted from tomato peels using a food-grade solvent (ethanol), while high-quality edible oil with high content of linoleic acid was extracted from tomato seeds. Subsequently, the extraction solid residues (i.e., the solid leftovers after the extraction of valuable compounds from tomato peels and tomato seeds) were mixed and pretreated to produce a biosorbent that was successfully applied for the removal of acidic dyes. In conclusion, this study demonstrated that tomato pomace can be entirely valorized as antioxidant compounds and edible oil as well as a sequential low-cost biosorbent. This would not only result in economic benefits for processed tomato producers but also contribute to environmental protection and resource conservation.
References
Abid Y, Azabou S, Jridi M, Khemakhem I, Bouaziz M, Attia H (2017) Storage stability of traditional Tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem 233:476–482
Allison BJ, Simmons CV (2017) Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenergy 105:331–341
AOAC (2000) Association of Official Analytical Chemists. Official methods of analysis, 17th ed. Washington
AOCS (1997) American Oil Chemists’ Society. Official and Recommended Practices of the AOCS, Champaign
Arco-Perez A, Ramos-Morales E, Yanez-Ruiz DR, Abecia L, Martin-Garcia AI (2017) Nutritive evaluation and milk quality of including of tomato or olive byproducts silages with sunflower oil in the diet of dairy goats. Anim Feed Sci Technol 232:57–70
Augustin MA, Sanguansri L, Fox EM, Cobiac L, Cole MB (2020) Recovery of wasted fruit and vegetables for improving sustainable diets. Trends Food Sci Tech 95:75–85
Azabou S, Abid Y, Sebii H, Felfoul I, Gargouri A, Attia H (2016) Potential of the solid-state fermentation of tomato by products by Fusarium solani pisi for enzymatic extraction of lycopene. LWT - Food Sci Technol 68:280–287
Azabou A, Ben Taheur F, Jridi M, Bouaziz M, Nasri M (2017) Discarded seeds from red pepper (Capsicum annum) processing industry as a sustainable source of high added-value compounds and edible oil. Environ Sci Pollut Res 24:22196–22203
Azabou Samia, Sebii Haïfa, Taheur Fadia Ben, Abid Yousra, Jridi Mourad, Nasri Moncef (2020) Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction solvents and potential application in refined olive oils. Food Bioscience 36:100664
Bakić MT, Pedisić S, Zorić Z, Dragović-Uzelac V, Grassino AN (2019) Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim Slov 66:367–377
Barka N, Abdennouri M, El Makhfouk M, Qourzal S (2013a) Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J Environ Chem Eng 1:144–149
Barka N, Ouzaouit K, Abdennouri M, Makhfouk ME (2013b) Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J Taiwan Inst Chem Eng 44:52–60
Bersuder P, Hole M, Smith G (1998) Antioxidant from a heated histidine-glucose model system. I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc 75:181–187
Biondi L, Luciano G, Cutello D, Natalello A, Mattioli S, Priolo A, Lanza M, Morbidini L, Gallo A, Valenti B (2020) Meat quality from pigs fed tomato processing waste. Meat Sci 159:107940
Butsat S, Siriamornpun S (2016) Effect of solvent types and extraction times on phenolic and flavonoid contents and antioxidant activity in leaf extracts of Amomum chinense C. Int Food Res J 23:180–187
CAC (1999) Codex standard for named vegetable oils, (http://wenku.baidu.com/view/966a07ffc8d376eeaeaa3167.html Accessed 02.07.14)
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Cheikh-Rouhou S, Besbes S, Hentati B, Blecker C, Deroanne C, Attia H (2007) Nigella sativa L.: chemical composition and physicochemical characteristics of lipid fraction. Food Chem 101:673–680
Cicco N, Lanorte M, Paraggio M, Viggiano M (2009) A reproductible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolic of plant methanol extract. Microchem J 91:107–110
Coelho M, Pereira R, Rodrigues AS, Teixeira JA, Pintado ME (2019) Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod Process 117:329–339
Elbadrawy E, Sello A (2016) Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab J Chem 9:1010–1018
Giuffrè AM, Capocasale M (2016) n-Alkanes in tomato (Solanum lycopersicum L.) seed oil: the cultivar effect. Int Food Res J 23:979–985
Goula AM, Lazarides HN (2015) Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: the cases of olive mill and pomegranate wastes. J Food Eng 167:45–50
Grassino AN, Halambek J, Djaković S, Brnčić SR, Dent M, Grabarić Z (2016) Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll 52:265–274
Grassino AN, Djaković S, Bosiljkov T, Halambek J, Zorić Z, Dragović-Uzelac V, Petrović M, Brnčić SR (2019) Valorisation of tomato peel waste as a sustainable source for pectin, polyphenols and fatty acids recovery using sequential extraction. Waste Biomass Valori. https://doi.org/10.1007/s12649-019-00814-7
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal–a review. J Environ Manag 90:2313–2342
Heguy JM, Cassinerio CA, Fadel JG, Asmus J, Taylor SJ, De Peters EJ (2015) Nutrient composition and total-tract apparent digestibility of whole tomato seeds by sheep. PAS 31:462–466
Isik F, Topkaya C (2016) Effects of tomato pomace supplementation on chemical and nutritional properties of crackers. Ital J Food Sci 28:525–535
Juric S, Ferraric G, Velikov KP, Donsi F (2019) High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J Food Eng 262:170–180
Kehili M, Choura S, Zammel A, Allouche N, Sayadi S (2018) Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem 246:295–304
Kehili M, Sayadi S, Frikha F, Zammel A, Allouche N (2019) Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod Process 117:321–328
Koleva II, Van Beek TA, Linssen JPH, Groot A, Evstatieva LN (2002) Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Analysis 13:8–17
Kumar V, Surasani R, Kudre T, Ballari RV (2018) Recovery and characterization of proteins from pangas (Pangasius pangasius) processing waste obtained through pH shift processing. Environ Sci Pollut R 25:11987–11998
Li Y, Li Y, Zhang D, Li G, Lu J, Li S (2016) Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour Technol 217:50–55
Lu Zhiqiang, Wang Jiajia, Gao Ruiping, Ye Fayin, Zhao Guohua (2019) Sustainable valorisation of tomato pomace: A comprehensive review. Trends in Food Science & Technology 86:172–187
Luten J, Crews E, Flynn A, Van DP, Hurrell R, Deelstra H, Shen L, Fairweather-Talt S, Hickson K, Faire R, Schleminer U, Frochlich W (1996) Interlaboratory trial on the determination of the in vitro iron dialysability from food. J Sci Food Agric 72:415–424
Najafi H, Pajootan E, Ebrahimi A, Arami M (2016) The potential application of tomato seeds as low-cost industrial waste in the adsorption of organic dye molecules from colored effluents. Desalin Water Treat 57:15026–15036
Navarro-González I, García-Valverde V, García-Alonso J, Jesús Periago M (2011) Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res Int 44:1528–1535
Pataro G, Carullo D, Falcone M, Ferrari G (2020) Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov Food Sci Emerg 63:102369
Peláez-Cid AA, Velázquez-Ugalde I, Herrera-González AM, García-Serrano J (2013) Textile dyes removal from aqueous solution using Opuntia ficus-indica fruit waste as adsorbent and its characterization. J Environ Manag 130:90–97
Perea-Dominguez XP, Hernandez-Gastelum LZ, Olivas-Olguin HR, Espinosa-Alonso LG, Valdez-Morales M, Medina-Godoy S (2018) Phenolic composition of tomato varieties and an industrial tomato by-product: free, conjugated and bound phenolics and antioxidant activity. J Food Sci Technol 55:3453–3461
Prosky P, Asp NG, Schweizer TF, Devries JW, Furda I (1988) Determination of insoluble, soluble, and total dietary fiber in foods, food products: “interlaboratory study”. J Assoc Off Anal Chem 71:1017–1023
Savadkoohi S, Hoogenkamp H, Shamsi K, Fa A (2014) Color, sensory and textural attributes of beef frankfurter, beef ham and meat-free sausage containing tomato pomace. Meat Sci 97:410–418
Scaglia B, D’Incecco P, Squillace P, Dell’Orto M, De Nisi P, Pellegrino L, Botto A, Cavicchi C, Adani F (2020) Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J Clea Prod 243:118650
Strati IF, Oreopoulou V (2011a) Process optimisation for recovery of carotenoids from tomato by-products. Food Chem 129:747–752
Strati IF, Oreopoulou V (2011b) Effect of extraction parameters on the carotenoid recovery from tomato waste. Int J Food Sci Technol 46:23–29
Szabo K, Cătoi AF, Vodnar DC (2018) Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant Foods Hum Nutr 73:268–277
Szabo K, Dulf FV, Diaconeasa Z, Vodnar DC (2019) Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT - Food Sci Technol 116:108558
Toor RK, Savage GP (2005) Antioxidant activity in different fractions of tomatoes. Food Res Int 38:487–494
Yargic AS, Yarbay Sahin RZ, Ozbay N, Onal E (2015) Assessment of toxic copper (II) biosorption from aqueous solution by chemically-treated tomato waste. J Clean Prod 88:152–159
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49:4083–4089
Yilmaz E, Aydeniz B, Guneser O, Arsunar E (2015) Sensory and physico-chemical properties of cold press-produced tomato (Lycopersicon esculentum L.) seed oils. J Am Oil Chem Soc 92:833–842
Acknowledgments
The authors would like to express their gratitude to Professor Slah Kammoun from the National Engineering School of Sfax for helping in performing FT-IR spectra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azabou, S., Louati, I., Ben Taheur, F. et al. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ Sci Pollut Res 27, 39402–39412 (2020). https://doi.org/10.1007/s11356-020-09835-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09835-5