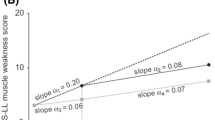
Opinion statement
Treatment of transthyretin familial amyloid polyneuropathy (TTR FAP) must be tailored to disease stage. Patients with early stage disease (i.e., without major impairment in walking ability), especially younger patients, should be referred as soon as possible for liver transplantation (LT) in the absence of major comorbid conditions. LT remains the most effective treatment option to date and should be offered to these patients as early as possible. Bridging therapy with an oral TTR stabilizer (tafamidis or diflunisal, according to local access to these treatments) should be started as soon as the diagnosis of TTR FAP is established. Early stage patients who do not wish to or have contraindications to LT should be treated with an oral TTR stabilizer or get access to the newly developed therapeutic options (IONIS TTR-Rx, patisiran, doxycycline/TUDCA). Late stage patients (presenting with significant walking impairment) are usually older and notoriously difficult to treat. They should be offered an oral TTR stabilizer but are not candidates for LT due to a significant rate of perioperative complications and increased risk of progressive neurological and especially cardiac disease despite LT. Access to the different therapies in development should also be considered depending on respective inclusion and exclusion criteria. The abovementioned treatment options were mostly validated in Val30Met mutation patients, but should also be offered to non-Val30Met patients, although mortality rates after LT are higher in these patients. Treatment decisions should be made on an individual basis. Screening for heart, eye, and renal involvement is mandatory for every patient at disease diagnosis and regularly thereafter, even in transplanted patients. Symptomatic treatment should be offered as needed, as well as genetic counseling to at-risk family members. Asymptomatic mutation carriers should benefit from regular screening for early symptoms of disease. Current therapeutic management of TTR FAP will hopefully be changed in the near future with data from the ongoing phase 2/3 studies testing the TTR gene silencing agents. In the longer term, it is likely that combined therapeutic approaches will be necessary to reverse the disease process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloid disorders are a heterogeneous group of diseases that can either be focal or systemic. They are due to the tissue deposition of abnormally folded proteins, leading to organ dysfunction. Different mechanisms may underlie the propensity of a protein to misfold, for example its intrinsic biochemical properties, increased protein synthesis, and/or pathogenic gene mutations. Amyloidoses belong to the broader group of conformational diseases, which also includes common diseases such as Alzheimer’s and Parkinson’s diseases [1]. Unlike what is found in these neurodegenerative disorders, however, in the systemic amyloidoses, the pathogenic protein precursors substantially circulate in the blood before accumulating in target tissues, thus offering a potential window for therapeutic intervention.
Transthyretin familial amyloid polyneuropathy (TTR FAP) is an autosomal dominant disorder caused by mutations in the TTR gene, that present either as sporadic cases or as familial cases, then often in geographical clusters such as Northern Portugal, Sweden, or Japan. Pathogenic mutations (mostly Val30Met) lead to a progressive sensorimotor and autonomic neuropathy as well as amyloid heart disease. Other clinical features of TTR FAP are summarized in Table 1. Death occurs within circa 10 years from disease onset, either because of the generalized neuropathy or because of life-threatening arrhythmias and/or conduction abnormalities [2]. Commonly used clinical scoring systems help describe the different stages of TTR FAP in terms of disability progression (Table 2). As the liver is the main source of mutated TTR, liver transplantation became an early treatment option in the 1990s. However, many patients are not candidates for this invasive procedure mainly because of age and/or advanced disease. Furthermore, as TTR is also secreted by the eye and the central nervous system. Accordingly a patient might be at risk of developing late complications in these organs, including cerebral amyloid angiopathy [5•].
In this review, we will present current and investigational approaches for the etiological treatment of TTR FAP, which can be divided as follows: suppression of variant TTR synthesis, stabilization of variant TTR protein, and clearance of established tissue amyloid deposits (Fig. 1).
Suppression of variant TTR synthesis
Liver transplantation
Orthotopic liver transplantation (LT) became available for the suppression of mutant TTR synthesis in the early 1990s, through the surgical removal of the source organ and its replacement by a liver secreting wild-type TTR. The primary aim of LT is to stop disease progression, not to reverse established symptoms. Hence, LT should be considered early in the disease course, i.e., before relevant difficulties walking have occurred. Other than the inability to walk unaided, the main adverse prognostic factors include advanced age, denutrition/cachexia as measured by the modified body mass index, advanced amyloid cardiomyopathy, and severe autonomic neuropathy [6, 7, 8••]. Genetic status also has prognostic significance, as patients carrying the Val30Met mutation have a better overall survival after LT than non-Val30Met patients according to data from the FAP World Transplant Registry (FAP WTR, accessible at www.fapwtr.org) [7, 8••]. However, prognosis varies among the different non-Val30Met mutations, which should not be considered as a homogeneous group of patients [9••]. Asymptomatic mutation carriers are not candidates for LT, as disease penetrance is known to be incomplete and to vary between populations. The need for early detection of symptomatic FAP in mutation carriers has prompted studies to identify objective early markers of neuropathy [10, 11, 12•]. In selected patients with advanced amyloid restrictive cardiomyopathy, a combined liver and heart transplantation can be considered.
LT has been proven to prolong survival of FAP patients [13, 14]. The FAP WTR reports overall survival rates of 77 % at 5 years and 55.3 % at 20 years after LT [7, 8••]. Better patient selection has allowed for increased life expectancy after LT. Survival was reported to be 100 % after 10 years according to a recent series from Japan [14]. However, periprocedural complications may occur. The main causes of death after LT are infections and cardiac-related causes, keeping in mind that LT does not always halt the progression of amyloid heart involvement, especially in non-Val30Met patients. This is thought to be due to the continued deposition of wild-type TTR on established heart amyloid deposits [15, 16], a phenomenon that has also been observed in the peripheral nerves of patients whose neuropathy progressed despite LT [17, 18•].
The occurrence of late cardiac deaths in transplanted patients should prompt clinicians to monitor these patients closely for conduction disorders and arrhythmias, at least on an annual basis. The continued secretion of mutated TTR in the eye and choroid plexus for the brain accounts for the ineffectiveness of LT in preventing disease progression in these organs. The late occurrence of cerebral amyloid angiopathy due to vascular deposition of mutated TTR has been reported [5•].
Organ donor shortage has led to the development to domino liver transplantation (DLT), a procedure where the explanted FAP liver is retransplanted to patients who would otherwise not benefit from a conventional LT due to the underlying disease (for instance, advanced malignancy). However, despite initial hope that FAP would not develop in these recipients before decades, it is now known that FAP can develop as soon as the first decade after LT [19, 20]. Increasing recipient age has recently been suggested to be a risk factor for the development of post-LT FAP [21]. Despite these drawbacks, DLT procedures are still occasionally performed. Whether the recipients of FAP livers would benefit from TTR stabilizers such as tafamidis or diflunisal (see below) is currently not documented [22].
Gene silencing
Advances in gene therapy have led to the development of gene silencing approaches to suppress the endogenous synthesis of mutated TTR in FAP patients. These techniques can be implemented independently from the underlying disease-causing mutation, and suppress both mutant and wild-type TTR protein synthesis. As TTR function seems dispensable in vivo, it is not expected that knocking down TTR gene expression will lead to serious adverse events in treated patients. Two types of gene silencing therapies are currently being evaluated in phase 2/3 clinical trials: antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs). As with LT, these approaches are expected to stop disease progression. However, by strongly reducing both mutant and wild-type TTR plasma levels, it is hoped that gene silencing therapies will further reduce disease progression compared to LT, where continued wild-type TTR deposition on established tissue amyloid deposits contributes to disease progression. The reversion of established manifestations might be observed in this context but this remains speculative. Finally, such procedures are much less invasive than LT.
Antisense oligonucleotides
Antisense oligonucleotides (ASOs) are short synthetic RNA sequences that bind to target mRNA in a sequence-specific manner, thereby specifically inhibiting its translation. Their potential to suppress TTR synthesis has been demonstrated in transgenic mouse models [23]. The leading ASO being evaluated in clinical trials is IONIS TTR-Rx (formerly ISIS TTR-Rx). It has been shown to reduce TTR protein plasma levels in a dose-dependent manner by up to 80 % in various animal models [24]. Similar results were obtained in healthy volunteers during a phase 1 study [24]. The treatment is currently undergoing evaluation in a phase 2/3 clinical trial, where IONIS TTR-Rx is administered as a weekly subcutaneous injection compared to placebo in a 2:1 ratio [25]. Primary outcomes measures are changes from baseline in neurological function and quality of life at 15 months, as measured by the modified Neuropathy Impairment Score +7 (mNIS + 7) and the Norfolk Quality of Life Diabetic Neuropathy (QOL-DN) Questionnaire, respectively. The study has finished enrolling patients with first results expected during the second half of 2017. Study participants are offered the possibility to enroll in an open-label extension study.
Small interfering RNAs
Small interfering RNAs (siRNAs) are double-stranded RNAs that knock down target mRNA in a sequence-specific manner by means of enzymatic degradation. The therapeutic use of siRNAs requires that they are delivered to the intracellular compartment, a problem that was solved by their formulation as lipid nanoparticles [26]. As such, the systemic delivery of patisiran, a siRNA that specifically knocks down both mutant and wild-type TTR gene expression, has been shown to greatly reduce TTR synthesis by the liver, although it does not appear to reduce TTR synthesis by the eye and central nervous system (CNS). In the phase 1 study, a single intravenous infusion of patisiran was shown to knock down serum TTR levels in healthy volunteers by more than 80 % in a dose-dependent manner [27••]. Nadir was reached at day 10, continued TTR knockdown was present at day 28, and TTR levels fully normalized at day 70. Only mild to moderate infusion-related side effects were observed.
Results from the patisiran phase 2 study were recently reported [28••]. Among 29 patients in the intention-to-treat population, 20 of them harbored the Val30Met mutation and were currently treated by a TTR stabilizer. Potent, dose-dependent serum TTR knockdown was confirmed, and the optimal dose was found to be 0.3 mg/kg administered intravenously (IV) every 3 weeks. This protocol allowed >80 % serum TTR knockdown, with efficacy maintained after the second infusion. Efficacy seemed independent from the underlying mutation and was similar among patients with and without TTR stabilizer treatments. Regarding safety, the main side effects were mild to moderate infusion-related events which occurred in a minority of patients. One patient experienced extravasation-related cellulitis and another patient experienced urinary tract infection, sepsis, and nausea/vomiting. No significant elevation of liver enzymes was noted.
Participants to the phase 2 study were offered the possibility to enroll in an ongoing open-label extension (OLE) study with clinical endpoints assessed every 6 months. Preliminary results show that neurologic impairment was stable at 18 months, suggesting that patisiran might slow disease progression [29]. An international, randomized, double-blind, placebo-controlled phase 3 study (the APOLLO trial) is currently evaluating the impact of patisiran on clinical outcomes, with the primary outcome measure being the mNIS + 7 at 18 months enrolment of patients is now completed and results are expected in the second half of 2017. Study participants are offered the possibility to enroll in an open-label extension study.
Stabilization of variant TTR protein
Pathogenic mutations increase the propensity of the TTR protein tetramers to dissociate into monomers, which in turn aggregate as soluble oligomers and then as insoluble amyloid fibrils. As TTR tetramer dissociation is considered a rate-limiting step in the process of amyloid fibril formation, oral treatments have been studied with the aim to stabilize TTR tetramers [30]. As with LT, these approaches are meant to stop disease progression and not to reverse established deficits. Two TTR stabilizers have completed evaluation in phase 3 clinical trials: diflunisal and tafamidis.
Diflunisal
A non-steroidal anti-inflammatory drug (NSAID) developed in the 1970s, diflunisal was shown to be a strong inhibitor of TTR amyloid fibril formation in vitro [31, 32]. Subsequent phase 1 and 2 studies confirmed the ability of diflunisal to stabilize TTR tetramers in healthy volunteers and FAP patients [33, 34].
The pivotal phase 3 study was a randomized, double-blind placebo-controlled trial that included 130 patients [35••]. A little more than 50 % of the participants exhibited the Val30Met mutation, and most (but not all) of the patients displayed an early stage polyneuropathy, i.e., remained able to walk unaided. Although there was a high drop-out rate in the placebo group that was mainly due to disease progression, diflunisal 250 mg bid showed its ability to significantly reduce disease progression compared to placebo as measured by the NIS + 7 score at 2 years, the study’s primary endpoint. Secondary efficacy endpoints including the SF-36 quality of life scale were also met.
Diflunisal was generally well tolerated, with no difference in serious adverse effects between treatment groups. Adverse effects leading to discontinuation of diflunisal occurred in four patients, including one with gastrointestinal bleeding and one with congestive heart failure, in accordance with the known side effects of NSAIDs [35••]. Overall, the study confirmed that diflunisal could significantly slow down FAP disease progression across a wide spectrum of TTR mutations and in patients at different stages of neuropathy, including perhaps some with more advanced disease.
The benefit of diflunisal seems to be maintained after 2 years of treatment [36]. A recent open-label study suggests that diflunisal might also have a positive impact on the autonomic neuropathy of FAP, although these data need to be confirmed [37]. Diflunisal has the advantage of being cheap and easily available worldwide, although it is currently not available in many European countries.
Tafamidis
Tafamidis can be administered orally and is a small molecule that binds to the thyoxine-binding site of TTR tetramers, thereby preventing their dissociation in monomers and the formation of soluble oligomers [38]. The pivotal multicentric phase 2/3 study compared tafamidis 20 mg qd to placebo in a 1:1 ratio over 18 months in 128 Val30Met patients presenting with a very early stage polyneuropathy [39]. Coprimary endpoints were the percentage of responder patients (defined as a <2 point worsening using the Neuropathy Impairment Score-Lower Limbs/NIS-LL) and mean change from baseline in the Norfolk QOL-DN Questionnaire at 18 months in the intention-to-treat population. Secondary endpoints included among others changes in neurological function and assessment of nutritional status as estimated by the modified body mass index (mBMI).
The study did not show a significant difference between treatment groups, although there was a trend towards a higher percentage of NIS-LL responder patients in the tafamidis group (45.3 versus 29.5 %, p = 0.068) and most secondary endpoints including nutritional status (an established prognostic factor in TTR FAP) favored tafamidis. In the efficacy-evaluable population (87 patients), both coprimary endpoints favored tafamidis in a statistically significant manner [39]. Treatment was generally well tolerated.
The 12-month OLE study showed stable rates of change in NIS-LL and QOL-DN scores under long-term tafamidis treatment [40•]. Placebo-treated patients that switched to tafamidis had slower rates of monthly change in NIS-LL and QOL-DN scores under tafamidis than under placebo, but overall had reduced preservation of neurological function than patients that received tafamidis early on. Post hoc analysis of nutritional status in the pivotal and OLE studies showed that mBMI was maintained or increased at 30 months in tafamidis-treated patients and placebo-treated patients that switched to tafamidis during the OLE study [41]. Overall, these results suggest sustained efficacy of tafamidis in long term treated patients and a greater benefit of treatment if given early in the disease course.
Tafamidis has also shown its ability to stabilize TTR tetramers from TTR FAP patients harboring mutations other than Val30Met [42•]. In this open-label study, the increase from baseline in NIS-LL was quite similar to that measured in the pivotal phase 3 study among tafamidis-treated patients. These preliminary data suggest that the benefit of tafamidis might extend to non-Val30Met TTR FAP patients. However, post-marketing studies on older Val30Met and non-Val30Met FAP patients showed a progression of the neuropathic and disability scores on the long term after a mean follow-up of 24 months [43, 44•, 45 ].
Tafamidis was approved in November 2011 by the European Medicines Agency for the treatment of stage 1 TTR FAP. It is currently available in Europe and Japan for the treatment of TTR FAP, but has not been approved in the USA. The effect of the drug on TTR cardiomyopathy has not been assessed in the initial trials dedicated to FAP. Consequently, an international multicentric placebo-controlled study (ATTRACT) evaluated the drug at 20 or 80 mg qd in FAC and SSA. Results will be known in 2018.
Clearance of established tissue amyloid deposits
These experimental therapeutic approaches target the late stages of amyloid formation (i.e., soluble oligomers and insoluble amyloid fibrils), without affecting upstream disease mechanisms (synthesis of unstable TTR tetramers and their dissociation as monomers). Two forms of treatments aimed at clearing established tissue amyloid deposits have undergone evaluation in phase 1 or 2 clinical trials: doxycycline/tauroursodeoxycholic acid (TUDCA) and serum amyloid P component (SAP) depletion.
Doxycycline/TUDCA
Transgenic Val30Met mouse models have helped establish the synergistic effects of doxycycline (a tetracycline antibiotic) and TUDCA (a biliary acid) on clearance of tissue amyloid deposits: doxycycline seems to act mainly as a TTR fibril disrupter, while TUDCA reduces non-fibrillar TTR tissue deposits [46, 47]. A monocentric, open-label phase 2 study is currently evaluating the impact of doxycycline 100 mg bid + TUDCA 250 mg tid during 12 months on disease progression of TTR amyloidosis patients (NCT01171859, accessible at ClinicalTrials.gov). The primary endpoint among patients with neuropathy is the proportion of responder patients, defined as those displaying a <2 points increase in the NIS-LL and a less than 10 % decrease in mBMI. Secondary endpoints include change from baseline in QOL scores using the SF-36 scale as well as neurological assessments using the Kumamoto neurologic scale [48]. Among 40 enrolled patients, 24 completed the 12-month treatment period [49]. Among them, 13 were evaluable for neurological outcomes: 6/13 could be defined as responders (increase <2 points in the NIS-LL). mBMI and QOL were maintained during the study period. Further data are needed to assess the impact of doxycycline/TUDCA on disease progression.
SAP depletion
Another proposed approach to remove tissue amyloid deposits consists in targeting SAP, a plasma protein that avidly but reversibly binds to tissue amyloid fibrils. A pilot study assessed the efficacy of SAP depletion in amyloid patients using the subcutaneous administration of CPHPC [(R)-1-[6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexa-noyl]pyrrolidine-2 carboxylic acid)] at 1 mg/kg per day in two divided doses [50]. Thirty-one patients were included, among them four patients with TTR FAP. Treatment was well tolerated and produced a >95 % depletion of plasma SAP. Some patients showed a reduction in whole-body amyloid load (as assessed by 123I SAP scintigraphy), in others amyloid load was not increased. In the subgroup of TTR FAP patients, neither change in whole-body amyloid load nor change in heart function occurred. Neurological function was not specifically assessed.
In order to further enhance the removal of amyloid deposits, CPHPC has been administered in combination with anti-SAP IgG antibodies. Proof of concept studies in mice treated with combination therapy showed the safe and effective clearance of tissue amyloid deposits in a complement and macrophage dependent manner [51]. This approach was recently tested in a phase 1, open-label trial that included 16 patients with systemic amyloidosis (mainly of the light-chain type) [52••]. The study regimen included pre-treatment with intravenous CPHPC, followed by a single intravenous injection of a humanized monoclonal IgG1 anti-SAP antibody. Some patients showed reduced liver amyloid load as assessed by 123I SAP scintigraphy. Treatment was generally well tolerated. No patient with TTR FAP was included in that study.
Interestingly, a similar approach is being tested in AL amyloidosis in a phase 2/3 multi-center trial that evaluates NEOD001 (Prothena, Inc), a humanized monoclonal antibody that specifically targets both the circulating soluble amyloid and deposited insoluble amyloid that accumulates in AL amyloidosis. A multi-center phase 1/2 clinical trial has shown acceptable safety, tolerability, pharmacokinetics, and immunogenicity of NEOD001 in patients with AL amyloidosis and persistent organ dysfunction [53••]. Most recently, conformation-specific anti-TTR antibodies have been developed that selectively bind amyloidogenic but not native TTR. The potential of these novel antibodies in preventing deposition and promoting clearance of TTR amyloidosis is being investigated [54].
Other principles of treatment
The main organs affected by TTR FAP are the peripheral nerves, the heart, the eyes, and the kidneys. As such, the follow-up of TTR FAP patients requires a multidisciplinar team. Particular care must be taken to detect heart rhythm and/or conduction disturbances, in order to prevent the occurrence of life-threatening arrhyhtmias and/or high-degree conduction blocks [54]. Diagnostic procedures such as endocavitary ECG recordings and treatment options like pacemaker and cardioverter defibrillator implantations should be considered as clinically indicated. Symptomatic treatment of dysautonomia and pain is of paramount importance [4]. Nutritional status should be evaluated on a regular basis.
As TTR FAP is an autosomal dominant disease, another important aspect of therapy is to provide appropriate genetic counseling to at-risk family members of patients. At-risk adult individuals should be offered the possibility to undergo genetic testing [55•]. Asymptomatic mutation carriers should undergo regular clinical follow-up, with the hope that early treatment interventions can be implemented as soon as the disease gets symptomatic, i.e., before irreversible disability occurs. As it might be challenging to assert the conversion from asymptomatic to early symptomatic status in the individual patient, advanced neurophysiological techniques have been advocated to help detect early stage neuropathy [10, 11, 12•]. As the penetrance of TTR FAP is quite variable depending on geographical areas, the onset and frequency of clinical follow-up should be tailored according to local penetrance rates [56, 57]. Collaborative multicentric studies are ongoing to better define the natural history of TTR FAP and help guide treatment decisions [58•, 59, 60].
Conclusion and perspectives
TTR FAP is a multivisceral, life-threatening disease that has benefited from innovative treatment approaches in the last decades. Currently available therapies are based on the suppression of variant TTR synthesis by means of LT in selected patients, and on the stabilization of variant TTR by means of tafamidis and diflunisal (with variations in access to these treatments according to countries). Although effective, many issues regarding currently available therapies still need to be addressed: progression of disease despite LT, role of TTR stabilizers in presymptomatic mutation carriers and LT patients, and long-term clinical impact of TTR stabilizers on organs other than the peripheral nerves.
Suppression of variant TTR synthesis by means of gene silencing (IONIS TTR-Rx, patisiran) is undergoing advanced evaluation in phase 2/3 clinical trials. It is hoped that these approaches will create new treatment options for TTR FAP patients and address the problems of variant TTR synthesis in other source organs (i.e., the eyes and CNS) and continued wild-type TTR deposition on established amyloid deposits. Generic strategies to clear established tissue amyloid deposits (doxycycline/TUDCA, CPHPC/anti-SAP antibodies) are in an earlier stage of development, but if successful might apply to virtually any type of systemic amyloidosis (genetic or acquired). It is likely that a combination of different approaches will be necessary to cure this aggressive disorder.
TTR FAP can be seen as a model disease for the broader group of conformational diseases, which include common conditions such as Alzheimer’s and Parkinson’s diseases. As such, it is hoped that knowledge gained from the treatment of this rare disease will benefit patients with other forms of protein misfolding diseases.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96.
Plante-Bordeneuve V, Kerschen P. Transthyretin familial amyloid polyneuropathy. Handb Clin Neurol. 2013;115:643–58.
Coutinho P, Martins da Silva A, Lopes Lima J, Resende Barbosa A. Forty years of experience with type I amyloid neuropathy. Review of 483 cases. In: Glenner GG, Pinho e Costa P, Falcao de Freitas A, editors. Amyloid and amyloidosis. Amsterdam: Excerpta Medica; 1980. p. 88–98.
Ando Y et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31.
•Maia LF et al. CNS involvement in V30M transthyretin amyloidosis: clinical, neuropathological and biochemical findings. J Neurol Neurosurg Psychiatry. 2014;86:159–67. This study gives new insights into the development of CNS complications in long-term TTR FAP survivors, including pathological data.
Adams D et al. The course and prognostic factors of familial amyloid polyneuropathy after liver transplantation. Brain. 2000;123(Pt 7):1495–504.
Herlenius G, Wilczek HE, Larsson M, Ericzon BG. Ten years of international experience with liver transplantation for familial amyloidotic polyneuropathy: results from the Familial Amyloidotic Polyneuropathy World Transplant Registry. Transplantation. 2004;77:64–71.
••Ericzon BG et al. Liver transplantation for hereditary transthyretin amyloidosis: after 20 years still the best therapeutic alternative? Transplantation. 2015;99:1847–54.
••Suhr OB, Larsson M, Ericzon BG, Wilczek HE. Survival after transplantation in patients with mutations other than Val30Met: extracts from the FAP World Transplant Registry. Transplantation. 2016;100:373–81. References 8 and 9 provide the latest data from the FAP World Transplant Registry with 20 years of follow-up. An invaluable tool to inform treatment decisions for Val30Met and non-Val30Met patients.
Heldestad V, Nordh E. Quantified sensory abnormalities in early genetically verified transthyretin amyloid polyneuropathy. Muscle Nerve. 2007;35:189–95.
Conceicao IM, Castro JF, Scotto M, de Carvalho M. Neurophysiological markers in familial amyloid polyneuropathy patients: early changes. Clin Neurophysiol. 2008;119:1082–7.
•Lefaucheur JP, Ng Wing Tin S, Kerschen P, Damy T, Plante-Bordeneuve V. Neurophysiological markers of small fibre neuropathy in TTR-FAP mutation carriers. J Neurol. 2013;260:1497–503. An interesting study highlighting the value of electrophysiological tests specifically assessing small nerve fiber function to diagnose very early stage TTR FAP.
Okamoto S et al. Liver transplantation for familial amyloidotic polyneuropathy: impact on Swedish patients’ survival. Liver Transpl. 2009;15:1229–35.
Yamashita T et al. Long-term survival after liver transplantation in patients with familial amyloid polyneuropathy. Neurology. 2012;78:637–43.
Yazaki M et al. Progressive wild-type transthyretin deposition after liver transplantation preferentially occurs onto myocardium in FAP patients. Am J Transplant. 2007;7:235–42.
Liepnieks JJ, Benson MD. Progression of cardiac amyloid deposition in hereditary transthyretin amyloidosis patients after liver transplantation. Amyloid. 2007;14:277–82.
Liepnieks JJ, Zhang LQ, Benson MD. Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology. 2010;75:324–7.
•Oshima T et al. Changes in pathological and biochemical findings of systemic tissue sites in familial amyloid polyneuropathy more than 10 years after liver transplantation. J Neurol Neurosurg Psychiatry. 2014;85:740–6. A study providing important informations regarding the composition of amyloid deposits in long-term survivors after liver transplantation, suggesting potential mechanisms of disease progression after transplantation.
Stangou AJ, Heaton ND, Hawkins PN. Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med. 2005;352:2356.
Llado L et al. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2010;16:1386–92.
Misumi Y, et al. Recipient aging accelerates acquired transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2016;22(5):656–64.
Carvalho A, Rocha A, Lobato L. Liver transplantation in transthyretin amyloidosis: issues and challenges. Liver Transpl. 2015;21:282–92.
Benson MD et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–18.
Ackermann EJ et al. Clinical development of an antisense therapy for the treatment of transthyretin-associated polyneuropathy. Amyloid. 2012;19 Suppl 1:43–4.
Benson M, Kincaid JC, Ackermann EJ, Monia BP. A phase 3 study to evaluate ISIS-TTRRx in patients with transthyretin familial amyloid polyneuropathy (TTR-FAP): study design and baseline demographics. Neurology. 2015;84:Supplement S50.006.
Love KT et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–9.
••Coelho T et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. Phase 1/2 studies demonstrating the safety and efficacy of siRNA therapy to knockdown TTR protein synthesis in vivo in TTR FAP patients.
••Suhr OB et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109. Phase 1/2 studies demonstrating the safety and efficacy of siRNA therapy to knockdown TTR protein synthesis in vivo in TTR FAP patients.
Suhr O, et al. Phase 2 open-label extension study of patisiran, an investigational RNAi therapeutic for the treatment of hereditary ATTR amyloidosis with polyneuropathy. ISA meeting, Uppsala, Sweden July 3–7 2016 (abstract); 2016.
Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–6.
Baures PW, Oza VB, Peterson SA, Kelly JW. Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the non-steroidal anti-inflammatory drug, flufenamic acid. Bioorg Med Chem. 1999;7:1339–47.
Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab Invest. 2004;84:545–52.
Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–49.
Tojo K, Sekijima Y, Kelly JW, Ikeda S. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci Res. 2006;56:441–9.
••Berk JL et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–67. The pivotal phase 3 trial demonstrating the efficacy of diflunisal in slowing TTR FAP disease progression.
Sekijima Y, Tojo K, Morita H, Koyama J, Ikeda S. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. Amyloid. 2015;22:79–83.
Takahashi R et al. Efficacy of diflunisal on autonomic dysfunction of late-onset familial amyloid polyneuropathy (TTR Val30Met) in a Japanese endemic area. J Neurol Sci. 2014;345:231–5.
Bulawa CE et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109:9629–34.
Coelho T et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–92.
•Coelho T et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–14. This open-label extension study confirms the results of the pivotal phase 3 trial and demonstrates sustained benefit of tafamidis in long term treated patients.
Suhr OB et al. Post hoc analysis of nutritional status in patients with transthyretin familial amyloid polyneuropathy: impact of tafamidis. Neurol Ther. 2014;3:101–12.
•Merlini G et al. Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis. J Cardiovasc Transl Res. 2013;6:1011–20. This study provides information on the effect of tafamidis in non-Val30Met TTR FAP patients.
Lozeron P et al. Effect on disability and safety of tafamidis in late onset of Met30 transthyretin familial amyloid polyneuropathy. Eur J Neurol. 2013;20:1539–45.
•Cortese A et al. Monitoring effectiveness and safety of tafamidis in transthyretin amyloidosis in Italy: a longitudinal multicenter study in a non-endemic area. J Neurol. 2016;263:916–24. A study evaluating the impact of tafamidis on the long term in the setting of Val30Met and non-Val30Met TTR FAP.
Plante-Bordeneuve V, et al. Treating transthyretin familial amyloid polyneuropathy: long term experience with tafamidis. 2015 PNS Meeting, Quebec City, Canada, June 28–July 2 2015 (abstract); 2015.
Cardoso I, Martins D, Ribeiro T, Merlini G, Saraiva MJ. Synergy of combined doxycycline/TUDCA treatment in lowering transthyretin deposition and associated biomarkers: studies in FAP mouse models. J Transl Med. 2010;8:74.
Cardoso I, Saraiva MJ. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. Faseb J. 2006;20:234–9.
Obici L et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid. 2012;19 Suppl 1:34–6.
Obici L, et al. A phase II study of doxycycline plus tauroursodeoxycholic acid in transthyretin amyloidosis. XIVth International Symposium on Amyloidosis (ISA), Indianapolis, USA; 27 April–1 May 2014 (abstract OP–68); 2014.
Gillmore JD et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br J Haematol. 2010;148:760–7.
Bodin K et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93–7.
••Richards DB et al. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373:1106–14.
••Gertz MA, et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol. 2016. References 52 and 53, proof-of-concept studies, explore the potential of monoclonal antibodies to remove established tissue amyloid deposits. However, no patient with TTR FAP was included in these studies.
Higaki JN et al. Novel conformation-specific monoclonal antibodies against amyloidogenic forms of transthyretin. Amyloid. 2016;23:86–97.
•Obici L et al. Recommendations for presymptomatic genetic testing and management of individuals at risk for hereditary transthyretin amyloidosis. Curr Opin Neurol. 2016;29 Suppl 1:S27–35. The latest recommendations on genetic counseling for at-risk relatives of TTR FAP patients.
Plante-Bordeneuve V et al. Genetic study of transthyretin amyloid neuropathies: carrier risks among French and Portuguese families. J Med Genet. 2003;40:e120.
Hellman U et al. Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid. 2008;15:181–6.
•Plante-Bordeneuve V et al. The Transthyretin Amyloidosis Outcomes Survey (THAOS) registry: design and methodology. Curr Med Res Opin. 2013;29:77–84. This article presents the THAOS registry, a worldwide effort to delineate the phenotype and natural history of TTR FAP across a wide range of mutations.
Coelho T, Maurer MS, Suhr OB. THAOS—The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29:63–76.
Adams D et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85:675–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Philippe Kerschen declares no conflict of interest.
Violaine Planté-Bordeneuve has received non-financial support from Pfizer and Alnylam and has done consulting for Ionis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neurologic Manifestations of Systemic Disease
Rights and permissions
About this article
Cite this article
Kerschen, P., Planté-Bordeneuve, V. Current and Future Treatment Approaches in Transthyretin Familial Amyloid Polyneuropathy. Curr Treat Options Neurol 18, 53 (2016). https://doi.org/10.1007/s11940-016-0436-z
Published:
DOI: https://doi.org/10.1007/s11940-016-0436-z