Abstract
Purpose of review
Management of patients with subdural hematomas starts with Emergency Neurological Life Support guidelines. Patients with acute or chronic subdural hematomas (SDHs) associated with rapidly deteriorating neurologic exam, unilaterally or bilaterally dilated nonreactive pupils, and extensor posturing are considered imminently surgical; likewise, SDHs more than 10 mm in size or those associated with more than 5-mm midline shift are deemed operative.
Recent findings
While twist drill craniostomy and placement of subdural evacuating vport system (SEPS) are quick, bedside procedures completed under local anesthesia and appropriate for patients with chronic SDH or patients that cannot tolerate anesthesia, these techniques are not optimal for patients with acute SDH or chronic SDH with septations. Burr hole SDH evacuation under conscious sedation or general anesthesia is an analogous technique; however, it requires basic surgical equipment and operating room staff, with a focus on a closed system with burr hole followed by rapid drain placement to avoid introduction of air into the subdural space, or multiple burr holes with extensive irrigation to reduce pneumocephalus and continue SDH evacuation via drain for several days. Acute SDH associated with significant mass effect and cerebral edema requires aggressive decompression via craniotomy with clot evacuation and frequently a craniectomy. Chronic SDHs that fail conservative management and progress clinically or radiographically are addressed with craniotomy with or without membranectomy.
Summary
Surgical SDH management is variable depending on its characteristics and etiology, patient’s functional status, comorbidities, goals of care, institutional preferences, and availability of specialized surgical equipment and adjunct therapies. Rapid access to surgical suites and trained staff to address surgical hemorrhages in a timely manner, with appropriate post-operative care by a specialized team including neurosurgeons and neurointensivists, is of paramount importance for successful patient outcomes. Here, we review various aspects of surgical SDH management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Indication for surgical interventions
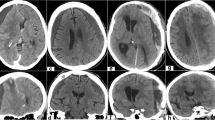
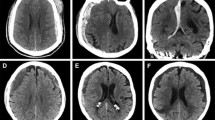
While asymptomatic subdural hematomas (SDHs) are treated conservatively, SDHs that result in worsening clinical symptoms or exhibit radiographic evidence of significant mass effect are managed surgically (Fig. 1). SDH recurrence is defined as a re-accumulation of SDH that becomes symptomatic, requiring intervention. SDHs requiring immediate attention are associated with acute clinical deterioration, e.g., mental status changes, unilateral or bilateral dilated pupils, or extensor posturing consistent with brainstem herniation. Although not imminently surgical, SDHs causing more than 5-mm midline shift or over 10 mm in size are considered operative, in principle. Surgical approaches to SDH treatment include twist drill craniostomy, including subdural evacuating port system (SEPS), burr hole(s) allowing for active or passive drainage with or without irrigation and drain placement, craniotomy to span the extent of SDH and allow for membrane removal with bone replaced at the end of surgery, or decompressive hemicraniectomy for acute SDH with significant mass effect and cerebral edema, while keeping the bone off to allow for cerebral edema resolution.
Twist drill craniostomy
Twist drill craniostomy (TDC) for chronic SDH was described in 1960; the use of closed drainage system was trialed in late 1970s (Table 1). Analogous pediatric procedures (not discussed) include percutaneous tapping and subdural shunt placement [1,2,3,4]. TDC variations introduced over time include a variety of drilling angles, craniostomy entrance points, mechanical vs automated drilling, insertion of hollow screws, cannulas or trocars with closed suction systems, and attachment of irrigation ports. TDC complication rates are low and include infection, seizures, and SDH re-accumulation, less commonly serious conditions, e.g., tension pneumocephalus [5, 6]. Other studies report 8% complications [7], including development of new acute SDH/EDH requiring surgical evacuation due to damaged dural/cortical artery or bridging veins, or violation of brain parenchyma by improper drain placement [8, 9]. SEPS introduced in 1999 resulted in clinical outcome improvement and reduced side effects associated with drain placement; a hollow port with self-tapping threads is introduced over the thickest SDH portion without entering the subdural space, allowing for slow drainage via mild negative pressure [10,11,12]. Neal et al. reported a 77.8% success rate with SEPS, less likely with mixed density or septated SDH [13]. Miele et al. concluded that the degree of head elevation does not affect drainage or recurrence [14]. An RCT by Sindou et al. showed similar success rates with 48- vs 96-h drainage, with twice complications (26.9%) and mortality (11.4%) with longer drainage [15]. A study of older SDH patients by Miranda et al. (> 65 years, mean 80) demonstrated a ~ 10% recurrence with TDC and 26.5% 6-month mortality [16]. Escosa et al. reported closed drain system to be beneficial, while presence of post-operative midline shift > 5 mm, SDH > 10 mm and neurologic deficits were associated with recurrence, regardless of dexamethasone, aspirin, or AC use [17]. Krieg et al. reported a 63.3% success with hollow screws; 16.2% patients required repeat screw placement and 20.5% required burr holes with membranectomy [18].
TDC under local or monitored anesthesia care (MAC) may be optimal in older patients with comorbidities; bilateral SDH drainage is accomplished without position change. Yadav et al. proposed modified angle TDC, using an infant feeding tube over guidewire for drain placement to avoid violating parenchyma [19]. Balser et al. compared the time to intervention and length of stay with SEPS vs burr holes, confirming that TDC is rapid and safe [20]; other studies suggest shorter procedure times but longer drainage with TDC [21]. SDH entry site and continued drainage may play a role. Jablawi et al. reported 67% effectiveness with TDC, 43% patients requiring open evacuation; entry points were over thickest SDH width rather than along the superior temporal line 1 cm anterior to the coronal suture, and scalp incision was closed without drain placement [22]. Further modifications include irrigation ports allowing instillation of adjunct therapies into SDH cavity. Wang et al. reported SEPS-like system with two ports, one for drainage and one for irrigation [23]. Neils et al. evaluated intra-cavity tPA with TDC, reporting 0% recurrence with tPA vs 30% without tPA; the mean drainage volume increased from 90 to 427.33 cm3 [24]. Urokinase likewise decreased duration of indwelling drain and hospital stay, with 0.43% recurrences [25]. Traumatic brain injury (TBI) causes tissue plasminogen activator release, increased plasmin levels, activation of fibrinolysis, inflammatory response, and increased vascular permeability via kallikrein system; tranexamic acid (TXA) counteracts this by binding to lysine sites on plasminogen with a decrease in plasmin levels [26]. The use of oral TXA 650 mg daily as SEPS adjunct resulted in a ~ 40.74% SDH reduction upon SEPS placement, with an additional 91.31% with continued TXA [27].
Burr hole evacuation
Over 700 articles and 25 clinical trials are published on burr hole SDH evacuation (BHE) (Table 2). OR environment is required due to need for perforator drills, electrocautery, and other surgical instruments; the remainder is flexible to tailor to patient needs. The standard burr hole diameter (14 mm) allows for larger dural opening, better visualization of clot and membranes, easier access to control bleeding, and possible introduction of additional instruments, e.g., endoscopes. While TDC introduces minimal air due to small cranial opening and closed drainage, BHE allows significant air into SDH space, requiring irrigation and drain placement for evacuation.
Anesthesia
Similar to TDC, BHE can be performed under local or MAC. Khadka et al. described BHE under local anesthesia with 98.6% evacuation and 4.7% recurrence [28]. Guzel et al. described successful BHE via single burr hole under MAC with no complications, beneficial for patients who cannot tolerate intubation or general anesthesia [29].
Single vs multiple BHE
Studies from 1970s describe chronic SDH patients treated with multiple BHE with 41% improvement, 25% deterioration, and 22.7% death [4]. Taussky et al. compared single vs multiple BHE in chronic SDH patients, reporting 29.4 vs 4.8% recurrence, respectively [30]. Zumofen et al. reported 86.9% success with 13.1% recurrence with BHE and overlying subgaleal drain; patients received antibiotics and phenytoin intra-operatively after reversal of asa/AC and remained flat for 48 h with drains below the head [31]. This study reported 6.6% seizures and 1.6% infections [31]. An RCT by Nayil et al. compared one vs two burr holes and reported 4–6% recurrences; patients remained flat for 48 hours [32]. Prospective study of BHE with irrigation and drain placement reported 100% SDH resolution with 3.2% recurrence [33].
Continued drainage
Drain placement is key, associated with lower recurrence of 3.1–10.5% with BHE and drain placement vs 17–33% without [34,35,36,37]. An RCT by Santarius et al. reported less recurrence with subdural drain placement (24 vs 9.3%); the trial was stopped early due to clear difference [38]. While Javadi et al. showed similar recurrence [39], Ramcharan et al. reported 4% recurrence with drain placement and 30% without [40]. An RCT by Singh et al. reported less recurrence with drain placement (26 vs 9%), with similar complications and mortality [41•]. An RCT by Kutty and Johny reported lower recurrence with single BHE with drain placement vs two BHE (1.4 vs 15.7%) [42]. Guilfoyle et al. published long-term follow-up from the Cambridge Chronic SDH Trial (CCSHT), indicating improved evacuation, less recurrence, and survival benefit 5 years post-operatively [43]. The timing of drainage may matter; Yu et al. reported 6.6% recurrence with drain placement, ranging from 16.3% with drain removal prior to 3 days to 1.3% if removed thereafter [44]. Drain location may likewise matter; subperiosteal drainage reduced seizure and infection risks avoiding SDH capsule, but was associated with higher recurrence [31]. Bellut et al. reported 1.8% recurrence with subperiosteal drain and 3.1% with subdural drains [45]. Subgaleal drains with tip [37] or holes overlying burr hole [46] reduced recurrence as compared to no drain, avoiding injury to SDH membranes/parenchyma and pneumocephalus [46]. An RCT by Kaliaperumal et al. compared subdural vs subperiosteal drain placement with no recurrences with either, but better functional recovery with subperiosteal drains [47•].
Open vs closed system
Weir compared BHE with closed drainage vs catheter placement with passive drainage, former being superior [48]. An RCT by Laumer et al. compared closed drainage BHE vs implantation of silicone catheter with Rickham reservoir within burr hole, reporting similar recurrence; patients with Rickham required fourfold less repeat surgical interventions due to bedside access to reservoir [49]. Kwon et al. reported > 200-cm3 drainage with closed system BHE associated with 0% recurrence, < 200-cm3 drainage with open system BHE with 4.1% recurrence [50]. Kuroki et al. compared BHE with rapid drain placement vs BHE with irrigation and drain placement and found 1.8 vs 11.1% recurrence, respectively [51]. Kwon et al. further supported the reverse correlation between the amount of drainage and recurrence: re-accumulation was common with mixed density SDH and drainage volume < 200 cm3 [50].
Active vs passive irrigation
Post-operative pneumocephalus is reduced by irrigation, supine positioning while filling SDH cavity, drain placement, and avoidance of intraoperative nitrous oxide [52]. Kitakami et al. reported BHE with saline irrigation followed by CO2 resulting in rapid SDH cavity disappearance [53]. Hennig and Kloster reported lower (2.6%) recurrence with irrigation; BHE without irrigation showed high recurrence (32.6 vs 23.8%) with or without drain; 44.4% patients with recurrence required craniotomies [54]. Ishibashi et al. also reported 2.9% recurrence post-BHE with irrigation, as compared to 10.3% without irrigation [55]. A study of BHE by Zakaraia et al. with closed drainage with or without irrigation showed comparable recurrence [56].
Mobilization
Abouzari et al. evaluated BHE with irrigation and closed drainage; patients were randomized to remain flat for 3 days vs mobilized to 30–40° [57]. Patients remaining flat had 2.3% SDH recurrence, while those mobilized had 19% recurrence; pulmonary/thrombotic complications were similar [57]. Adeolu et al. compared mobilization day 2 vs 7, reporting no recurrence in either group [58]. Tsutsumi et al. reported higher recurrence with T1 hypointense SDH (11.6 vs 3.4%) [35]. Kaplan et al. used transcranial Dopplers to assess ipsilateral middle cerebral artery (MCA) flow upon evacuation of > 2 cm SDH; MCA flow normalized with improvement in mental status and contralateral weakness [59].
Schwarz et al. reported midline shift, hypertension, bilateral SDH, and vitamin K antagonists as recurrence predictors [60]. Nakaguchi et al. reported 12% recurrence with < 10-mm SDH vs 45% with > 10-mm SDH, 26% recurrence with pneumocephalus vs 8% without, and 48% recurrence if > 30% SDH cavity was air by volume [61]. Patients with frontal drains had 5% recurrence vs 38% with parietal, 36% with occipital, and 33% with temporal drains; patients with frontal drains had the least subdural air [61]. Unterhofer et al. reported comparable recurrence with or without membranectomy (21 vs 28%) [62•]. Mobbs and Khong suggested endoscopic visualization and subdural catheter placement, with a sharp division or electrocautery of membranes [63].
Adjunct therapies
Platelet-activating factor (PAF) levels are increased within acute SDH, localizing to perisinusoidal vessels in SDH membranes [64]. Hirashima et al. reported 0% recurrence with 1.5–3mg daily etizolam (PAF-dependent platelet aggregation inhibitor) vs 29.2% in controls [65]. Sun et al. reported less recurrence with dexamethasone [66]. Neils et al. evaluated intra-cavity tPA, reporting 30% recurrence with TDC, 11.8% with BHE, and 0% with BHE and tPA; the mean evacuated volume increased from 26 to 100 cm3 [24]. Shimamura et al. RCT reported a fivefold reduction in recurrence (25.6 vs 5.5%) with BHE, drain, and SDH cavity irrigation with 100 u/ml thrombin regardless of systemic anti-platelets; TXA was administered for 24 h [67]. Thrombin may aid hemostasis by inducing vessel constriction and fibrin deposition by binding its receptor on sinusoidal endothelium that undergoes microhemorrhaging in SDH hyperfibrinolytic state [68]. Yeon et al. reported no change in SDH recurrence if warfarin was restarted 3 days post-operatively [69].
Twist drill vs burr hole evacuation
Smely et al. reported 18.2% recurrence and 6% death with TDC and 33% recurrence and 10% death with BHE [70]. Williams et al. reported 63.6% deterioration with TDC, compared to 16% deterioration with BHE and 7% with BHE and drain placement [71]. An RCT by Muzii et al. compared SEPS vs BHE with irrigation and drain placement; recurrence, mortality, and recovery were similar (18.2 vs 33.3%) [72]. Gokmen et al. reported similar SDH recurrences, 2.6% in TDC and 6.3% in BHE group [73]. Lin reported improved cure rate with TDC vs BHE (88.8 vs 75.5%) with less recurrence requiring repeat interventions (7.9 vs 11.9%) and less complications (7.9 vs 20.7%) [74]. Kim et al. reported similar outcomes [75]. Wang et al. treated patients with twist drill YL-1 needle vs BHE with irrigation, reporting 100 vs 94.3% improvement and 13.2 vs 17% recurrence; another prospective study reported 18.4 vs 11.1% recurrence with TDC vs BHE [23, 76]. Finally, Brennan et al. reported 2% mortality, 14% morbidity, 9% recurrences, and 22% unfavorable outcomes associated with BHE, with patient age, bed rest, and single burr hole being independent predictors of poor functional outcome, and failure to insert the drain as a predictor of recurrence and unfavorable functional outcome [77].
Craniotomy for acute SDH
Craniotomy is useful for treating acute SDH with thick clot and chronic or subacute SDH that failed other treatments with significant radiographic progression, or SDH with septations (Table 3). Management of acute SDH depends on patient prognosis, goals of care, and hospital course; it is life-threatening due to the underlying structural TBI associated with up to 92% mortality. Surgical evacuation via open craniotomy is indicated for acute SDH with > 1-cm thickness or > 5-mm midline shift [78], deteriorating patient with the Glasgow Coma Scale (GCS) < 8, unilateral or bilateral fixed dilated pupils, or evidence of elevated increased intracranial pressure (ICP) > 20 [79]. Modifications include craniotomy or craniectomy, with or without partial or complete resection of SDH membranes. SDH etiology, patient age, time to surgical treatment, pre- and post-operative ICPs, and extent of craniotomy or decompressive craniectomy affect outcomes. Subdural hygromas require surgical interventions if behaving aggressively, including evacuation, placement of ventriculoperitoneal or subduroperitoneal shunt, or cranioplasty.
Morbidity and mortality in acute SDH patients remain high due to underlying parenchymal injury, loss of autoregulation, poor preoperative neurologic state, and uncontrolled post-operative ICPs. Britt and Hamilton proposed large decompressive craniectomy for acute SDH in 1978 [80]. Koc et al. reported 38% functional recovery in 91% acute SDH patients with GCS 9–15, with a mortality over 90% in patients with GCS 3–4, bilaterally nonreactive pupils, or intracerebral hematomas; 80% mortality was reported in patients with unilaterally nonreactive pupil or subarachnoid hemorrhage [81]. Guilburd and Sviri described acute SDH evacuation through multiple dural fenestrations with > 80% success; however, 51.6% expired and over third sustained persistently elevated ICPs > 25 refractory to medical management [82]. An RCT by Jiang et al. investigated large frontotemporoparietal decompression vs temporoparietal decompression in patients with severe TBI and refractory ICPs [83]. More patients with wide decompression showed improvement (39.8 vs 28.6%) with better survival and less neurodeficits [83]. Smaller studies showed no difference in outcomes [84].
A study of severe TBI patients by Ucar et al., conducted to identify GCS cutoff for patients that benefit from decompression and address its timing, indicated that decompression should occur ≤ 4 h from injury and is futile with GCS ≤ 5 [85]. Woertgen et al. reported no difference in outcomes in acute SDH patients undergoing craniotomy or decompressive hemicraniectomy; however, mortality due to herniation was higher with decompression (53 vs 32.3%) [86]. Missori et al. used double dural sheets with decompression to aid with temporalis dissection during cranioplasty [87]. Nguyen et al. performed fenestration on bone prior to replacement, with improved post-operative SDH volumes, better drainage, and reduced recurrences [88]. Other studies described floating or hinge craniotomies (bone attached to cranium with sutures [89], Y-shaped titanium plates [90], or temporalis muscle [90, 91]), or using divided bone flaps with multiple hinges [92].
Craniotomy for chronic SDH
Chronic SDH evacuation via craniotomy is associated with better outcomes. Tabaddor and Shulmon reported 39.3% clinical improvement, 21.4% deterioration, and 28.6% mortality; a later study by Hamilton et al. reported 71.7% improvement, 10.6% recurrence, and 14.9% complications [4, 93]. Ernestus et al., Callovini et al., and Van der Veken reported similar numbers [94,95,96]. Godlewski et al. compared patients with acute, subacute, and chronic SDH; 83.5% chronic, 75% subacute, and 10.5% acute SDH patients underwent BHE, with 37.1, 26.7, and 25% recurrences, respectively; the remaining patients were treated with craniotomy/craniectomy [97]. In craniotomy/craniectomy group, 5.9% of acute SDH, 60% of subacute, and 42.9% of chronic SDH patients had recurrences [97]. Craniotomy/craniectomy patients with acute SDH did well, while subacute/chronic SDH patients best responded to BHE.
Extensive craniectomy may not be important in treating chronic SDH patients. Beatty et al. reported 91% improvement with 8.7% mortality post-minicraniectomy [98]. A study by Lee et al. comparing BHE vs 3-cm craniotomy with membranectomy vs extended craniotomy with membranectomy reported 16, 18 and 23% recurrences, respectively [99]. Lindval and Koskinen likewise reported 17% recurrences irrespective of anti-platelet use [100]. Mahmoud et al. reported 2.86% recurrence with minicraniectomies [101]. Horn et al. reported 8% recurrence in BHE vs craniotomy groups [102], while White et al. reported 17.7 vs 19.8% recurrence, with 2.5-fold higher (17.2%) mortality in the latter [103]. Regan et al. showed 6.6% recurrence and 3.3% mortality in patients undergoing BHE; recurrence post-minicraniotomy was 24.1% with mortality of 6.9% [104•]. Mondorf et al. reported 27.8 vs 14.3% recurrence with craniotomy vs BHE [105]. Kim et al. reported ~ 90% improvement regardless of technique; patients undergoing BHE had 8.9% recurrences with 8.1% mortality, vs 50% recurrence post minicraniotomy, and 9.5% recurrence with 9.5% mortality after extensive craniotomy [106].
Membranectomy may be beneficial. Tanikawa et al. reported 0% recurrence with patients undergoing craniotomy, including chronic SDH patients with multiple membranes [107]. Mohamed et al. reported no recurrence with craniotomy, durectomy, outer membranectomy, and drain placement, with two patients requiring percutaneous tapping [108]. Rocchi et al. reported zero recurrence with membranectomy, with 21.4% complications including seizures and a hemorrhagic infarct [109]. Balevi et al. reported 11.9% recurrence following large craniectomy with membranectomy within 24 h; 22.8% patients developed tension pneumothorax, 25% had seizures, and 14.28% expired [110]. An RCT by Unterhofer et al. reported a comparable 21.4% recurrence with minicraniotomy vs 28.6% with additional membranectomy, possibly due to inability of atrophied brain to re-expand and formation of neomembranes [62•].
Bilateral chronic SDH
Bilateral chronic SDHs have an incidence of 15–25% and are associated with higher recurrence and worse outcomes, with advanced age, diabetes, coagulation abnormalities, altered mental status, gait instability, and less midline shift associated with bilateral SDH [111,112,113,114]. Hsieh et al. reported age as a risk factor, with similar complication and recurrence in patients with unilateral vs bilateral BHE [115]. Lee and Park reported similar recurrence post-BHE with bilateral vs unilateral SDH (21.4 vs 16.3%), with favorable outcome and improvement less likely with bilateral SDH [113]. Fujitani et al. reported that SDH’s iso- or hypointensity on MRI were a single predictor of contralateral SDH enlargement in patients with bilateral chronic SDH undergoing unilateral BHE [116].
Few reports describe SAH, IPH, and acute SDH after bilateral BHE; neurologic deterioration may also occur after bilateral craniotomies due to brain sag [117, 118]. Decision-making is complicated if significant differences in SDH size/thickness or lateralization of symptoms are present, suggesting that one SDH is asymptomatic. Lee et al. described TDC for bilateral SDH, 20% patients requiring reoperation [119]. Recent review reported 21.6% recurrences in 136 unilaterally treated patients with bilateral SDH, 28.7% requiring repeat treatment; the absence of post-operative drainage and mixed density SDH were independent predictors for retreatment [120]. A review of 500 chronic SDH patients by Mori et al. showed 49 recurrences, 18 being contralateral to the operated side, and 14 of these having a contralateral thin SDH/effusion on preoperative CT [121]. SDH recurrences may result from ICP alterations and decreased tamponade effect due to brain displacement toward the surgical side, allowing re-expansion of contralateral SDH [121].
Conclusions
Optimizing surgical management for various types of subdural hemorrhages is needed. Recurrence rates are highest with twist drill craniostomies, although they have significant advantages of procedure completion at bedside or under MAC. Closed system, active irrigation, continued drainage, and mobilization have been associated with better outcomes with twist drill and burr hole SDH evacuation. Craniotomy carries the highest morbidity and mortality, especially in older patients who are more likely to be on anti-platelets or anticoagulation, but should be the method of choice in treating recurrent chronic SDH with multiple membranes, or acute SDH in deteriorating patients. Management of bilateral chronic SDH remains under investigation. Patients with SDH are served best by a specialized multidisciplinary team, individualized approach, and rapid surgical interventions, if needed.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:• Of importance
Rand BO, Ward AA Jr, White LE Jr. The use of the twist drill to evaluate head trauma. J Neurosurg. 1966;25(4):410–5.
Burton C, Brisman R. Neurosurgical indications for the use of a compact hand twist drill. Johns Hopkins Med J. 1968;123(1):17–22.
Burton C. The management of chronic subdural hematoma using a compact hand twist drill. Mil Med. 1968;133(11):891–5.
Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46(2):220–6.
Tindall GT, Payne NS 2nd, O’Brien MS. Complications of surgery for subdural hematoma. Clin Neurosurg. 1976;23:465–82.
Caron JL, Worthington C, Bertrand G. Tension pneumocephalus after evacuation of chronic subdural hematoma and subsequent treatment with continuous lumbar subarachnoid infusion and craniostomy drainage. Neurosurgery. 1985;16(1):107–10.
Sharma BS, Tewari MK, Khosla VK, Pathak A, Kak VK. Tension pneumocephalus following evacuation of chronic subdural haematoma. Br J Neurosurg. 1989;3(3):381–7.
Reinges MH, Hasselberg I, Rohde V, Küker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000;69(1):40–7.
Yoshino Y, Aoki N, Oikawa A, Ohno K. Acute epidural hematoma developing during twist-drill craniostomy: a complication of percutaneous subdural tapping for the treatment of chronic subdural hematoma. Surg Neurol. 2000;53(6):601–4.
Emonds N, Hassler WE. New device to treat chronic subdural hematoma—hollow screw. Neurol Res. 1999;21(1):77–8.
Asfora WT, Schwebach L, Louw D. A modified technique to treat subdural hematomas: the subdural evacuating port system. S D J Med. 2001;54(12):495–8.
Asfora WT, Schwebach L. A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol. 2003;59(4):329–32.
Neal MT, Hsu W, Urban JE, Angelo NM, Sweasey TA, Branch CL Jr. The subdural evacuation port system: outcomes from a single institution experience and predictors of success. Clin Neurol Neurosurg. 2013;115(6):658–64.
Miele VJ, Sadrolhefazi A, Bailes JE. Influence of head position on the effectiveness of twist drill craniostomy for chronic subdural hematoma. Surg Neurol. 2005;63(5):420–3.
Sindou M, Ibrahim I, Maarrawi J. Chronic sub-dural hematomas: twist drill craniostomy with a closed system of drainage, for 48 hours only, is a valuable surgical treatment. Acta Neurochir. 2010;152(3):545–6.
Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114(1):72–6.
Escosa Baé M, Wessling H, Salca HC, de Las Heras Echeverría P. Use of twist-drill craniostomy with drain in evacuation of chronic subdural hematomas: independent predictors of recurrence. Acta Neurochir. 2011;153(5):1097–103.
Krieg SM, Aldinger F, Stoffel M, Meyer B, Kreutzer J. Minimally invasive decompression of chronic subdural haematomas using hollow screws: efficacy and safety in a consecutive series of 320 cases. Acta Neurochir. 2012;154(4):699–705.
Yadav YR, Yadav S, Parihar VS. Modified twist drill technique in the management of chronic subdural hematoma. Turk Neurosurg. 2013;23(1):50–4.
Balser D, Rodgers SD, Johnson B, Shi C, Tabak E, Samadani U. Evolving management of symptomatic chronic subdural hematoma: experience of a single institution and review of the literature. Neurol Res. 2013;35(3):233–42.
Wang K, Chen D, Cao X, Gao L. A prospective comparative study of twist drill craniostomy versus burr hole craniostomy in patients with chronic subdural hematoma. Turk Neurosurg. 2017;27(1):60–5.
Jablawi F, Kweider H, Nikoubashman O, Clusmann H, Schubert GA. Twist drill procedure for chronic subdural hematoma evacuation: an analysis of predictors for treatment success. World Neurosurg. 2017;100:480–6.
Wang QF, Cheng C, You C. A new modified twist drill craniostomy using a novel device to evacuate chronic subdural hematoma. Medicine (Baltimore). 2016;95(10):e3036.
Neils DM, Singanallur PS, Wang H, Tracy P, Klopfenstein J, Dinh D, et al. Recurrence-free chronic subdural hematomas: a retrospective analysis of the instillation of tissue plasminogen activator in addition to twist drill or burr hole drainage in the treatment of chronic subdural hematomas. World Neurosurg. 2012;78(1–2):145–9.
Lu J, Shen D, Hu F, Zhou J, Lan F, Guo D, et al. An improved electronic twist-drill craniostomy procedure with post-operative urokinase instillation in treating chronic subdural hematoma. Clin Neurol Neurosurg. 2015;136:61–5.
Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5(5):467–73.
Tanweer O, Frisoli FA, Bravate C, Harrison G, Pacione D, Kondziolka D, et al. Tranexamic acid for treatment of residual subdural hematoma after bedside twist-drill evacuation. World Neurosurg. 2016;91:29–33.
Khadka NK, Sharma GR, Roka YB, Kumar P, Bista P, Adhikari D, et al. Single burr hole drainage for chronic subdural haematoma. Nepal Med Coll J. 2008;10(4):254–7.
Guzel A, Kaya S, Ozkan U, Ufuk Aluclu M, Ceviz A, Belen D. Surgical treatment of chronic subdural haematoma under monitored anaesthesia care. Swiss Med Wkly. 2008;138(27–28):398–403.
Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008;22(2):279–82.
Zumofen D, Regli L, Levivier M, Krayenbühl N. Chronic subdural hematomas treated by burr hole trepanation and a subperiostal drainage system. Neurosurgery. 2009;64(6):1116–21.
Nayil K, Altaf R, Shoaib Y, Wani A, Laharwal M, Zahoor A. Chronic subdural hematomas: single or double burr hole-results of a randomized study. Turk Neurosurg. 2014;24(2):246–8.
Park SH, Kang DH, Park J, Hwang JH, Hwang SK, Sung JK, et al. Fibrinogen and D-dimer analysis of chronic subdural hematomas and computed tomography findings: a prospective study. Clin Neurol Neurosurg. 2011;113(4):272–6.
Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochiai C, Nagai M. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990;26(5):771–3.
Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg. 1997;87(6):870–5.
Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag O, Kars HZ. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 2007;34(1):84–7.
Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir. 2007;149(5):487–93.
Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067–73.
Javadi A, Amirjamshidi A, Aran S, Hosseini SH. A randomized controlled trial comparing the outcome of burr-hole irrigation with and without drainage in the treatment of chronic subdural hematoma: a preliminary report. World Neurosurg. 2011;75(5–6):731–6.
Ramachandran R, Hegde T. Chronic subdural hematomas—causes of morbidity and mortality. Surg Neurol. 2007;67:367–73.
• Singh AK, Suryanarayanan B, Choudhary A, Prasad A, Singh S, Gupta LN. A prospective randomized study of use of drain versus no drain after burr-hole evacuation of chronic subdural hematoma. Neurol India. 2014;62(2):169–74. An RCT supporting the importance of drain placement to minimize SDH recurrence.
Kutty SA, Johny M. Chronic subdural hematoma: a comparison of recurrence rates following burr-hole craniostomy with and without drains. Turk Neurosurg. 2014;24(4):494–7.
Guilfoyle MR, Hutchinson PJ, Santarius T. Improved long-term survival with subdural drains following evacuation of chronic subdural haematoma. Acta Neurochir. 2017;159(5):903–5.
Yu GJ, Han CZ, Zhang M, Zhuang HT, Jiang YG. Prolonged drainage reduces the recurrence of chronic subdural hematoma. Br J Neurosurg. 2009;23(6):606–11.
Bellut D, Woernle CM, Burkhardt JK, Kockro RA, Bertalanffy H, Krayenbühl N. Subdural drainage versus subperiosteal drainage in burr-hole trepanation for symptomatic chronic subdural hematomas. World Neurosurg. 2012;77(1):111–8.
Yadav YR, Parihar V, Chourasia ID, Bajaj J, Namdev H. The role of subgaleal suction drain placement in chronic subdural hematoma evacuation. Asian J Neurosurg. 2016;11(3):214–8.
• Kaliaperumal C, Khalil A, Fenton E, Okafo U, Kaar G, O'Sullivan M, et al. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir. 2012;154(11):2083–8. An RCT comparing different locations for drain placement, suggesting better functional recovery with subperiosteal drain placemen.
Weir BK. Results of burr hole and open or closed suction drainage for chronic subdural hematomas in adults. Can J Neurol Sci. 1983;10(1):22–6.
Laumer R, Schramm J, Leykauf K. Implantation of a reservoir for recurrent subdural hematoma drainage. Neurosurgery. 1989;25(6):991–6.
Kwon TH, Park YK, Lim DJ, Cho TH, Chung YG, Chung HS, et al. Chronic subdural hematoma: evaluation of the clinical significance of postoperative drainage volume. J Neurosurg. 2000;93(5):796–9.
Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir. 2001;143(10):1041–4.
Nagata K, Asano T, Basugi N, Tango T, Takakura K. Studies on the operative factors affecting the reduction of chronic subdural hematoma, with special reference to the residual air in the hematoma cavity. No Shinkei Geka. 1989;17(1):15–20.
Kitakami A, Ogawa A, Hakozaki S, Kidoguchi J, Obonai C, Kubo N. Carbon dioxide gas replacement of chronic subdural hematoma using single burr-hole irrigation. Surg Neurol. 1995;43(6):574–7.
Hennig R, Kloster R. Burr hole evacuation of chronic subdural haematomas followed by continuous inflow and outflow irrigation. Acta Neurochir. 1999;141(2):171–6.
Ishibashi A, Yokokura Y, Adachi H. A comparative study of treatments for chronic subdural hematoma: burr hole drainage versus burr hole drainage with irrigation. Kurume Med J. 2011;58(1):35–9.
Zakaraia AM, Adnan JS, Haspani MS, Naing NN, Abdullah JM. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: an analysis. Surg Neurol. 2008;69(6):608–15.
Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61(4):794–7.
Adeolu AA, Rabiu TB, Adeleye AO. Post-operative day two versus day seven mobilization after burr-hole drainage of subacute and chronic subdural haematoma in Nigerians. Br J Neurosurg. 2012;26(5):743–6.
Kaplan M, Erol FS, Bozgeyik Z, Koparan M. The effectiveness of simple drainage technique in improvement of cerebral blood flow in patients with chronic subdural hemorrhage. Turk Neurosurg. 2007;17(3):202–6.
Schwarz F, Loos F, Dünisch P, Sakr Y, Safatli DA, Kalff R, et al. Risk factors for reoperation after initial burr hole trephination in chronic subdural hematomas. Clin Neurol Neurosurg. 2015;138:66–71.
Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93(5):791–5.
• Unterhofer C, Freyschlag CF, Thomé C, Ortler M. Opening the internal hematoma membrane does not alter the recurrence rate of chronic subdural hematomas: a prospective randomized trial. World Neurosurg. 2016;92:31–6. An RCT that assessed the importance of removal of SDH membranes.
Mobbs R, Khong P. Endoscopic-assisted evacuation of subdural collections. J Clin Neurosci. 2009;16(5):701–4.
Hirashima Y, Nagahori T, Nishijima M, Endo S, Takaku A, Nakagawa Y. Analysis of plasma and hematoma lipids related to choline glycerophospholipid in patients with chronic subdural hematoma. Neurol Med Chir (Tokyo). 1994;34(3):131–5.
Hirashima Y, Kuwayama N, Hamada H, Hayashi N, Endo S. Etizolam, an anti-anxiety agent, attenuates recurrence of chronic subdural hematoma—evaluation by computed tomography. Neurol Med Chir (Tokyo). 2002;42(2):53–5.
Sun TF, Boet R, Poon WS. Non-surgical primary treatment of chronic subdural haematoma: preliminary results of using dexamethasone. Br J Neurosurg. 2005;19(4):327–33.
Shimamura N, Ogasawara Y, Naraoka M, Ohnkuma H. Irrigation with thrombin solution reduces recurrence of chronic subdural hematoma in high-risk patients: preliminary report. J Neurotrauma. 2009;26(11):1929–33.
Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002;96(5):877–84.
Yeon JY, Kong DS, Hong SC. Safety of early warfarin resumption following burr hole drainage for warfarin-associated subacute or chronic subdural hemorrhage. J Neurotrauma. 2012;29(7):1334–41.
Smely C, Madlinger A, Scheremet R. Chronic subdural haematoma--a comparison of two different treatment modalities. Acta Neurochir. 1997;139(9):818–25.
Williams GR, Baskaya MK, Menendez J, Polin R, Willis B, Nanda A. Burr-hole versus twist-drill drainage for the evacuation of chronic subdural haematoma: a comparison of clinical results. J Clin Neurosci. 2001;8(6):551–4.
Muzii VF, Bistazzoni S, Zalaffi A, Carangelo B, Mariottini A, Palma L. Chronic subdural hematoma: comparison of two surgical techniques. Preliminary results of a prospective randomized study. J Neurosurg Sci. 2005;49(2):41–6.
Gökmen M, Sucu HK, Ergin A, Gökmen A, Bezircio Lu H. Randomized comparative study of burr-hole craniostomy versus twist drill craniostomy: surgical management of unilateral hemispheric chronic subdural hematomas. Zentralbl Neurochir. 2008;69(3):129–33.
Lin X. Comparing twist-drill drainage with burr hole drainage for chronic subdural hematoma. Chin J Traumatol. 2011;14(3):170–3.
Kim GH, Kim BT, Im SB, Hwang SC, Jeong JH, Shin DS. Comparison of the indications and treatment results of burr-hole drainage at the maximal thickness area versus twist-drill craniostomy at the pre-coronal point for the evacuation of symptomatic chronic subdural hematomas. J Korean Neurosurg Soc. 2014;56(3):243–7.
Wang W, Liu H, Yang J. Burr hole craniostomy irrigation with and without drainage during surgical treatment of chronic subdural hematoma: a retrospective study of 87 cases. Turk Neurosurg. 2017;
Brennan PM, Kolias AG, Joannides AJ, Shapey J, Marcus HJ, Gregson BA, et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017;127(4):732–9.
Servadei F, Nasi MT, Cremonini AM, Giuliani G, Cenni P, Nanni A. Importance of a reliable admission Glasgow Coma Scale score for determining the need for evacuation of posttraumatic subdural hematomas: a prospective study of 65 patients. J Trauma. 1998;44(5):868–73.
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–24.
Britt RH, Hamilton RD. Large decompressive craniostomy in the treatment of acute subdural hematoma. Neurosurgery. 1978;2(3):195–200.
Koç RK, Akdemir H, Oktem IS, Meral M, Menkü A. Acute subdural hematoma: outcome and outcome prediction. Neurosurg Rev. 1997;20(4):239–44.
Guilburd JN, Sviri GE. Role of dural fenestrations in acute subdural hematoma. J Neurosurg. 2001;95(2):263–7.
Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22(6):623–8.
Schulz C, Mauer UM. Postoperative course after acute traumatic subdural hematoma in the elderly. Does the extent of craniostomy influence outcome? Z Gerontol Geriatr. 2011;44(3):177–80.
Ucar T, Akyuz M, Kazan S, Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005;22(11):1311–8.
Woertgen C, Rothoerl RD, Schebesch KM, Albert R. Comparison of craniostomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci. 2006;13(7):718–21.
Missori P, Polli FM, Peschillo S, D’Avella E, Paolini S, Miscusi M. Double dural patch in decompressive craniectomy to preserve the temporal muscle: technical note. Surg Neurol. 2008;70(4):437–9.
Nguyen HS, Doan N, Wolfla C, Pollock G. Fenestration of bone flap during decompressive craniostomy for subdural hematoma. Surg Neurol Int. 2016;7:16.
Mezue WC, Ndubuisi C, Ohaegbulam SC, Chikani M, Erechukwu U. Cranial bony decompressions in the management of head injuries: decompressive craniostomy or craniectomy? Niger J Clin Pract. 2013;16(3):343–7.
Schmidt JH 3rd, Reyes BJ, Fischer R, Flaherty SK. Use of hinge craniostomy for cerebral decompression. Technical note. J Neurosurg. 2007;107(3):678–82.
Adeleye AO, Azeez AL. Decompressive craniectomy bone flap hinged on the temporalis muscle: a new inexpensive use for an old neurosurgical technique. Surg Neurol Int. 2011;2:150.
Valença MM, Martins C, da Silva JC. “In-window” craniostomy and “bridgelike” duraplasty: an alternative to decompressive hemicraniectomy. J Neurosurg. 2010;113(5):982–9.
Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniostomy reevaluated. Neurosurgery. 1993;33(1):67–72.
Ernestus R-I, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3):220–5.
Callovini GM, Bolognini A, Callovini G, et al. Primary enlarged craniostomy in organized chronic subdural hematomas. Neurol Med Chir (Tokyo). 2014;54(5):349–56.
Van Der Veken J, Duerinck J, Buyl R, et al. Mini- craniostomy as the primary surgical intervention for the treatment of chronic subdural hematoma–a retrospective analysis. Acta Neurochir. 2014;156(5):981–7.
Godlewski B, Pawelczyk A, Pawelczyk T, et al. Retrospective analysis of operative treatment of a series of 100 patients with subdural hematoma. Neurol Med Chir (Tokyo). 2013;53(1):26–33.
Beatty RA. Subdural haematomas in the elderly: experience with treatment by trephine craniostomy and not closing the dura or replacing the bone plate. Br J Neurosurg. 1999;13(1):60–4.
Lee KS, Bae WK, Park YT, Yun IG. The pathogenesis and fate of traumatic subdural hygroma. Br J Neurosurg. 1994;8(5):551–8.
Lindvall P, Koskinen L-OD. Anticoagulants and anti-platelet agents and the risk of development and recurrence of chronic subdural haematomas. J Clin Neurosci. 2009;16(10):1287–90.
Mahmood SD, Waqas M, Baig MZ, Darbar A. Mini-craniostomy under local anesthesia for chronic subdural hematoma: an effective choice for elderly patients and for patients in a resource-strained environment. World Neurosurg. 2017;106:676–9.
Horn EM, Feiz-Erfan I, Bristol RE, Spetzler RF, Harrington TR. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 2006;65(2):150–3.
White M, Mathieson CS, Campbell E, Lindsay KW, Murray L. Treatment of chronic subdural haematomas, a retrospective comparison of minicraniectomy versus burr-hole drainage. Br J Neurosurg. 2010;24(3):272–5.
• Regan JM, Worley E, Shelburne C, et al. Burr hole washout versus craniostomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. 2015;10(1):e0115085. An RCT suggesting that BHE is a better technique for chronic SDH evacuation rather than craniostomy.
Mondorf Y, Abu-Owaimer M, Gaab MR, Oertel JMK. Chronic subdural hematoma—craniostomy versus burr hole trepanation. Br J Neurosurg. 2009;23(6):612–6.
Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniostomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50(2):103–8.
Tanikawa MMM, Yamada K, Yamashita N, Matsumoto T, Banno T, Miyati T. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir. 2001;143(6):613–8.
Mohamed EE. Chronic subdural haematoma treated by craniostomy, durectomy, outer membranectomy and subgaleal suction drainage. Personal experience in 39 patients. Br J Neurosurg. 2003;17(3):244–7.
Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007;67(4):374–80.
Balevi M. Organized chronic subdural hematomas treated by large craniostomy with extended membranectomy as the initial treatment. Asian J Neurosurg. 2017;12(4):598–604.
Penchet G, Loiseau H, Castel JP. Chronic bilateral subdural hematomas. Neurochirurgie. 1998;44(4):247–52.
Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6):1125–9.
Lee J, Park JH. Clinical characteristics of bilateral versus unilateral chronic subdural hematoma. Korean J Neurotrauma. 2014;10(2):49–54.
Park HS, Park ES, Park JB, Kwon SC, Lyo IU, Kim MH, et al. Chronic subdural hematomas: comparison between unilateral and bilateral involvement. Korean J Neurotrauma. 2014;10(2):55–9.
Hsieh CT, Su IC, Hsu SK, Huang CT, Lian FJ, Chang CJ. Chronic subdural hematoma: differences between unilateral and bilateral occurrence. J Clin Neurosci. 2016;34:252–8.
Fujitani S, Ishikawa O, Miura K, Takeda Y, Goto H, Maeda K. Factors predicting contralateral hematoma growth after unilateral drainage of bilateral chronic subdural hematoma. J Neurosurg. 2017;126(3):755–9.
Liu JKC. Neurological deterioration due to brain sag following bilateral craniostomy for subdural hematoma evacuation. World Neurosurg. 2018.
Seung WB, Jeong JH. Postoperative subarachnoid hemorrhage and multipunctate Intracerebral hemorrhages following evacuation of bilateral chronic subdural hematomas. Korean J Neurotrauma. 2017;13(2):149–52.
Lee SJ, Hwang SC, Im SB. Twist-drill or burr hole craniostomy for draining chronic subdural hematomas: how to choose it for chronic subdural hematoma drainage. Korean J Neurotrauma. 2016;12(2):107–11.
Andersen-Ranberg NC, Poulsen FR, Bergholt B, Hundsholt T, Fugleholm K. Bilateral chronic subdural hematoma: unilateral or bilateral drainage? J Neurosurg. 2017;126(6):1905–11.
Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001;41(8):371–81.
Acknowledgements
The editors would like to thank Dr. Myrna Rosenfeld for taking the time to review this manuscript.
Funding
Elena I Fomchenko, Charles C Matouk, and Jason L Gerrard are supported by Yale Neurosurgery. Emily J Gilmore and Kevin N Sheth are supported by Yale Department of Clinical Neurosciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elena I Fomchenko, Emily J Gilmore, Charles C Matouk, and Jason L Gerrard each declare no conflict of interest. Kevin N Sheth is a section editor for Current Treatment Options in Neurology.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Fomchenko, E.I., Gilmore, E.J., Matouk, C.C. et al. Management of Subdural Hematomas: Part II. Surgical Management of Subdural Hematomas. Curr Treat Options Neurol 20, 34 (2018). https://doi.org/10.1007/s11940-018-0518-1
Published:
DOI: https://doi.org/10.1007/s11940-018-0518-1