Opinion statement
Atherosclerotic disease, a primary cause of stroke and myocardial infarction, is the most common underlying cause of death worldwide. While atherosclerosis was formerly considered to be a relatively inert structural abnormality, decades of research have since shown that it is a biologically active process, driven by active inflammation. In concert with this conceptual shift, newer strategies to image vascular lesions have evolved. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging has been validated as a non-invasive tool to characterize atherosclerotic inflammation. It is hypothesized that a combination of structural and biological (e.g., inflammatory) imaging may provide better means to assess clinical risk, to assess efficacy of therapy, and to identify new, effective treatments. Limitations remain, however, and further advances in technology and tracer development are required before FDG PET imaging will contribute a significant clinical impact at the level of the individual patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advances in non-invasive cardiovascular imaging techniques have improved risk prediction and allow earlier detection of pathological lesions. Previously, direct visualization of the coronary arteries was limited to angiography, which is able only to diagnose obstructive lesions and is too invasive to function as a screening tool. At-risk lesions, however, may be non-obstructive and can be better identified by techniques that characterize wall morphological features, in concert with molecular markers, such as: surrounding inflammation, thrombosis, apoptosis, vessel formation, and hemorrhage [1].
This review focuses on the utility of positron emission tomography (PET)-based atherosclerotic plaque imaging using 18F-fluorodeoxyglucose (FDG) in combination with computed tomography (CT) to jointly characterize the biological and morphological features the arterial wall. The ability of PET/CT imaging to define arterial inflammation and its relationship to risk of cardiovascular disease events and the role of imaging to evaluate the impact of therapy will be discussed. Potential clinical advancements using novel tracers and a brief introduction to other non-invasive cardiac imaging modalities will be outlined.
Plaque development
A significant amount of research spanning decades has greatly enhanced our understanding of how atherosclerotic plaques develop, rupture, and precipitate atherothrombotic events. Briefly, endothelial dysfunction, a result of vascular risk factors, is associated with aggregation of lipids, monocytes, and other inflammatory cells, along the vessel wall and intima. Collections of these cells lead to atheromatous plaques with a fibrous cap which generally do not cause flow-limiting lesions; they are, however, imbued with microscopic calcifications and proteolytic enzymes released by macrophages undergoing apoptosis [2••]. Though this process occurs over many years, acute events may occur at any time. As these enzymes degrade the fibrous cap within the coronary arteries, unstable plaques rupture and can precipitate thrombi that cause ischemia, leading to acute myocardial infarction [3]. In other vessels, such as the carotid arteries, this sequence of events may lead to ischemic stroke; atherosclerosis in the aorta may cause reduced blood flow to other tissue beds supplied by the systemic vasculature. The development of imaging that can differentiate high-risk, non-flow limiting plaques from others has the potential to alter management of atherosclerosis on a large scale.
Features of high-risk plaques
An expansive body of research has yielded a set of atherosclerotic plaque features that associate with atherothrombosis. While overall plaque burden and high-grade obstruction and ischemia are of clear importance, additional morphological and biological features have more recently been found to independently predict myocardial infarction. Thin-cap fibroatheromas, lesions with a fibrous cap <65 μm in thickness, are thought to be the precursor lesion to most plaque rupture events [4]. Other characteristics of these lesions noted on pathology after cardiac events include necrotic core, macrophage infiltration, mild calcification, and neovascularization [5]. Additionally, bleeding into the necrotic core via the vasa vasorum may predispose to plaque rupture. Moreover, macrophage activity in these lesions is known to be a key driver of atherosclerosis. Inflammatory cells produces metalloproteinases that degrade the structural integrity of the plaque, cause thinning of the fibrous cap and predispose to rupture [6••].
Basis for FDG-PET imaging of inflamed tissues
PET imaging, while most commonly associated with cancer screening and diagnosis, offers a sensitive method to detect tracer uptake in the arterial system. Use of this tracer, 18F-flurodeoxyglucose (FDG), offers the unique opportunity to identify metabolically active tissues [7]. FDG is a glucose analogue that accumulates in cells in proportion to their glycolytic rate. Since inflammatory cells, especially activated ones, have particularly high rates of glycolysis, FDG preferentially enters these cells in order to participate in glycolysis [8, 9]. Furthermore, as macrophages do not store glycogen, they rely on glucose obtained from external sources [10]. However, unlike glucose, once phosphorylated by hexokinase, FDG cannot be metabolized further and is effectively trapped within the cells; its level of accumulation reflects the increased glucose requirement of inflammatory cells [11]. Further, the rate of macrophage glycolysis is proportional to its level of pro-inflammatory activation [12]. Moreover, the relationship between FDG uptake and macrophage density has also been confirmed by histology of tissues [10].
FDG accumulation in tissues can be non-invasively measured by taking advantage of the unique properties of PET. PET imaging uses ionizing radiation in the form of a radiolabeled tracer, most often 18F-labeled radioligands that typically have short half-lives. The injected radiotracers accumulate in tissues after which they release positrons that travel a minute distance then interact with electrons, leading to an annihilation event. At this point, two annihilation photons are created at a 180-degree angle. These photons can then be detected by the PET scanner, which recognizes two photons that arrive simultaneously at 180 degrees apart as having the same tissue origin (thus allowing localization and quantitation) [4, 11]. The distribution of the initial tracer within the body can then be reconstructed digitally.
PET imaging displays greater sensitivity than other imaging modalities, permitting the use of smaller quantities of contrast agents [13]. One disadvantage, however, of nuclear imaging is that the resolution is much less than that of CT or magnetic resonance imaging (MRI). Given that each imaging modality has its own disparate advantages, the combined use of PET/CT or PET/MRI offers the highest quality images in terms of sensitivity and resolution. Unlike PET/CT imaging, PET and MRI imaging sequences may be obtained simultaneously, which may reduce motion artifact and decrease variability between images [11].
Arterial inflammation assessed with PET relates to plaque progression and risk of CVD events
Given the importance of inflammation as a driver of atherosclerosis, PET imaging has been developed and validated as a means to characterize the process. Studies have shown an association between greater FDG PET activity and high-risk plaque morphology in the carotid arteries. In one such study, there was a positive correlation between FDG PET activity and the number of high-risk features present [1, 6••]. Notably, FDG uptake is not simply associated with plaque size; instead, it is the macrophage content of plaques that leads to increased FDG signal [6••, 10]. Furthermore, carotid artery FDG uptake is increased in symptomatic lesions, when compared to asymptomatic lesions [6••]. Similarly, FDG uptake in culprit coronary artery plaques is increased shortly after myocardial infarction [14].
FDG PET measures of arterial inflammation also predict the future behavior of the underlying atheroma. Animal studies have shown that rupture-prone plaques can be identified using FDG PET. In one such study of New Zealand rabbits, plaque rupture was triggered by administration of Russell’s viper venom and histamine. In these rabbits, only plaques with high levels of baseline FDG uptake became atherothrombotic [15]. Similarly, human studies have linked high arterial FDG uptake with subsequent plaque changes. Abdelbaky et al. observed that arterial locations with greater atherosclerotic inflammation later manifest more atherosclerotic plaque progression (as calcium deposition) compared to less-inflamed neighboring locations [16]. Further, short-term changes in arterial inflammation appear to predict future changes in atheroma growth. Joseph et al. recently reported that plaques with short term increases (compared to those with decreases) in inflammation subsequently grow relatively rapidly in size (measured as increased plaque thickness, by MRI) [17].
Moreover, imaging measures of plaque inflammation have been shown to independently predict future clinical events. One study, comprised of patients with known malignancy, suggested that increased FDG uptake may be used to predict cardiovascular events [18]. Another retrospective study showed, in individuals without active cancer, that arterial FDG uptake predicts future cardiovascular disease events and does so beyond clinical risk calculators and calcium scoring [19••]. Moreover, small prospective studies are now demonstrating a similar ability to predict future events. A small study of individuals presenting with acute stroke demonstrated that carotid FDG uptake robustly predicts future stroke risk [20]. Larger prospective studies are needed to better delineate the value of FDG PET imaging for prediction of atherothrombotic risk.
Impact of therapy on PET imaging signal
FDG PET imaging of arterial inflammation has been shown to be highly reproducible in clinically stable patients, with low inter- and intraobserver variability [21]. Such reproducibility offers a potential role for the evaluation of therapeutic interventions. Early animal studies using atherosclerotic animal models demonstrated that lipid lowering agents can lead to a reduction in FDG signal on PET imaging [22]. Likewise, the administration of an atherogenic diet led to increased FDG uptake in the aorta, a signal which could again be reduced after the animals are returned to a normal diet [23]. Human studies have shown similar results. In one such study, simvastatin therapy attenuated plaque uptake of FDG; this effect was not seen with placebo treatment [24]. A multicenter, double-blinded trial that compared the effects of high- versus low-dose atorvastatin showed a dose-effect of statins on the artery wall signal and also demonstrated that anti-inflammatory actions could be seen in individuals already taking low dose statins [25•]. Further, non-pharmacologic lipid-lowering interventions (e.g., apheresis) have also been shown to rapidly reduce the arterial inflammatory signal [26]. Such findings of a beneficial impact of statins are consistent with clinical endpoint trial findings [27].
Further research has shown that concordant imaging and outcomes findings are seen with other drug classes. A 2011 study comparing the effects of pioglitazone to glimepiride on atherosclerotic plaque inflammation in patients with diabetes illustrated attenuation of FDG signal in the carotid arteries and ascending aorta [28]; this finding correlated well with the PROactive study, a 2005 randomized controlled trial that revealed a reduction in macrovascular events among diabetic patients treated with pioglitazone [29]. Conversely, imaging studies that fail to demonstrate a significant change in FDG signal may predict a lack of clinical benefit to therapy, as evidenced by multiple trials involving drugs such as dalcetrapib and darapladib [30–33]. Together, these data suggest that changes in the FDG signal may predict clinical effectiveness of anti-atherosclerotic interventions.
Using PET to derive novel physiologic insights
The scientific value of FDG PET imaging goes beyond the characterization of clinical risk and discovery of novel therapies. Multi-modality imaging with PET can be used to derive important physiologic insights. Disorders of inflammation, including rheumatoid arthritis (RA) and psoriasis or infectious disorders including human immunodeficiency virus (HIV) infection and prosthetic valve endocarditis, have been widely studied using FDG PET/CT imaging.
The most studied of these conditions is HIV infection, which is associated with early atherosclerotic disease and higher risk of cardiac events [34, 35]. Notably, PET imaging displays increased arterial inflammation even in young patients with undetectable levels of viremia and low Framingham Risk Scores [36•]. One recent study showed that FDG signal in lymph nodes can be substantially attenuated in treatment-naïve HIV-infected patients after treatment with antiretroviral therapy [37]. This finding was expected. However, the authors also reported that antiretroviral therapy failed to improve arterial inflammation at all. These findings suggest that efforts to reduce viral activity in HIV may not translate into improvement of arterial inflammation (or the substantial CVD risk seen in HIV).
Though some of the findings of this study were unexpected, they speak to how much research is yet to be done, both in cases of HIV infection and in more generalized disorders of systemic inflammation. FDG PET imaging is likely to be used as a major tool to further this area of discovery (Figs. 1 and 2). Currently, two studies are examining the impact of anti-inflammatory treatments to reduce arterial inflammation in HIV; one investigates the use of low-dose methotrexate (NCT02312219) while the other uses the IL1beta antagonist canakinumab (NCT02272946). It is hoped that insights from trials such as those will one day result in an attenuation of ASCVD risk in HIV-infected individuals.
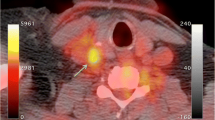
Arterial inflammation (by FDG PET) predicts subsequent CVD risk. FDG uptake was measured in the aortas of 513 individuals without prior history of cardiovascular disease (CVD). a During follow-up (median 4.2 years), 44 participants developed CVD. The aortic inflammatory signal strongly predicted subsequent CVD independent of traditional risk factors (hazard ratio 4.71; 95 % confidence interval [CI] 1.98 to 11.2; p < 0.001) and after further adjustment for coronary calcium score (hazard ratio 4.13; 95 % CI 1.59 to 10.76; p = 0.004). Furthermore, incorporation of the inflammatory signal into a model with Framingham Risk Score (FRS) variables improved delineation of risk. Net reclassification improvements were 27.48 % (95 % CI 16.27 to 39.92) and 22.3 % (95 % CI 11.54 to 35.42) for the 10 and 6 % intermediate-risk cut points, respectively. b Additionally, TBR was inversely associated with the timing of CVD (beta −0.096; p < 0.0001). Adapted with permission from Figueroa et al. JACC Imaging 2013.
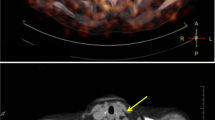
Arterial inflammation is increased in HIV-infected individuals. a Sample images from HIV-infected and non-infected individuals (matched for conventional risk factors) are displayed. b Group mean data shows substantially increased arterial inflammation in HIV-infected individuals compared to age, gender, and FRS-matched controls. c Arterial inflammation in HIV relates to markers of monocyte activation. Adapted with permission from Subramanian et al. JAMA 2012.
Limitations of PET imaging
PET imaging allows clinicians and researchers to visualize arterial inflammation and may presage the response to therapeutic interventions. However, despite its many potential uses, it has several notable limitations. Importantly, lesion detection using PET relies inherently on the spatial resolution the PET scanner [38]. Traditional PET scanners have spatial resolution of approximately 4–6 mm, which makes imaging of small coronary arteries difficult. Furthermore, respiratory motion introduces artifact into any image; displacement due to respiratory motion ranges from less than 10 mm to greater than 20 mm depending on the area that is being imaged. Additional limitations of PET imaging have already been described, including the natural uptake of FDG into myocardium surrounding the coronary arteries [39]. Finally, the cost to the patient associated with PET imaging, both financially and in terms of radiation exposure, must be included when assessing the risks and benefits of using imaging as a screening and diagnostic tool.
Novel tracers and next steps
Attempts to reduce the limitations of PET imaging have led to the development of novel tracers as well as technological refinements. Improvements in PET scanners have led to improvements in spatial resolution and more rapid imaging, both of which can modify the effects of respiratory motion artifact [38, 40]. Additional research has focused on the utilization of novel tracers, such as 18-sodium fluoride (18F-NaF). Studies have shown a substantial increase of 18F-NaF at sites of plaque rupture, both within the coronary arteries and within the larger vasculature [41••]. Furthermore, 18F-NaF can potentially differentiate between active and inactive coronary calcification [2••]. Notably, while traditional FDG PET imaging may produce signal in normal or stressed myocytes, myocardial uptake of 18F-NaF is low [2••, 11]. Concurrently, several novel agents targeting inflammation are being explored; these have the potential to provide better specificity for inflammation, along with less myocardial uptake compared to FDG.
Could PET imaging of inflammation be used as a clinical tool?
Improvements in non-invasive imaging for the evaluation of high-risk atherosclerotic plaque could lead to a shift in how ASCVD is treated. In the current era, with new, more expensive drugs being used to treat chronic atherosclerosis (including the PCSK9 antagonists, which cost tens of thousands of dollars per year), there is a growing interest in methods to refine clinical risk assessment. While FDG PET has the potential to provide such enhanced risk prediction, we await the results of prospective trials to better delineate the value of such multi-modality imaging. Ongoing research in this area may provide a clinically useful method by which risk can be stratified appropriately.
Conclusion
Imaging arterial inflammation using FDG PET CT has matured as a scientific tool. The imaging approach is being used to assess novel anti-atherosclerotic therapies and to yield new physiologic insights. Ongoing and future trials are assessing its role in clinical risk assessment.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imag. 2013;6:1327–41.
•• Dweck MR, Chow MWL, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–48. The first study to measure 18F-NaF uptake in the coronary arteries with PET/CT.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66.
Tarkin JM, Joshi FR, Rudd JHF. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–57.
Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–8.
•• Rudd JHF, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–11. The first study to demonstrate that FDG PET imaging may be use to identify and quantify plaque inflammation.
James OG, Christensen JD, Wong TZ, Borges-Neto S, Koweek LM. Utility of FDG PET/CT in inflammatory cardiovascular disease. Radiographics. 2011;31:1271–86.
Rudd JHF, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography. Ready for prime time? J Am Coll Cardiol. 2010;55:2527–35.
Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–14.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12:294–301.
Joseph P, Tawakol A. Imaging atherosclerosis with positron emission tomography. Eur Heart J. 2016. doi:10.1093/eurheartj/ehw147.
Tawakol A, Singh P, Mojena M, et al. HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–71.
Rudd JHF, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1009–16.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of fdg imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imag. 2010;3:388–97.
Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation. 2008;117:2061–70.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imag. 2013;6:747–54.
Joseph P, Ishai A, Mani V, Kallend D, Rudd J, Fayah ZTA. Short-term changes in arterial inflammation predict long-term changes in atherosclerosis progression. Eur J Nucl Med. 2016.
Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy JP, Maunoury C, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15:209–17.
•• Figueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imag. 2013;6:1250–9. Arterial inflammation as measured by PET/CT predicts and improves CVD risk discrimination beyond traditional risk factors.
Marnane M, Merwick A, Sheehan OC, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–18.
Rudd JHF, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible. Implications for Atherosclerosis Therapy Trials. J Am Coll Cardiol. 2007;50:892–6.
Ogawa M, Magata Y, Kato T, Hatano K, Ishino S, Mukai T, et al. Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med. 2006;47:1845–50.
Worthley SG, Worthley SG, Zhang ZY, et al. In vivo non-invasive serial monitoring of fdg-pet progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging. 2014;25:251–7.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation. Evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31.
• Tawakol A, Fayad Z, Mogg R. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multi-center FDG-PET/CT feasibility study. J Am. 2013; 62. Use of statin medication reduces FDG uptake in a dose-dependent manner, suggesting a reduction in atherosclerotic plaque inflammation in patients on appropriate medical therapy.
Van Wijk DF, Sjouke B, Figueroa A, et al. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol. 2014;64:1418–26.
La Rosa JC, Conti CR. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35.
Mizoguchi M, Tahara N, Tahara A, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes: a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imag. 2011;4:1110–8.
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89.
Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–59.
Tawakol A, Singh P, Rudd JHF, et al. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. J Am Coll Cardiol. 2014;63:86–8.
White HD. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014; 140330050005008.
O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. Jama. 2014;312:1006–15.
Hsue PY, Waters DD. What a cardiologist needs to know about patients with human immunodeficiency virus infection. Circulation. 2005;112:3947–57.
Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12.
• Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–86. HIV infection is associated with significant arterial inflammation, even in patients with undetectable viremia and few traditional risk factors for cardiac disease.
Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016. doi:10.1001/jamacardio.2016.0846.
Polycarpou I, Tsoumpas C, King AP, Marsden PK. Impact of respiratory motion correction and spatial resolution on lesion detection in PET: a simulation study based on real MR dynamic data. Phys Med Biol. 2014;59:697–713.
Nahrendorf M, Frantz S, Swirski FK, et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol. 2015;65:1583–91.
Tarkin JM, Rudd JHF. Techniques for noninvasive molecular imaging of atherosclerotic plaque. Nat Rev Cardiol. 2015;12:79.
•• Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13. 18F-NaF PET allows identification of culprit coronary lesions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shawnbir Gogia and Yannick Kaiser each declare no potential conflicts of interest.
Ahmed Tawakol reports grants from Actelion, Genetech, and Takeda and personal fees from Actelion, Amgen, Astra Zeneca, and Takeda.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Gogia, S., Kaiser, Y. & Tawakol, A. Imaging High-Risk Atherosclerotic Plaques with PET. Curr Treat Options Cardio Med 18, 76 (2016). https://doi.org/10.1007/s11936-016-0495-1
Published:
DOI: https://doi.org/10.1007/s11936-016-0495-1