Abstract
Effect of heat treatment on colour stability of dried salak fruit during storage was investigated by using hot air (40–90 °C), heat pump (isothermal and intermittent modes, 26–37 °C) and freeze-drying. Influence of pre-treatment on the colour property was studied as well by blanching the sample at three levels of temperature (50–70 °C). Total colour change (∆E*) was used to assess the colour degradation kinetics and quantify the degree of browning during processing and storage. It was found that the dried pre-treated sample under heat pump isothermal drying recorded the highest ∆E* value during storage followed by non-pre-treated samples under heat pump intermittent drying, freeze-drying, hot air-drying and heat pump isothermal drying. Weibull model is found to better fit the experimental data as compared with zero-order and first-order kinetics models. Analysis on the kinetics constants reveals that the heat treatment could affect the microstructure, water sorption properties and concentration of reacting species of the dried product. This in turn contributes to the colour changes of the dried product during storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colour is an important quality attribute in food products as it affects the visual appeal of the finished product as perceived by consumers. Changes of colour in food, in either positive or negative way, are a common phenomenon occurring during processing and storage. In fact, colour development is a result of various chemical and biochemical reactions that occur at cellular level. In general, discolouration is often accompanied by some other deleterious effects such as off-flavour development and loss of nutrients. Therefore, the loss of phytochemicals and other nutrients in food can be closely related to the discolouration rate of the product.
Drying is an effective method to preserve agricultural products. Removal of moisture from fresh products may deactivate enzymes and microorganisms that often cause undesirable biochemical reactions in food, thus prolonging the shelf life of natural products (Fernandes et al. 2010; Mujumdar and Law 2010). However, thermal effect may destruct heat-sensitive substances in biological products and result in degradation of product quality, such as the colour, during drying process and in subsequent storage period. In most cases, the rates of discolouration often follow zero-order or first-order kinetics models, and the dependence of degradation rate constant on temperature can be described by Arrhenius-type equation (Goula et al. 2006; Koca et al. 2007; Lau et al. 2000; Saxena et al. 2010).
While extensive works have been carried out to investigate the effect of pre-treatment and drying method on discolouration rate of food product during processing (Aversa et al. 2009; Chong et al., 2008a; Chong et al., 2008b; Ganjloo et al. 2009; Gokhale and Lele 2010), study on colour stability during storage is very scarce particularly for heat pump-drying and intermittent drying. In a recent study, it was reported that browning rate in dried apple during storage could be closely related to microstructure modification during pre-treatment and drying (Acevedo et al. 2008). Meanwhile, degradation of important phytochemicals and nutrients in dried products was reported to correlate well with colour changes during storage (Arabhosseini et al. 2007; Niamnuy et al. 2008; Topuz et al. 2009). Hence, study on colour kinetics is essential in predicting product quality and minimising undesired changes of food property during storage. Besides the common kinetics models reported in literatures (e.g., the zero-order and first-order models), Weibull model has been proposed as an alternative approach to determining the browning rate and shelf life of food products (Corradini and Peleg 2004; Mizrahi 2004; Peleg et al. 2002). The model is also very useful in describing thermal degradation of heat-labile vitamins and colour pigments (Cunha et al. 1998; Odriozola-Serrano et al. 2009; Oms-Oliu et al. 2009a).
Salak fruit, or domestically known as snake fruit in Malaysia, is a peculiar fruit that can also be found in Indonesia, Thailand and the Philippines. Despite the unique taste and smell, salak fruit contains valuable bioactive antioxidants such as vitamin A, vitamin C and phenolic compounds (Leong and Shui 2002; Leontowicz et al. 2007; Setiawan et al. 2001). However, salak fruit has an inherent short shelf life of less than a week. Drying is therefore an option to preserve the fruit not only in dried form, but to retain the bioactive compounds that are beneficial to human health.
The objective of this study was to investigate the effect of pre-treatment (blanching), drying method (freeze-drying, hot air-drying and heat pump-drying) and drying mode (isothermal and intermittent drying) on colour stability of dried salak fruit during storage. Kinetics of total colour change (∆E*) was investigated to determine discolouration rate of dried salak fruit during storage.
Materials and methods
Sample preparation
Fresh salak fruit was purchased from a local fruit supplier (The Federal Agricultural Marketing Authority, Selayang, Selangor, Malaysia). Fruits with similar size (ca. 50–70 × 50 mm) and skin colour (dark brown with average L*, a* and b* values of 22.37 ± 1.68, 1.05 ± 0.90 and 8.66 ± 2.11, respectively) were selected for all experiments. The fresh fruits were cleaned with a paper towel and kept in refrigerator at about 10 °C until experiments were conducted (with storage period no longer than 7 days). In each experiment, salak fruit was peeled and the cloves were separated. The individual cloves were cut longitudinally into equal halves, and the seeds at centre were removed. The halves were then cut to the required dimension and thickness (40 × 20 × 3 mm).
Pre-treatment
Pre-treatment was performed, when necessary, to inactivate potential enzyme activities (ascorbate oxidase and polyphenol oxidase) that may cause degradation of colour during drying and storage. Samples were blanched in hot water at 50 °C, 60 °C and 70 °C for 5 min, then immediately cooled under running cold water to stop further heating. Blanching water was drained, and excess water on sample surface was superficially dried with paper towel before subjected to drying. Relatively low blanching temperature (50–70 °C) was used because it could minimise the degradation of nutrition components and colour changes (Jayaraman and Das Gupta 2006; Ong and Law 2010a; Rahman and Perera 2007).
Drying procedure and measurements
Hot air-drying, freeze-drying and heat pump-drying were performed by using a laboratory-scale oven (Memmert, UFP500, Germany), a laboratory-scale freeze dryer (Martin Christ, Alpha 1–2 LD Plus, Germany) and a pilot-scale heat pump-assisted dryer prototype (I-Lab instrument, Malaysia), respectively. Basically, heat pump-drying is a type of convective drying but with lower air relative humidity as compared with hot air-drying at same level of temperature. The heat pump-assisted dryer prototype (Fig. 1) consists of three main parts: a heat pump system, a heat recovery unit and drying chambers. The heat pump system is comprised of an evaporator (refrigerant R-22), a primary condenser (water-cooled), a secondary condenser (air-cooled), an expansion valve and a compressor. Moisture-laden air exhausted from drying chamber was first directed to the cool surface of evaporator where the air was cooled down to its saturated dew point and condensed. Temperature of the dehumidified air was then elevated by the hot water heat exchanger and an auxiliary heater (when higher temperature was required). A blower was used to supply constant forced air in the drying chambers.
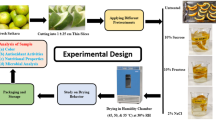
Figure 2 illustrates the experimental works in this study while Table 1 shows the drying conditions and total drying time of the respective trials. Isothermal drying trials were performed at four levels of temperature in hot air-drying (HA40, HA50, HA70 and HA90) and two levels of temperature in heat pump-drying (HP26 and HP37). Two intermittent modes were investigated namely periodic heat supply (HP37HP37) and step-up air temperature (HP26HP37). Both have been reported to be efficient drying techniques in preserving the colour of dehydrated product (Chin and Law 2010; Chua et al. 2001b, 2002; Hii et al. 2011). For the periodic heat supply mode, dehumidified air at 37 °C and 17% RH was supplied to the drying chamber intermittently at 1-h intervals. Meanwhile, in the step-up temperature mode, dehumidified air at 26 °C (27% RH) was used in the initial stage of drying (the first 3 h), and the air temperature was increased to 37 °C (17% RH) thereafter until the fruit sample reached its equilibrium moisture content. Freeze-drying was performed at main drying temperature of −30 °C (coil temperature) for 24 h followed by final drying temperature of −50 °C (coil temperature) for 7 h. Fresh samples were frozen in a freezer (Ardo, S320, Italy) for at least 24 h before freeze-drying. For each drying trial, about 80 g of salak slices were arranged in thin-layer drying trays where another 20 g of random sample was placed separately in a sample holder that was made from wire mesh. With the exception of freeze-dried samples, weight loss of sample in the wire mesh holder was recorded hourly off-line until constant weight was obtained in three consecutive measurements by using a digital balance (Adventurer OHAUS, AR3130, USA). Moisture content at this stage was marked as the final moisture content (also known as equilibrium moisture content). Removing, weighing and returning of sample during the off-line weighing were performed in less than 15 s in order to avoid interference on the drying process (Janjai et al. 2008; Madamba et al. 1996; Özdemir and Onur Devres 1999; Wongwises and Thongprasert 2000). Dry matter weight was determined by drying the sample in an oven at 105 °C for 24 h, and water activity of dried sample was measured by using a water activity metre (Pawkit, Decagon, USA).
Storage
About six to ten pieces of dried samples (selected randomly from bulk samples) were packed in a high-density polyethylene plastic bag with dimension of 135 × 76 mm and thickness of 60 μm. The plastic bag was then thermally sealed by using a heat sealer (Fei Ying, KS-100, China). The seal point was carefully examined to ensure proper sealing and no leakage. The samples were kept in dark at room temperature (25 °C, 55% RH) for up to 6 months, and colour analysis was performed at a monthly basis. At the end of storage, samples were analysed for final moisture content and water activity using a handheld water activity metre (Pawkit, Decagon, USA).
Colour measurement
Colour of dried salak was measured by using a handheld colour metre (AccuProbe, HH06, USA). About six pieces of dried samples were arranged in a Petri dish to form a thin-layer sheet that could cover the nosecone of the colour metre sensor. Nine measurements were taken at different spots on both sides of the surface, by flipping over the samples when necessary, in order to get an average reading. Colours of the sample were indicated by CIELab colour scales L*, a* and b*. Total colour change, ∆E*, was calculated according to Eq. 1.
Kinetics models and parameters
Three kinetics models were evaluated in order to relate the colour change of product during storage to its heat treatment history. Zero-order kinetics model for the total colour change is shown in Eq. 2. Whilst adapted first-order kinetics model and Weibull model that incorporate with the concept of fractional conversion are shown in Eqs. 3 and 4, respectively (Oms-Oliu et al. 2009b; Topuz 2008; Topuz et al. 2009). Parameter b, which is temperature-independent in the Weibull model, indicates concavity or convexity of the kinetics curve when it takes value below and above unity, respectively (Corradini and Peleg 2004; Odriozola-Serrano et al. 2009).
Modelling was carried out by performing nonlinear least-square regression with MS Excel SOLVER tool (Microsoft Office Professional 2003, USA). Statistical parameters such as coefficient of determination (R 2), chi-square (χ 2) and root mean square error (RMSE) were used as criteria to determine the goodness of fit. Model with the highest R 2 and lowest χ 2 and RSME values was chosen as the best model to describe the colour change kinetics of the dried sample during storage (Odriozola-Serrano et al. 2009).
Statistical analysis
All experiments were conducted as a completely randomised experiment and performed in triplicate. Data were analysed by using one-way ANOVA, and means comparisons were determined by Duncan Multiple Range Test at 95% confidence level using statistical software, SAS for Window (Version 9.1, SAS Institute, USA).
Results and discussion
Effect of pre-treatment and drying method on product colour before storage
The colour of fresh salak fruit is similar to fresh garlic, with average L*, a* and b* values of 55.46 ± 2.07, −3.27 ± 0.36 and 27.96 ± 0.60, respectively. Generally, browning effect was observed in all samples after drying. However, the browning indices varied among the dried samples depending on the pre-treatment temperature and drying method. Hot air-dried samples (HA40, HA50, HA70 and HA90) were darker and redder as compared with dried samples from other drying methods. It can be seen from Fig. 3 that ∆E* values of the hot air-dried samples (7.30–32.06) increase significantly (p < 0.05) with hot air temperature. The evolution of colour at the high drying temperature is probably caused by formation of a series of brown pigments, for instance, melanoindins, due to thermal effect (Maskan 2001).
On the other hand, colour of dried samples by heat pump-drying (HP26, HP37, HP37HP37 and HP26HP37) remained almost the same as the fresh fruit. With the exception of sample HP26, total colour change of the heat pump-dried samples was relatively low during drying as compared with the hot air-dried samples (Fig. 3). This suggests that heat pump-drying could preserve product colour better by avoiding serious Maillard browning reaction which often occurs at high temperature drying (Chua et al. 2000; Chua et al. 2001a).
Based on Fig. 3, it can be seen that ∆E* values of dried samples from heat pump intermittent drying (HP37HP37 and HP26HP37) are significantly (p < 0.05) lower than that in heat pump isothermal drying (HP26 and HP37). This could be due to the reduced heating period on the product by cutting off the heat flux during intermittent drying and hence diminish the browning effect. Nonetheless, when comparing the total colour change among the samples of heat pump isothermal drying, ∆E* value of HP26 (8.17) is higher than HP37 (6.67). This is probably due to the longer drying time in HP26 as compared with HP37, even though drying temperature is lower in the former. The non-pre-treated sample (HP26) could have suffered from enzymatic browning during the long processing hours. On the contrary, total colour change of pre-treated samples (HP37B50, HP37B60 and HP37B70) is relatively low during drying. The results reveal that pre-treatment could minimise the enzymatic browning in samples effectively during drying. Nevertheless, the pre-treatment procedure needs to be improved in order to reduce the colour degradation that is incurred during the pre-treatment. It was found that total colour change of the dried pre-treated samples was partly contributed by the pre-treatment (prior drying), where the ∆E* values (14.65–18.57) had increased significantly (p < 0.05) with blanching temperature. The colour degradation could be due to browning reactions that are caused by enzymatic activity after tissue disruption and prior to denaturation of enzymes in hot water.
Effect of pre-treatments and drying methods on product colour during storage
All dried samples turned darker and redder after 6 months storage with some light sour off-odour similar to a fermented plum. However, no formation of moulds or fungi was observed on the surface of the dried samples. Browning effects during storage were different among the dried samples depending on their heating history. Referring to Fig. 3, appreciable colour change was observed in all the dried pre-treated samples (HP37B50, HP37B60 and HP37B70) during storage, where significantly (p < 0.05) high ∆E* values (24.56–25.50) was detected. Apparently, although enzymes had been inactivated during pre-treatment, many other chemical reactions took place during storage. This could probably due to the higher proportion of cellular collapse and impaired cells that increase the effective release of reacting species in the pre-treated sample (e.g., amino acids, reducing sugars, ascorbic acid and phenolic compounds), thus facilitating the non-enzymatic browning reactions (Acevedo et al. 2008). On the other hand, noticeable total colour change was observed in samples of heat pump intermittent drying (HP37HP37 and HP26HP37) and freeze-drying (FD) as well, with ∆E* values ranging from 20.78 to 24.99 and 19.10, respectively (Fig. 3). This could be due to the intrinsic moisture sorption properties of the dried samples. Freeze-dried and heat pump-dried products are hygroscopic and rehydrate faster as compared with others. Hence, the moisture content and water activity of these samples increase rapidly during storage. This in turn alleviates the diffusion of reacting species and promotes browning reactions (Labuza 1977). On the contrary, the ∆E* values (11.64–14.24) of samples by heat pump isothermal drying (HP26 and HP37) was found to be relatively low as compared with other samples. This could be due to the mild temperature in the heat pump-drying that helps in maintaining integrity of cells and thus avoid some undesired enzymes and/or chemical activities that usually occur in broken cells. With the exception of sample HA90, the hot air-dried samples had shown higher ∆E* values (13.21–18.64) as compared with the dried samples by heat pump isothermal drying. This could be due to the cellular collapses and tissue disruptions that occur during the high temperature processing. Total colour change in sample HA90 during storage was lower simply because of the lower concentration of reacting species prior storage due to high depletion rate in the drying process.
Table 2 shows the moisture content and water activity values of all dried samples before and after storage. It can be seen that moisture content and water activity values of all dried samples increased significantly after 6 months of storage. Basically, the high-density polyethylene plastic bag is a common packaging material for foods, but the film is water-vapour-permeable (Park 1999; Phillips 1996; Sandhya 2010; Techavises and Hikida 2008). At the same time, dried products tend to absorb moisture from the surrounding atmosphere due to their low equilibrium moisture content and hygroscopic properties. Hence, increment of moisture content and water activity in dried products is common during storage (Azeredo et al. 2006; Daramola et al. 2010; Kumar and Mishra 2004; Rahman et al. 2007). Nevertheless, moisture gaining rates were found different among the dried samples. Moisture content of dried samples with pre-treatment (HP37B50, HP37B60 and HP37B70), heat pump intermittent drying (HP26HP37 and HP37HP37) and FD were found slightly higher as compared with other samples, with values ranging from 0.30 to 0.34, 0.33 to 0.35 and 0.29 g water/g dry solid, respectively. Meanwhile, lower moisture content was recorded in samples of heat pump isothermal drying (HP26 and HP37) and hot air-drying (HA40, HA50, HA70 and HA90), with values ranging from 0.26 to 0.28 and 0.16 to 0.24 g water/g dry solid, respectively. Apparently, the heating treatments had affected the moisture sorption characteristics of the dried products. This in turn resulted in different moisture gaining rates as well as discolouration rates during storage. Furthermore, it was observed that water activity values of all dried samples increased during storage. Basically, the increased water activity values were due to increased moisture content in the dried samples. It seems that the water activity values of the dried samples were within the range that is optimum for various deleterious reactions (0.55–0.65; Leung 1987; Ong and Law 2010b; Villamiel et al. 2006). Hence, degradation of colour during storage could be associated with moisture sorption properties of the dried samples.
Kinetics of total colour change ∆E*
Colour change of dried salak fruits was further studied by analysing the kinetics of the total colour change (∆E*) with reference to storage time. Increment in the ∆E* value would depict the colour change of the dried sample from light yellow to dark brown colour. It can be seen from Fig. 4 that the ∆E* values of all samples increase rapidly during the first few months of storage before reaching a plateau in the later storage period. The slow colour development towards the end of storage is probably due to exhaustion of browning reaction substrates in sample after long storage period (Arabhosseini et al. 2007; Soliva et al. 2000). Furthermore, it can be seen from Fig. 4 that the ∆E* values of dried samples evolve at different rates, although all dried samples were kept under the same storage conditions. The results show that the heat treatment history have significantly influenced the moisture sorption property as well as stability of phytochemicals in the dried samples and hence led to different degrees of colour change during storage. Table 3 presents the kinetic constants and respective R 2 values of parameter ∆E* that were estimated from the zero-order, first-order and Weibull models. It is apparent that Weibull model could describe the kinetics of all the colour parameters better than zero-order and first-order models, with higher values in R 2 (0.949–1.000) and lower values in χ 2 (0.001–0.009; data not shown) and RMSE (0.008–0.080; data not shown).
Nevertheless, interpretation and comparison of the degradation kinetics across the samples based on Weibull’s kinetic constants could be practically challenging. Both the scale factor (k a) and shape factor (b) need to be considered simultaneously when examining the data. Basically, b constants in Weibull model would indicate that the kinetics curve of a colour parameter is either a tail-forming (b < 1) or shoulder-forming (b > 1) growth curve. Value of b > 1 can be interpreted as increasing degradation over the entire storage period while b < 1 indicates fast degradation in initial storage period but ceasing degradation in later period of storage (Buzrul and Alpas 2007; Buzrul et al. 2005; Oms-Oliu et al. 2009b). When magnitude of the b constant is decreasing, then a more pronounced tailing will be observed. Indirectly, the b constant would also indicate kinetics pattern of chemical events that occur at cellular level and controlling colour degradation (Peleg et al. 2002). It can be observed from Fig. 4 that ∆E* value of sample HA90 increase rapidly in the first few months and then maintains at the maximum value over the remaining storage period. In accordance with the Weibull kinetic constants (Table 3), sample HA90 possesses the highest k a value (1.038) and the lowest b value (0.629) which indicates rapid degradation at initial period and long-tailing at the later stage. Information on this kinetic pattern cannot be seen from the first-order model, although sample HA90 has recorded the highest k 1 value (0.818) as well in the first-order model. Nevertheless, it is worthy to note that HA90 has the lowest ∆E* value during storage even though it has a high kinetic constant.
Referring to Table 3, b constant of Weibull model reveals that samples that were dried at higher temperature (e.g. HA70 and HA90) would possess higher degradation rates in colour during the initial storage period while samples that were dried at lower temperature (e.g. HA40 and HA50) would have lower degradation rates during the initial storage period. Similar result can be observed in samples by heat pump isothermal drying where b constant value of sample HP37 is lower than sample HP26. This could be due to the high temperature drying that led to broken cells in the samples and consequently resulted in instant oxidation and chemical reactions of the reacting species in the samples during storage. On the contrary, intact cells in samples that were processed at mild temperature might take some time to collapse and release the reacting species, thus delaying the browning reactions during storage.
Generally, kinetics of total colour change during storage is relatively high in all dried pre-treated samples (HP37B50, HP37B60 and HP37B70) with k a values ranging from 0.407 to 0.616 and b values ranging from 0.922 to 1.633. The results show that the pre-treatment has induced adverse impact on the sample colour development, although it is designed to inactivate enzymatic browning during long period of drying and storage. It seems that blanching in hot water has resulted in denaturation of cell membranes and bursting of cell walls that consequently led to release of reacting species in the cell tissues and thus facilitating non-enzymatic browning during storage (Acevedo et al. 2008; Aguilera and Stanley 1999; Bondaruk et al. 2007). On the contrary, the dried non-pre-treated sample (HP37) under the same drying condition has lower degradation kinetics during storage as compared with the dried pre-treated sample (HP37B50, HP37B60 and HP37B70) with k a and b values of 0.371 and 1.219, respectively. Therefore, the pre-treatment procedure needs to be improved (e.g. shorter blanching time) or even excluded, as it has caused serious browning in the sample during blanching and storage. Pre-treatment is not necessary in the present study as the enzymatic browning effect is not significant under the heat pump isothermal drying.
Conclusions
Colour changes of dried salak fruit during storage is closely related with its heat treatment history. Pre-treatment and drying conditions could significantly affect the microstructure, concentration of reacting species and moisture sorption of products which in turn influence the stability of product colour during storage period. It was found that freeze-drying produced dried products with minimum colour change, but the colour degradation rate of the freeze-dried samples during storage was relatively high. Hot air-dried samples (except 40 °C) experienced severe colour change during drying as well as during storage (except 90 °C). As a result, total colour change of hot air-dried samples especially those dehydrated at high temperature gave relatively high overall total colour change. Although pre-treated samples showed minimal colour change during drying, the sample experienced significant colour change during pre-treatment and also in the subsequent storage period. Therefore, the overall total colour change for all pre-treated samples has high overall total colour change. Dried samples by heat pump intermittent drying possessed appealing product colour after drying, but colour degradation rates were quite high during storage. On the other hand, isothermal heat pump-dried samples have relatively low total colour change in drying as well as in storage, and thus gave low overall total colour change if compared with all samples. Therefore, heat pump isothermal drying could be considered as a good drying method for the fruit processing industry, particularly salak fruit if overall colour change during drying and storage is of main concern. Weibull model was found to be a better kinetics model as compared with the zero-order and first-order models. It is very useful in estimating the colour degradation kinetics during storage.
Abbreviations
- a*:
-
Colour parameter (dimensionless, positive value indicates red and negative value indicates green)
- b :
-
Weibull model’s shape factor
- b*:
-
Colour parameter (dimensionless, positive value indicates yellow and negative value indicates blue)
- C :
-
Value of colour parameter
- ∆E* :
-
Total colour change (dimensionless)
- k 0 :
-
Zero-order kinetic constant (1/month)
- k 1 :
-
First-order kinetic constant (1/month)
- k a :
-
Weibull kinetic constant (1/month)
- L*:
-
Colour parameter (dimensionless, 0 for black to 100 for white)
- R 2 :
-
Coefficient of determination
- RMSE:
-
Root mean square error
- t :
-
Storage time (month)
- χ 2 :
-
Chi-square
- 0:
-
Initial value
- ∞:
-
Equilibrium value after long storage period
References
Acevedo, N. C., Briones, V., Buera, P., & Aguilera, J. M. (2008). Microstructure affects the rate of chemical, physical and color changes during storage of dried apple discs. Journal of Food Engineering, 85(2), 222–231.
Aguilera, J. M., & Stanley, D. W. (1999). Microstructural principles of food processing and engineering. USA: An Aspen Publication.
Arabhosseini, A., Huisman, W., van Boxtel, A., & Müller, J. (2007). Long-term effects of drying conditions on the essential oil and color of tarragon leaves during storage. Journal of Food Engineering, 79(2), 561–566.
Aversa M, Curcio S, Calabrò V & Iorio G (2009) Experimental evaluation of quality parameters during drying of carrot samples. Food and Bioprocess Technology, Online First™, 9 November 2009.
Azeredo, H. M. C., Brito, E. S., Moreira, G. E. G., Farias, V. L., & Bruno, L. M. (2006). Effect of drying and storage time on the physico-chemical properties of mango leathers. International Journal of Food Science & Technology, 41(6), 635–638.
Bondaruk, J., Markowski, M., & Blaszczak, W. (2007). Effect of drying conditions on the quality of vacuum-microwave dried potato cubes. Journal of Food Engineering, 81(2), 306–312.
Buzrul, S., & Alpas, H. (2007). Modeling inactivation kinetics of food borne pathogens at a constant temperature. LWT - Food Science and Technology, 40(4), 632–637.
Buzrul, S., Alpas, H., & Bozoglu, F. (2005). Use of Weibull frequency distribution model to describe the inactivation of Alicyclobacillus acidoterrestris by high pressure at different temperatures. Food Research International, 38(2), 151–157.
Chin, S. K., & Law, C. L. (2010). Product quality and drying characteristics of intermittent heat pump drying of Ganoderma tsugae Murrill. Drying Technology: An International Journal, 28(10–12), 1457–1465.
Chong, C. H., Law, C. L., Cloke, M., Abdullah, L. C., & Daud, W. R. W. (2008). Drying kinetics, texture, colour and determination of effective diffusivities during sun drying of chempedak. Drying Technology: An International Journal, 26(10), 1286–1293.
Chong, C. H., Law, C. L., Cloke, M., Hii, C. L., Abdullah, L. C., & Daud, W. R. W. (2008). Drying kinetics and product quality of dried chempedak. Journal of Food Engineering, 88(4), 522–527.
Chua, K. J., Mujumdar, A. S., Chou, S. K., Hawlader, M. N. A., & Ho, J. C. (2000). Convective drying of banana, guava and potato pieces: Effect of cyclical variations of air temperature on drying kinetics and color change. Drying Technology: An International Journal, 18(4), 907–936.
Chua, K. J., Mujumdar, A. S., Hawlader, M. N. A., Chou, S. K., & Ho, J. C. (2001a). Batch drying of banana pieces—Effect of stepwise change in drying air temperature on drying kinetics and product colour. Food Research International, 34(8), 721–731.
Chua, K. J., Mujumdar, A. S., Hawlader, M. N. A., Chou, S. K., & Ho, J. C. (2001b). Convective drying of agricultural products. Effect of continuous and stepwise change in drying air temperature. Drying Technology: An International Journal, 19(8), 1949–1960.
Chua, K. J., Hawlader, M. N. A., Chou, S. K., & Ho, J. C. (2002). On the study of time-varying temperature drying—Effect on drying kinetics and product quality. Drying Technology: An International Journal, 20(8), 1559–1577.
Corradini, M. G., & Peleg, M. (2004). A model of non-isothermal degradation of nutrients, pigments and enzymes. Journal of the Science of Food and Agriculture, 84(3), 217–226.
Cunha, L. M., Oliveira, F. A. R., & Oliveira, J. C. (1998). Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function. Journal of Food Engineering, 37(2), 175–191.
Daramola, O. A., Idowu, M. A., Atanda, O. O., & Oguntona, C. R. B. (2010). Effects of packaging material on the quality of “pupuru” flour during storage. African Journal of Food Science, 4(5), 258–263.
Fernandes, F., Rodrigues, S., Law, C., & Mujumdar, A. (2010). Drying of exotic tropical fruits: A comprehensive review. Food and Bioprocess Technology, 4(2), 163–185.
Ganjloo A, Russly AR, Osman A, Bakar J & Bimakr M (2009) Kinetics of crude peroxidase inactivation and color changes of thermally treated seedless guava (Psidium guajava L.). Food and Bioprocess Technology, Online First™, 25 August 2009.
Gokhale S & Lele S (2010) Optimization of convective dehydration of Beta vulgaris for color retention. Food and Bioprocess Technology, Online First™, 23 April 2010.
Goula, A. M., Adamopoulos, K. G., Chatzitakis, P. C., & Nikas, V. A. (2006). Prediction of lycopene degradation during a drying process of tomato pulp. Journal of Food Engineering, 74(1), 37–46.
Hii, C. L., Law, C. L., Cloke, M., & Sharif, S. (2011). Improving Malaysian cocoa quality through the use of dehumidified air under mild drying conditions. Journal of the Science of Food and Agriculture, 91(2), 239–246.
Janjai, S., Lamlert, N., Intawee, P., Mahayothee, B., Haewsungcharern, M., Bala, B. K., et al. (2008). Finite element simulation of drying of mango. Biosystems Engineering, 99(4), 523–531.
Jayaraman, K. S., & Das Gupta, D. K. (2006). Drying of fruits and vegetables. In A. S. Mujumdar (Ed.), Handbook of industrial drying (pp. 606–631). USA: Taylor & Francis Group.
Koca, N., Burdurlu, H. S., & Karadeniz, F. (2007). Kinetics of colour changes in dehydrated carrots. Journal of Food Engineering, 78(2), 449–455.
Kumar, P., & Mishra, H. N. (2004). Storage stability of mango soy fortified yoghurt powder in two different packaging materials: HDPP and ALP. Journal of Food Engineering, 65(4), 569–576.
Labuza, T. P. (1977). The properties of water in relationship to water binding in foods: A review. Journal of Food Processing and Preservation, 1(2), 167–190.
Lau, M. H., Tang, J., & Swanson, B. G. (2000). Kinetics of textural and color changes in green asparagus during thermal treatments. Journal of Food Engineering, 45(4), 231–236.
Leong, L. P., & Shui, G. (2002). An investigation of antioxidant capacity of fruits in Singapore markets. Food Chemistry, 76(1), 69–75.
Leontowicz, M., Leontowicz, H., Drzewiecki, J., Jastrzebski, Z., Haruenkit, R., Poovarodom, S., et al. (2007). Two exotic fruits positively affect rat's plasma composition. Food Chemistry, 102(1), 192–200.
Leung, H. K. (1987). Influence of water activity on chemical reactivity. In L. B. Rockland & L. R. Beuchat (Eds.), Water activity: Theory and application to food (pp. 27–50). Inc, New York: Marcel Dekker.
Madamba, P. S., Driscoll, R. H., & Buckle, K. A. (1996). The thin-layer drying characteristics of garlic slices. Journal of Food Engineering, 29(1), 75–97.
Maskan, M. (2001). Kinetics of colour change of kiwifruits during hot air and microwave drying. Journal of Food Engineering, 48(2), 169–175.
Mizrahi, S. (2004). Accelerated shelf-life tests. In R. Steele (Ed.), Understanding and measuring the shelf-life of food (pp. 317–337). England: Woodhead Publishing Limited.
Mujumdar, A., & Law, C. (2010). Drying technology: Trends and applications in postharvest processing. Food and Bioprocess Technology, 3(6), 843–852.
Niamnuy, C., Devahastin, S., Soponronnarit, S., & Vijaya Raghavan, G. S. (2008). Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. Journal of Food Engineering, 87(4), 591–600.
Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2009). Influence of storage temperature on the kinetics of the changes in anthocyanins, vitamin C, and antioxidant capacity in fresh-cut strawberries stored under high-oxygen atmospheres. Journal of Food Science, 74(2), C184–C191.
Oms-Oliu, G., Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2009a). Effects of high-intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chemistry, 115(4), 1312–1319.
Oms-Oliu, G., Odriozola-Serrano, I., Soliva-Fortuny, R., & Martín-Belloso, O. (2009b). Use of Weibull distribution for describing kinetics of antioxidant potential changes in fresh-cut watermelon. Journal of Food Engineering, 95(1), 99–105.
Ong, S. P., & Law, C. L. (2010a). Drying kinetics and antioxidant phytochemicals retention of salak fruit under different drying and pretreatment conditions. Drying Technology: An International Journal, 29(4), 429–442.
Ong SP & Law CL (2010b) Hygrothermal properties of various foods, vegetables and fruits. In: Jangam SV, Chung Lim L & Mujumdar AS (ed) Drying of foods, vegetables and fruits, pp 31–58. Singapore.
Özdemir, M., & Onur Devres, Y. (1999). The thin layer drying characteristics of hazelnuts during roasting. Journal of Food Engineering, 42(4), 225–233.
Park, H. J. (1999). Development of advanced edible coatings for fruits. Trends in Food Science & Technology, 10(8), 254–260.
Peleg, M., Engel, R., Gonzalez-Martinez, C., & Corradini, M. G. (2002). Non-Arrhenius and non-WLF kinetics in food systems. Journal of the Science of Food and Agriculture, 82(12), 1346–1355.
Phillips, C. A. (1996). Review: Modified atmosphere packaging and its effects on the microbiological quality and safety of produce. International Journal of Food Science and Technology, 31(6), 463–479.
Rahman, M. S., & Perera, C. O. (2007). Drying and food preservation. In M. S. Rahman (Ed.), Handbook of food preservation (pp. 404–427). USA: Taylor & Francis Group.
Rahman, M. M., Kibria, G., Bhuiyan, Z. H., Chowdhury, K., Khan, S., & Karim, R. (2007). Studies on the relation between water activity and sensory qualities as well as chemical changes of coriander (Coriandrun sativum) powder in flexible packaging films. Bangladesh Journal of Scientific & Industrial Research, 42(1), 53–58.
Sandhya, S. (2010). Modified atmosphere packaging of fresh produce: Current status and future needs. LWT-Food Science and Technology, 43(3), 381–392.
Saxena A, Maity T, Raju P & Bawa A (2010) Degradation kinetics of colour and total carotenoids in jackfruit (Artocarpus heterophyllus) bulb slices during hot air drying. Food and Bioprocess Technology, 1–8.
Setiawan, B., Sulaeman, A., Giraud, D. W., & Driskell, J. A. (2001). Carotenoid content of selected Indonesian fruits. Journal of Food Composition and Analysis, 14(2), 169–176.
Soliva, R. C., Elez, P., Sebastián, M., & Martín, O. (2000). Evaluation of browning effect on avocado purée preserved by combined methods. Innovative Food Science & Emerging Technologies, 1(4), 261–268.
Techavises, N., & Hikida, Y. (2008). Development of a mathematical model for simulating gas and water vapor exchanges in modified atmosphere packaging with macroscopic perforations. Journal of Food Engineering, 85(1), 94–104.
Topuz, A. (2008). A novel approach for color degradation kinetics of paprika as a function of water activity. LWT - Food Science and Technology, 41(9), 1672–1677.
Topuz, A., Feng, H., & Kushad, M. (2009). The effect of drying method and storage on color characteristics of paprika. LWT-Food Science and Technology, 42(10), 1667–1673.
Villamiel, M., del Castillo, M. D., & Corzo, N. (2006). Browning reactions. In Y. H. Hui (Ed.), Food biochemistry & food processing (pp. 71–102). USA: Blackwell Publishing.
Wongwises, S., & Thongprasert, M. (2000). Thin layer and deep bed drying of long grain rough rice. Drying Technology: An International Journal, 18(7), 1583–1599.
Acknowledgements
The authors are grateful to the Ministry of Agriculture for the financial support through e-science funding (05-02-12-SF0014) and support from the University of Nottingham, Malaysia Campus. Special thanks are also dedicated to Mr. Chor Ah Be from FAMA Selayang and Mr. Chee Pok Chay from Sungai Jan Jerantut for their valuable technical information.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, S.P., Law, C.L. & Hii, C.L. Effect of Pre-treatment and Drying Method on Colour Degradation Kinetics of Dried Salak Fruit During Storage. Food Bioprocess Technol 5, 2331–2341 (2012). https://doi.org/10.1007/s11947-011-0647-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0647-y