Abstract
Chemical (pH 3.3, 70 °C, 85 °C; pH 12, 70 °C, 85 °C) and biochemical (Alcalase, Protamex, Neutrase, Flavourzyme, and Trypsin) hydrolysis of Persian sturgeon (Acipenser persicus) visceral protein was investigated. The results of this study revealed that there are significant differences between enzymes in terms of degree of hydrolysis (DH%; P < 0.05). Alcalase-hydrolyzed fish protein had the highest DH% (50.13%), and Trypsin-hydrolyzed fish protein had the minimum DH% (14.21%). The highest DH% in chemical hydrolysis was related to pH 3.3 at 85 °C (68.87%). The highest protein recovery (83.64%) and protein content (73.34) were related to enzymatic hydrolysis by Alcalase. The results of current study showed the significant effect of hydrolysis conditions on fish protein hydrolysate properties. Microbial enzymes could produce fish hydrolysates with higher degree of hydrolysis when compared to animal enzyme. Also, in chemical hydrolysis it is clear that hydrolysis at the lower pH and at higher temperature causes to more protein recovery and degree of hydrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing demand for protein in a global scale turns the focus on under-utilized protein sources (Liaset et al. 2000; Nolsøe and Undeland 2009). Novel processing methods are needed to convert seafood byproducts into more profitable and marketable products. Proteins from fish processing byproducts can be modified to improve their quality and functional characteristics by enzymatic hydrolysis. Proteolytic enzymes constitute one of the most important groups of commercial enzymes, which have ample utilization in industrial process (Brandelli 2008). Biochemical production of fish protein hydrolysates (FPH) may be carried out by employing an autolytic process by endogenous enzyme or an accelerated and controllable method using exogenous enzymes (Shahidi et al. 1995). The use of fish protein hydrolysates for maintaining the growth of different microorganisms (Gildberg et al. 1989; Safari et al. 2009) or food and feed ingredients (Kristinsson and Rasco 2000a) were investigated. Many enzymes have been described to be interesting in hydrolysis of fish proteins (Papain, Alcalase®, Protamex®, Flavourzyme®, Neutrase®, etc.; Aspmo et al. 2005). Enzymes from microbial origin have been also applied to the hydrolysis of fish. In comparison to animal- or plant-derived enzymes, microbial enzymes offer several advantages including a wide variety of available catalytic activities, greater pH, and temperature stabilities (Diniz and Martin 1996). Chemical hydrolysis of proteins that is achieved by cleaving peptide bonds with either acid or base is another way to produce fish protein hydrolysates. Several processes have been proposed for the acid or alkaline hydrolysis of fish. This has been the method of choice in the past for the industry primarily because it is relatively inexpensive and quite simple to conduct. There are, however, many limitations to food ingredients using this method. Chemical hydrolysis tends to be a difficult process to control and almost invariably leads to products with variable chemical composition and functional properties. Protein hydrolysis with strong chemicals and solvents is performed at extreme temperatures and pH and generally yield products with reduced nutritional qualities, poor functionality, and restricted to use as flavor enhancers (Kristinsson and Rasco 2000a).
Acid hydrolysis of proteins is used more commonly than hydrolysis under alkaline conditions. Although the process is harsh and difficult to control, it is still the preferred method for hydrolyzed vegetable proteins. Acid hydrolysis of fish protein has usually involved reacting fish proteins with hydrochloric acid, or in some cases sulfuric acid, and the proteins are completely hydrolyzed at high temperature and often high pressure. The use of alkali reactants (e.g., sodium hydroxide) to hydrolyze protein often results in poor functionality and more importantly can adversely affect the nutritive value of the hydrolysate. Despite this, limitation alkali treatment is used in the food industry to recover and solubilize a broad range of proteins. During alkaline hydrolysis of fish protein, rapid cleavage to large water-soluble polypeptides takes place, followed by further degradation at a slower rate. To date most of the studies on acid and alkaline processing have involved work with fish fillets (Nolsøe and Undeland 2009). Generally, using chemical hydrolysis tend to produce FPH with more ash content in comparison to enzymatic hydrolysis. This could be explained by needing neutralization in chemical hydrolysis to stop the reactions in which salt could be produced (Gildberg et al. 1989).
Sturgeon are important fish that are cultured or caught for both meat and caviar production in the world. Recent estimates of the international caviar trade, conducted largely by Iran, Russia, Kazakhstan, China, and Romania, are 114 tons/year of legal trade (CITES 2004). Caspian Sea sturgeon are often claimed to yield the highest quality caviar, and countries bordering the Caspian Sea have accounted for about 80% of the global trade.
Annually, approximately 330 tones of sturgeon are caught in Caspian Sea coast in Iran for producing 31.3 tones caviar. Almost 20–25% of the weight of Sturgeon is viscera, which are produced as byproduct of sturgeon caviar and meat process industries. Sturgeon viscera could be a rich source of protein, which can be used as animal feed and human food ingredient (Ovissipour et al. 2009). Thus, the aim of this study was to evaluate the effect of five protease enzymes and two pH at different temperatures on hydrolysis of Persian sturgeon (Acipenser persicus) visceral protein.
Materials and Methods
Materials
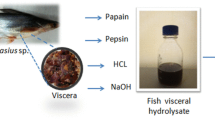
Persian sturgeon (A. persicus; total length 134 ± 10 cm, weight 27.56 ± 1.34 kg, n = 3, female) was provided by a local fisherman in Sari, Iran. Fresh viscera was immediately removed and frozen at −20 °C (at most 5 days). Prior to the hydrolysis process, fish viscera were thawed overnight in a refrigerator at 4 °C, and all three viscera were mixed together.
Microbial origin enzymes, Alcalase 2.4 L (2.4 AU/g; alkaline protease), Neutrase 0.8 L (0.8 AU/g; neutral protease), Flavourzyme (1.5 AU/g; exopeptidase), and Protamex (1.5 AU/g; combined proteases) were supplied by Iranian branch (Tehran, Iran) of Novo Co. (Novozymes, Bagsvaerd, Denmark). Animal origin enzyme, Trypsin (an alkaline protease; 200 FIP-U/g) was provided from Merck, Darmstadt, Germany.
In order to chemically hydrolyze viscera, hydrochloric acid (HCl) and sodium hydroxide (NaOH) were used to acidic and alkali hydrolysis, respectively, and were provided from Merck, Darmstadt, Germany. All chemical reagents used for experiments were of analytical grade.
Preparation of Fish Protein Hydrolysate
The fish viscera were first minced in a Moulinex® blender and then heated at 85 °C for 20 min to inactivate endogenous enzymes (Ovissipour et al. 2009). The cooked viscera were mixed with distilled water 1:2 (w/v) and homogenized in a Moulinex® blender for about 2 min at ambient temperature. The chemical and biochemical hydrolysis conditions are presented in Table 1. Novozymes enzymes (Alcalase, Neutrase, Flavourzyme, and Protamex) were added to substrate based on enzyme activity per kilogram crude protein (30 AU/kg protein), and Merck enzyme (Trypsin) was added based on mg/g protein (Shahidi et al. 1995; Vazquez and Murado 2008).
All reactions were performed in 250 mL glass vessels, in a shaking water bath with constant agitation (200 rpm). At the end of the enzymatic experiments, the reactions were terminated by heating the solution at 95 °C for 20 min (Ovissipour et al. 2009).
Also, in order to terminate the chemical reaction, neutralization was used. Thus, acidic hydrolysis terminated using 2 N NaOH, and alkaline hydrolysis terminated by 2 N HCl (Kristinsson and Rasco 2000a). All enzymatic and chemical experiments were performed during 18 h. After deactivation of the reactions, digests were centrifuged at 6,700×g at 10 °C for 20 min in a Hettich D-7200 (Tuttlingen, Germany) centrifuge to collect the supernatant.
Chemical Composition
The total crude protein (N × 6.25) in raw materials was determined using the Kjeldahl method (AOAC 2005). Protein in the fish hydrolysates was measured by the Biuret method in the supernatant following centrifugation (Layne 1957), using bovine serum albumin as a standard protein. Absorbance was measured at 540 nm in a UV/vis spectrophotometer. Protein recovery was calculated as the amount of protein present in the hydrolysate relative to the initial amount of protein present in the reaction mixture (Ovissipour et al. 2009). All experiments were performed in three assessments of the same sample.
Degree of Hydrolysis
Degree of hydrolysis was estimated according to Hoyle and Merritt (1994) as described previously (Ovissipour et al. 2009). Each run after the specified hydrolysis was terminated by the addition of 20% trichloroacetic acid (TCA) followed by centrifugation to collect the 10% TCA soluble material as the supernatant. Then degree of hydrolysis was computed as
As mentioned before, total nitrogen in hydrolysates was determined using Biuret method according to Layne (1957).
Statistical Analysis
The data obtained were subjected to one-way analysis of variance using SPSS statistical software, release 12.0 (SPSS Inc., Chicago, IL, USA). Duncan's new multiple range test was performed to determine the significant differences of the means at the 5% probability level.
Results and Discussion
Chemical Composition
Protein content of raw material and protein hydrolysate are shown in Table 2. The maximum protein content of the hydrolysates was related to Alcalase (73.34%) within the range reported by others of 63.4% to 90.8% (Bhaskar et al. 2008; Kristinsson and Rasco 2000a, b; Shahidi et al. 1995). The lowest protein content is observed in FPH produced by Trypsin (70.21%), but there was no significant difference between Protamex and Neutrase. The results of chemical hydrolysis revealed that the highest protein content was related to acidic hydrolysis at higher temperature (pH 3.3, 85 °C; P < 0.05).
The results revealed that fish protein hydrolysates produced by enzymatic reaction have more protein content compared to the ones produced by chemical hydrolysis (Table 2). There are some explanations about low protein content of hydrolysates by chemical reactions. One explanation is referring to low protein recovery ability by chemical hydrolysis from substrates with high connective tissues such as viscera (Nolsøe and Undeland 2009). Fonkwe and Singh (1996) reported low protein recovery by chemical hydrolysis of mechanically deboned turkey residues. Another explanation is because of neutralization in alkali and acidic hydrolysis to stop the reactions. Safari et al. (2009) mentioned that neutralization step in chemical hydrolysis results in high ash content, which limits the applicability of the fish protein hydrolysates to use in bacteria growth media.
Hydrolysis Condition
Enzymatic Hydrolysis
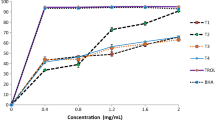
Figure 1 shows the progression of enzymatic hydrolysis of Persian sturgeon viscera using different enzymes (Alcalase, Protamex, Flavourzyme, Neutrase, and Trypsin) during 18 h. The DH% increased with increasing incubation time. All the curves show a high degree of hydrolysis during the first 3 h, followed by a decrease in the DH%. The highest DH% is related to Alcalase hydrolysates, and the lowest is related to Trypsin. Upon further incubation (3–18 h), the DH% for all enzymes slowed down. Also, the results of DH ratio showed that the lowest DH ratio is related to Trypsin (P < 0.05; Table 2). The same results could be observed by Guerard et al. (2002), Ovissipour et al. (2009), Pan et al. (2009), and Tang et al. (2009). Guerard et al. (2002) speculated that a reduction in the reaction rate may be due to the limitation of the enzyme activity by formation of reaction products at high degrees of hydrolysis. However, decrease in hydrolysis rate may also be due to a decrease in the substrate concentration, enzyme inhibition, and enzyme deactivation (Guerard et al. 2002). Tang et al. (2009) used six different enzymes to hydrolyze of hemp (Cannabis sativa) protein. Their results showed a higher DH% using Alcalase and the lowest using Trypsin. Also, they assumed that, with increasing hydrolysis time, the rate of hydrolysis decreased. These results agreed with our finding. At the end of the current study (18 h), the hydrolysates obtained with Alcalase, Protamex, Flavourzyme, Neutrase, and Trypsin had significantly different DH% values of 50.13%, 41.1%, 37.21%, 23.34%, and 14.21%, respectively.
Chemical Hydrolysis
The curves of chemical hydrolysis are presented in Fig. 2. The results of acidic and alkaline hydrolysis of Persian sturgeon visceral protein showed that the higher DH% could be achieved at higher temperature. The highest DH% was related to pH 3.3 at 85 °C with 68.87%, which was significantly higher than other conditions. The results of DH ratio are related to acidic hydrolysis at 85 °C (P < 0.05; Table 2).
There are, however, many limitations to food ingredients using this method. Chemical hydrolysis tends to be a difficult process to control and almost invariably leads to products with variable chemical composition and functional properties (Blenford 1994). Total hydrolysis of fish protein substrate can be achieved in 18 h at 118 °C in 6 N hydrochloric acid (Thomas and Loffler 1994). During alkaline hydrolysis of fish protein, rapid cleavage to large water-soluble polypeptides takes place, followed by further degradation at a slower rate (Kristinsson and Rasco 2000a). Tannenbaum et al. (1970a, b) have studied the alkaline process for hydrolyzing insoluble fish protein concentrate and its applications. They developed a small-scale batch process that utilizes high pH (12.5) and 95 °C for 20 min. The product consisted of large peptides, some relatively insoluble at the isoelectric point, but with an overall improvement in functionality with respect to the original FPC. The lower DH% in this study maybe related to the lower temperatures used for hydrolysis.
Protein Recovery
The results of protein recovery obtained by different enzymes and chemical conditions during 18 h are presented in Fig. 3. The results revealed that Alcalase-hydrolyzed fish protein has the highest protein recovery (83.64%) after 18 h, which significant differences clearly observed (P < 0.05). In enzymatic hydrolysis Trypsin has the lowest protein recovery (61.65%). These results agreed with Aspmo et al. (2005). Their findings showed that the highest recovery of hydrolysate is related to Alcalase in enzymatic hydrolysis of Atlantic cod (Gadus morhua) visceral protein.
The results of chemical hydrolysis showed that the highest protein recovery was at pH 3.3 and temperature of 85 °C (P < 0.05). The higher recovery in alkali hydrolysis was performed at pH 12 and higher temperature (85 °C) in comparison to 70 °C. Generally, protein recovery under alkali hydrolysis is low. Hence, limited alkali treatment is used in the food industry to recover and solubilize a broad range of proteins. Fonkwe and Singh (1996) discussed the use of alkali extraction to recover mechanically deboned turkey residue with an alkaline sodium chloride solution but found it to be unsuitable due to low recovery. The lower protein recovery in current study could be explained by the kind of raw materials as substrate. Nolsøe and Undeland (2009) in a review paper reported that the high protein recovery by chemical hydrolysis in fish fillets, while they also speculated that the protein recovery depends on substrate properties in terms of connection tissue content, which is high in fish viscera.
Conclusion
The hydrolysis of Persian sturgeon visceral waste protein using different enzymes and chemical reactions led to its hydrolysates with various degree of hydrolysis, protein content, and protein recovery. Indeed, hydrolysates produced by Alcalase had the highest protein content, DH%, and protein recovery. Also, chemical hydrolysis at higher temperature and lower pH caused hydolysates with higher protein content, DH%, and protein recovery. Generally, using microbial enzymes (Alcalase, Protamex, Neutrase, and Flavourzyme) to hydrolyze proteins have more benefits with respect to recovery and economic features compared to animal-derived enzymes (Trypsin) and chemical hydrolysis.
A comparison between FPH from chemical and biochemical hydrolysis revealed that in spite of good hydrolysis in both chemical and biochemical hydrolysations and more DH ratio in chemical hydrolysis, more protein content and protein recovery are notable in biochemical hydrolysation. In an economical point of view, more protein recovery, which results at FPH with higher protein content, is advisable for hydrolysation process plants. Biochemical hydrolysation results in FPH with higher protein content and no salt which can used as nitrogen source for bacteria (Safari et al. 2009) or animal feed (Kristinsson and Rasco 2000a).
References
AOAC. (2005). Official methods of analysis (16th ed.). Washington: Association of Official Analytical Chemists.
Aspmo, S. I., Horn, S. J., & Eijsink, V. G. H. (2005). Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochemistry, 40, 1957–1966.
Bhaskar, N., Benila, T., Radha, C., & Lalitha, R. G. (2008). Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresource Technolo5gy, 99(2), 335–343.
Blenford, D. E. (1994). Protein hydrolysates: Functionalities and uses in nutritional products. International Food Ingredients, 3, 45.
Brandelli, A. (2008). Bacterial keratinases: Useful enzymes for bioprocessing agroindustrial wastes beyond. Food and Bioprocess Technology, 1, 105–116.
CITES. (2004). The Convention on International Trade in Endangered Species of Wild Fauna and Flora. http://www.cites.org/eng/news/press/2004/pdf/1008sturgeonquota.pdf..
Diniz, F. M., & Martin, A. M. (1996). Use of response surface methodology to describe the combined effects of pH, temperature and E/S ratio on the hydrolysis of dogfish (Squalus acanthias). International Journal of Food Science and Technology, 31, 419–426.
Fonkwe, L. G., & Singh, R. K. (1996). Protein recovery from enzymatically deboned turkey residue by enzymic hydrolysis. Process Biochemistry, 31(6), 605.
Gildberg, A., Batista, I., & Strøm, E. (1989). Preparation and characterization of peptones obtained by a two-step enzymatic hydrolysis of whole fish. Biotechnology Applied Biochemistry, 11, 413–423.
Guerard, F., Guimas, L., & Binet, A. (2002). Production of tuna waste hydrolysates by a commercial neutral protease preparation. Journal of Molecular Catalysis B: Enzymatic, 19–20, 489–498.
Hoyle, N. T., & Merritt, J. H. (1994). Quality of fish protein hydrolysate from Herring (Clupea harengus). Journal of Food Science, 59, 76–79.
Kristinsson, H. G., & Rasco, B. A. (2000a). Fish protein hydrolysates: Production, biochemical and functional properties. Critical Reviews in Food Science and Nutrition, 40, 43–81.
Kristinsson, H. G., & Rasco, B. A. (2000b). Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. Journal of Agricultural and Food Chemistry, 48, 657–666.
Layne, E. (1957). Spectrophotometric and turbidimetric methods for measuring proteins. Methods in ensymology, vol. 3 (p. 450). New York: Academic.
Liaset, B., Lied, E., & Espe, M. (2000). Enzymatic hydrolysis of by-products from the fish-filleting industry; chemical characterization and nutritional evaluation. Journal of the Science of Food and Agriculture, 80, 560–581.
Nolsøe, H., & Undeland, I. (2009). The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food and Bioprocess Technology, 2, 1–27. doi:10.1007/s11947-008-0088-4.
Ovissipour, M., Abedian, A. M., Motamedzadegan, A., Rasco, B., Safari, R., & Shahiri, H. (2009). The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from the Persian sturgeon (Acipenser persicus) viscera. Food Chemistry, 115, 238–242.
Pan, M., Jiang, T. S., & Pan, J. L. (2009). Antioxidant activities of Rapeseed protein hydrolysates. Food and Bioprocess Technology, doi:10.1007/s11947-009-0206-y.
Safari, R., Motamedzadegan, A., Ovissipour, M., Regenstein, J. M., Gildberg, A., & Rasco, B. (2009). Use of hydrolysates from yellowfin tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food and Bioprocess Technology, doi:10.1107/s11947-009-0225-8.
Shahidi, F., Han, X. Q., & Syniwiecki, J. (1995). Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry, 53, 285–293.
Tang, C. H., Wang, X. S., & Yang, X. Q. (2009). Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chemistry, 114, 1484–1490.
Tannenbaum, S. R., Ahern, M., & Bates, R. P. (1970a). Solubilization of fish protein concentrate. I. An alkaline process. Food Technology, 24(5), 604.
Tannenbaum, S. R., Ahern, M., & Bates, R. P. (1970b). Solubilization of fish protein concentrate. II. Utilization of the alkaline-process product. Food Technology, 24(5), 607.
Thomas, D., & Loffler, F. (1994). Improved protein functionalities by enzymatic treatment. Food Marketing and Technology, 2, 2–4.
Vazquez, J. A., & Murado, M. A. (2008). Enzymatic hydrolysates from food wastewater as a source of peptones for lactic acid bacteria productions. Enzyme and Microbial Technology, 43, 66–72.
Acknowledgment
The authors thank Novozymes Co. branch in Iran, Dr. Barbara Rasco, and Dr. Turid Rustad for scientific supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ovissipour, M., Safari, R., Motamedzadegan, A. et al. Chemical and Biochemical Hydrolysis of Persian Sturgeon (Acipenser persicus) Visceral Protein. Food Bioprocess Technol 5, 460–465 (2012). https://doi.org/10.1007/s11947-009-0284-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0284-x