Abstract
Increasing recognition that apathy is one of the most prevalent behavioral and psychological symptoms of dementia and causes substantial caregiver distress has led to trials evaluating psychosocial and pharmacological treatments of apathy in dementia. We evaluated evidence of the efficacy of pharmacotherapies for apathy in dementia from studies since 2013. Previously reported benefits of acetylcholinesterase inhibitors and memantine were not replicated in recent studies. Antidepressants had mixed results with positive effects for apathy shown only for agomelatine, while stimulants, analgesics, and oxytocin study results were inconclusive. For some approaches, such as antipsychotic review, positive effects were found only in combination with nonpharmacological approaches. Relatively few studies assessed apathy outcomes specifically, complicating interpretation of potentially positive treatment effects; none dissected outcomes for emotional, motivational and behavioral components of apathy. Better trial design and more detailed analysis are needed in order to evaluate outcomes of pharmacological treatments for apathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We aim to review recent developments in pharmacotherapy for apathy in dementia. Apathy is the most common behavioral and psychological symptom of dementia (BPSD) [1, 2]. It is highly prevalent across different forms and stages of dementia, including in mild cognitive impairment (MCI), Alzheimer’s disease (AD), frontotemporal dementia (FTD), and vascular dementia, as well as in other neurodegenerative and psychiatric disorders such as Parkinson’s disease (PD), schizophrenia, stroke, multiple sclerosis, traumatic brain injury, and major depression [2, 3].
Various definitions have been used to identify and investigate apathy, and the term has been broadly applied to a wide range of states of being; in addition, it has been described as a symptom and increasingly as a syndrome [4]. An international task force recently proposed diagnostic criteria for apathy in neuropsychiatric disorders, the core feature of which is diminished motivation in at least two of three domains: (i) self-initiated or environment-stimulated goal-directed behavior, (ii) cognitive goal-directed behavior, including a loss of ideas and curiosity, and (iii) emotional goal-directed behavior, including loss of spontaneous emotion or emotional responsiveness [5, 6]. These symptoms must persist for at least 4 weeks and be accompanied by significant functional impairment.
Prevalence estimates differ across patient populations, settings, and definitions of apathy [2]. Apathy occurs in a significant percentage (1.4–15.8 %) of cognitively normal individuals. It is more common in neurological conditions such as stroke and dementia, in which severity and progression are strongly correlated with increased levels of apathy (reviewed in [7]). Apathy rates for cognitively normal individuals are generally higher when self-reported, while for people with MCI, informant-reported apathy rates are higher [8]. Apathy is linked to decreased function and higher caregiver burden and stress [9–11].
The neuropathology of apathy in dementia has been studied most extensively in AD where higher levels of apathy are linked to increased tangle burden [12], neuronal loss [13], and increased total and phospho-tau levels in the cerebrospinal fluid (CSF); associations to plaque burden or CSF Aβ levels have been inconsistent [14]. There is currently no mechanistic understanding of how these pathological observations are linked to apathy and whether the effect is independent of general atrophy and neurodegeneration [15].
The variety of apathetic symptoms in dementia suggests differential involvement of neuroanatomical structures involved in the three different apathy domains [16–18]. Lack of self-initiated goal-oriented behavior may be due to bilateral lesions in the prefrontal-basal ganglia circuits, affecting both associative and limbic territories, or in more severe dementia, the corpus callosum and internal capsule. Loss of cognitive goal-directed behavior involves the dorsal prefrontal cortex and the basal ganglia (e.g., dorsal caudate), while affective dysfunction in apathy is thought to arise through limbic dysfunction in the orbital-mesial frontal cortex and the basal ganglia (e.g., ventral striatum) [17, 19–21].
Cholinergic, dopaminergic, serotonergic, and GABAergic neurotransmitters have all been linked to apathy in AD via pharmacological, post-mortem, or imaging studies [22]. Cholinergic deficiency may affect limbic systems, while dopamine deficiency affects the reward system [23, 24]. Selective serotonergic reuptake inhibitors (SSRIs) affect both dopaminergic and serotonergic neurotransmisison and are commonly used to treat depression. The link between SSRIs and increased apathy [25] led to the hypothesis that balancing serotonin and dopamine levels in individuals with dementia may improve apathy. Dementia is also linked to a loss of GABAergic and noradrenergic neurons, and elevated plasma GABA levels have been associated with apathy in AD [26]. These neurochemical deficits underline the potential of pharmacological intervention for apathy.
The evaluation of efficacy of pharmacotherapies relies on clinical assessment measures, such as the Apathy Evaluation Scale (AES) and Apathy Scale (AS). The AES comprises 18 items assessing the three different domains of apathy (affective, behavioral, and cognitive) separately [27]. The AS is one of several adaptations of the AES, comprising 14 items [28, 29]. A more commonly used measure is the Neuropsychiatric Inventory (NPI; [30]), which assesses up to 11 other symptoms besides apathy. The apathy subscale consists of a screening question, and—if answered positively—the severity and frequency of symptoms are rated and combined in a single score [31]. The NPI, AES, and AS have all been validated in people living with dementia and in institutionalized patients [29].
A 2012 review of pharmacological treatments for apathy in dementia found limited evidence that acetylcholinesterase inhibitors, and possibly memantine, could be effective to treat apathy, and indications that stimulants, calcium antagonists, and antipsychotics might be useful [32]. Since 2012, publications concerning dementia and apathy have outstripped the growth in publications on dementia generally by 20 %. It is timely to provide an update and examine recent progress in pharmacological treatment of apathy in dementia.
Methods
Literature Search
We explored scientific databases for manuscripts published from January 1, 2013 to July 1, 2016. We used the search terms apathy, abulia, amotivation, or passivity in combination with any of the following: treatment, management, pharmacological, drug, cholinesterase inhibitor, donepezil, galantamine, rivastigmine, memantine, antipsychotic, amisulpride, risperidone, antidepressant, bromocriptine, d-amphetamine, methylphenidate, amantadine hydrochloride, gabapentin, modafinil, atomoxetine, cabergoline, pramipexole, ropinirole, apomorphine, rotigotine, or anticonvulsant. Searches in the Cochrane Database of Systematic Review, Medline, and Embase yielded 144, 673, and 1403 results, respectively. Abstracts were screened and full texts were accessed where relevant and internal references were screened for additional studies.
Inclusion and Exclusion Criteria
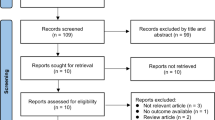
We included peer-reviewed manuscripts published in English that reported studies conducted in patients with dementia with pre- and post-treatment measures of apathy; cross-sectional studies were excluded. There were no restrictions in setting or dementia type. After exclusion, 24 studies were included for review (Table 1). Post hoc and case studies are indicated. Studies including five or fewer participants were considered as case reports and included in Tables 1 and 2 but not discussed individually.
Rating
The methodological quality of the studies included in this review was evaluated using the Cochrane Collaboration’s tool for bias risk assessment (Table 2) [33]. This approach was chosen over numerical rating systems to allow for a more informed review of the strengths and weaknesses of the included studies. Inter-rater reliability was established by two assessors rating all included studies on the Cochrane tool (kappa = .88).
Results
Anti-Dementia Medications
Acetylcholinesterase Inhibitors
The acetylcholinesterase inhibitors (AChEIs) rivastigmine, galantamine, and donepezil inhibit the breakdown of acetylcholine by acetylcholinesterase, thereby increasing acetylcholine neurotransmission. Although they are primarily used to treat the cognitive symptoms of AD, they also have potentially beneficial effects on BPSD, and all have been investigated for the treatment of apathy.
After promising effects of rivastigmine for BPSD in AD ([34], see also below), Oh et al. evaluated its efficacy in Parkinson’s disease dementia (PDD) in a small longitudinal, single-center, open-label trial [35•]. Twenty-three PDD patients were treated with rivastigmine for 24 weeks and followed up after 6 months. Apathy, as measured by the NPI, was the third most common neuropsychiatric symptom at baseline (15 days prior to starting rivastigmine), present in 56.5 % of the sample group, and had the third highest score for caregiver distress [35•]. There was no difference in apathy after rivastigmine treatment, but caregiver distress caused by apathy significantly declined.
The effects of galantamine treatment were evaluated in two studies: an open-randomized trial comparing galantamine and risperidone [36] and a cohort study evaluating the combination of galantamine and ambulatory rehabilitation [37•]. The randomized trial evaluated a wide array of neuropsychiatric symptoms based on the NPI in 91 patients with probable dementia or MCI. Apathy scores were lower after 12 weeks of treatment with either galantamine or risperidone (an antipsychotic medication), but only when combined with an occupational therapy intervention [36]. No information is provided on the statistical significance of this reduction; there were no significant differences between treatments and there was no control group [36]. The cohort study compared the efficacy of galantamine treatment alone against galantamine combined with physical, occupational, and speech therapy over a 6-month period. Apathy, as measured by the AS, was significantly lower at both 3 and 6 months in the combined therapy group compared to the galantamine-only group; however, it is unclear whether these findings were corrected for differences at baseline [37•].
Rea et al. evaluated apathy in 113 AD patients upon treatment with donepezil alone, or with a combination of donepezil and choline alphoscerate, a cholinergic precursor [38•]. The rationale was that the combination could sustain a more effective targeting of the cholinergic dysfunction associated with apathy. The results confirmed this hypothesis as after 12 months to the end of the trial at 24 months, the combination group had on average from 2 to 4 points lower apathy scores on the NPI than the group treated with donepezil alone. Caregiver distress was also lower in the combination group from 6 to 24 months. Long-term differences between both groups were mainly due to increased apathy in the donepezil-only group and can only be partly attributed to an improvement in the combination group, as their score reduction compared to baseline was not significant beyond 12 months [38•]. Sensitivity analysis showed that this effect was specific to participants with normal frontal function.
Cholinergic neurotransmission is affected to an even larger degree in persons with dementia with Lewy bodies (DLB) [39]. Earlier RCTs indicated beneficial effects of donepezil treatment on cognition and behavior in DLB [40, 41], leading to a phase 3 study with 100 participants [42]. Donepezil induced sustained cognitive improvement for up to 52 weeks. However, BPSD improved in all groups, including the placebo group, and the specific effects on apathy are unclear, as it is reported only in terms of incidence of adverse events.
A small open-label trial with 23 persons with FTD investigated the effect of discontinuation of treatment with donepezil. While there was a significant decrease on the total NPI score and improvements in delusions, agitation, irritability, and aberrant behavior, there was no significant change in apathy [43]. Together with earlier findings [44], these studies point to adverse effects of donepezil on BSPD in FTD. However, they remain to be validated in a blinded withdrawal RCT, and whether or not apathy is worse with donepezil treatment in FTD remains unclear as well.
Other studies have looked at combinations or switches between different AChEIs. Spalletta et al. evaluated switching between a rivastigmine transdermal patch and a non-rivastigmine oral AChEI in 422 persons with mild-to-moderate AD. A switch to, but not from, the transdermal rivastigmine patch stabilized cognitive symptoms based on the MMSE. However, using the NPI, no significant changes in apathy symptoms were observed [45]. Matsuzono et al. obtained similar results when comparing the effects of rivastigmine and donepezil in a sample of 146 persons with AD. Rivastigmine improved cognition at 3 and 6 months, but there were no differences in terms of apathy on the Apathy Scale [46].
A randomized three-arm trial comparing rivastigmine, galantamine, and donepezil, but without a placebo control group in 55 persons with AD, found no differences in apathy scores (as scored on the NPI) between baseline and 12 months for any of the treatment groups [47].
Memantine
Memantine affects glutamatergic transmission by blocking NMDA receptors, moderately reduces clinical deterioration, and is approved for moderate-to-severe AD. Cumbo et al. compared the effect of memantine on BPSD to that of each of the AChEIs in a four-arm, randomized, open-label trial. Treatment effects were compared across a total of 146 persons with AD over a 12-month period. All treatments except galantamine showed a significant improvement over time on the total NPI score; however, in terms of apathy, there were no significant improvements in any of the treatment groups [34].
The Okayama Memantine Study compared the combination of memantine with either donepezil or galantamine in 123 persons with AD. Apathy was assessed using the AS and found to remain stable in the group treated with donepezil and memantine, while apathy significantly worsened in the galantamine plus memantine group. In an older subgroup of participants aged 75+, the donepezil and memantine combination improved apathy symptoms [48].
Post hoc analyses of a double-blind RCT comparing the efficacy of memantine and donepezil in 167 persons with probable AD, which included the NPI as a secondary outcome measure, found no difference in apathy between the treatment groups over the 24-week trial [49].
Psychostimulants
Apathy is linked to a decrease in dopaminergic transmission in AD as well as in PD [50]. Dopaminergic circuits regulate motivation and reward; thus, pharmacological approaches that stimulate dopamine signaling could be effective to treat apathy. Methylphenidate is a psychostimulant, primarily used to treat attention deficit hyperactivity disorder (ADHD). An RCT trial sought to confirm promising results from an earlier cross-over trial with methylphenidate for the treatment of apathy in dementia [51, 52•]. Sixty participants with possible or probable AD and clinically significant apathy were recruited for a multi-center, double-blind trial, and randomly assigned to treatment with either 20 mg methylphenidate or placebo. After 6 weeks, a statistically significant difference between the groups was found on one primary outcome, the Clinical Global Impression of Change, but not the other, the AES score. However, the secondary outcome of the NPI apathy score was improved. While the outcome of this trial indicated positive global effects of methylphenidate in dementia, the results for apathy were inconclusive.
Caffeine modulates dopamine and acetylcholine neurotransmission, and its effects on behavioral symptoms have been extensively studied in healthy adults [53, 54]. An observational pilot study sought to determine whether caffeine was associated with behavioral symptoms as per the NPI, including apathy, in dementia. Caffeine consumption of 29 persons with dementia in an aged care facility was monitored over 96 h. Apathy was negatively correlated with concurrent caffeine consumption [55]: this correlation might be a cause-and-effect relationship as apathetic patients may have lacked motivation for consumption, which was not stimulated or regulated in any way. Further research is needed to establish whether caffeine is beneficial for apathy.
Antidepressants
Symptoms of apathy and depression overlap partially: dysphoria and low social engagement occur in both, while pessimism, feelings of guilt, low self-esteem, and suicidality are specific to depression [56]. This partial symptom overlap may indicate that depression treatment could also be beneficial for apathy, although conversely certain antidepressants have been shown to induce apathy [25, 57]. To investigate this relationship in the context of dementia, Leontjevas et al. compared the effects of activating strategies, psychotherapy, and an antidepressant medication stepped-care approach ((1) citalopram, (2) nortriptyline, and (3) psychiatrist consult) on apathy and depression in both dementia special care and somatic units (for people with physical disabilities) of residential care facilities [58]. Only participants with depressive symptoms or diagnosed depression after screening were treated with medication stepped-care. An overall reduction in apathy was found in dementia units (mainly attributed to activating strategies), but not in somatic units. Post hoc analysis showed that medication stepped-care treatment was associated with apathy worsening in both unit types.
Callegari et al. evaluated the use of agomelatine for apathy in 24 participants with bvFTD with no history of depression [59••]. Agomelatine is a structural analogue of melatonin and has antagonistic effects on melatonergic receptors and the serotonergic 5-HT2C receptor [60]. It has been approved for use in major depressive disorder and is used to treat disrupted circadian rhythms due to its effect on sleep regulation [61]. The authors hypothesized that agomelatine could reduce apathy by restoring prefrontal dopaminergic and noradrenergic tone via 5-HT2C antagonism. To control for potential effects via the melatonergic system, melatonin was used as the control treatment for 10 weeks, followed by a 10-week-crossover period. Agomelatine, but not melatonin, treatment was associated with significant decreases in AES—even when controlling for NPI-depression score—and also in caregiver-rated NPI-apathy and distress scores. Switching from melatonin to agomelatine was associated with a decrease in AES score; the reverse was associated with an increase in AES.
Antipsychotics
The effect of antipsychotic medication on apathy was examined in a cluster RCT of 191 persons with dementia in 16 aged care facilities which evaluated four interventions: (1) person-centered care, (2) antipsychotic review (based on NICE guidelines), (3) exercise, and (4) social interaction with pleasant activities. All facilities received person-centered care; the other three interventions were randomly assigned to eight facilities so that each combination of interventions was assigned to two facilities exclusively. Antipsychotic review alone, which resulted in a 50 % reduction in these medications, was associated with increased levels of NPI-apathy. However, when combined with either social interaction or exercise, antipsychotic review significantly reduced apathy, adjusting for numerous confounds including baseline depression [62].
Other Pharmacological Agents
A number of less conventional treatment options have also been explored for apathy. Kimura et al. reported results of an 8-week open-label trial of lavender therapy in patients with FTD [63]. Total NPI score of the 20 patients improved over a 4-week treatment with lavender aroma but returned to baseline levels after an additional 4 weeks without lavender aroma therapy. The same was true for NPI-apathy, but not for any of the other NPI domains. Since this small trial was open-label and did not include a control group or measures of cognition, function, quality of life, or caregiver stress, more rigorous studies are needed to evaluate the possible effect of lavender on apathy.
Yokukansan, more commonly referred to as Kampo, is a traditional Japanese medicine used to treat neurosis and insomnia, with benefits for BPSD [64]. Teranashi et al. compared its efficacy in 76 persons with AD, vascular dementia, or DLB who were randomized to fluvoxamine (an SSRI antidepressant), risperidone, or yokukansan [65]. Total NPI scores significantly decreased after 8 weeks of treatment in each intervention group; however, apathy did not significantly change [65].
Pain has been associated with mood symptoms such as depression in dementia [66], prompting the hypothesis that pain treatment could also affect BPSD. In an open-label one-arm study, Husebo et al. evaluated the effect of analgesia (with paracetamol, extended release morphine, buprenorphine transdermal patch, or pregabaline) for 8 weeks on a mood cluster of NPI domains [67]. Apathy was significantly improved in the pain intervention group; however, correction for differences in apathy levels between groups at baseline was not reported.
Oxytocin is a neuropeptide that has been successfully used to improve emotional expression processing, empathy, cooperative behavior, and motivation in healthy adults and persons with autism and schizophrenia [68–72], with beneficial effects on social behavior and BPSD in persons with FTD [73]. Finger et al. evaluated the safety and tolerability of three doses of intranasal oxytocin administered twice daily for 1 week in a phase 1 trial of 23 persons with bvFTD [74•]. There were preliminary indications that oxytocin could improve apathy outcomes on the NPI and FBI, which were included as secondary outcome measures, but they remain to be confirmed in a larger sample with longer treatment.
A small (11 participants with AD) open-label trial evaluated the effect of tetrahydrocannabinol (THC; medical cannabis oil) on BPSD as an add-on treatment for 4 weeks. Apathy symptoms were improved at midpoint evaluation; but over the 4-week period, the improvements were not statistically significant. Both the overall NPI score and caregiver distress were significantly lower at the end of the trial period [75].
Nonpharmacological Treatment
A review of nonpharmacological treatment of apathy in dementia [76] concluded that apathy was improved by multisensory stimulation, occupational therapy, social interaction or pet therapy, creative activities, exercise, staff education, or a combination of these. Many of these studies were small, qualitative and/or tailored to specific patients, making it difficult to form general conclusions. In addition, it is possible that a single intervention across a broad range of individuals with dementia and apathy may not be sufficient to demonstrate a clinically significant effect due to the importance of individualizing treatment strategies [15, 77]. Further, the long-term effect or sustainability of nonpharmacological interventions remains unclear [76] and combination of pharmacological and nonpharmacological interventions little studied.
Implications and Future Directions
Despite the recent increase in research interest into apathy in dementia, with a 20 % increase in apathy-specific dementia studies over the last few years, the number of randomized controlled trials specifically evaluating pharmacological treatment is small and consequently the evidence limited (Table 3). Earlier studies showed some benefit in apathy symptoms using AChEIs or memantine [32], though these effects have not been confirmed in more recent studies. This review indicates that evidence for other approaches, including melatonergic antidepressants, stimulants, analgesics, oxytocin, or reduction of antipsychotic medications, may be equally promising or at the least, have potential treatment effects that remain to be confirmed. SSRI antidepressants were confirmed as not beneficial or even deleterious. For antipsychotic reduction, positive effects for apathy were found only in combination with nonpharmacological approaches.
Apart from the shortcomings of study bias as outlined in Table 2, other limitations should be heeded in reviewing these findings. First, apathy was rarely the primary outcome in the studies. Second, measurement of apathy was limited and there were inconsistencies in the definition of apathy. Commonly, the single-item NPI-apathy domain score was used to assess change in apathy which does not allow for in-depth assessment of the domains of apathy. Only one in four included studies used an apathy-specific scale such as the AES or AS. Novel technology-based approaches, such as video games, eye trackers, or activity-monitoring devices, are currently under development and show potential as objective research tools for the measurement of apathy [15]. Third, the real-life impact and effect size of treatment were often unclear. Fourth, confounding factors were not always controlled for. A clear example is depression, which is also common in people living with dementia and has overlapping features with apathy. Although differential diagnosis can be challenging, it is critical in terms of treatment and management, as commonly used SSRI antidepressants do not improve apathy, but in fact may even worsen it [32, 58]. Other potential confounds are concurrent medications and medical comorbidities that also affect motivation. Antipsychotics, which are used in about a quarter to a third of persons with dementia in residential care, are associated with side effects of apathy, sedation, and fatigue [78]; many studies reviewed did not report whether their concurrent use was considered. Similarly, polypharmacy is common among persons with dementia and those living in residential aged care and has been associated with apathy [79, 80]. There is strong evidence for apathy following stroke [81], and it is also associated with common comorbidities such as hypertension and diabetes mellitus [82, 83].
Fifth and importantly, the clinical definition of apathy is parsed into three different components: self-initiated, cognitive, and emotional goal-directed behavior, and neuroanatomical insights suggest that different mechanisms may underlie each specific clinical aspect. It may, therefore, prove useful to analyze pharmacological effects on these aspects separately. Studying efficacy at this level would require a much more rigorous approach than those used in nearly all trials to date. Sixth, the setting could play an important role in the interpretation of trial findings. It may not be possible to generalize findings from studies in residential aged care facilities, where most studies to date have been performed, to hospital or community settings. Finally, the type and severity of dementia may influence responsiveness to pharmacological treatment. Similar research efforts are being undertaken for the treatment of apathy in other contexts, such as after stroke, in schizophrenia, or in dementia-free PD patients. Our mechanistic understanding of the role of apathy in the respective disease processes is currently too limited to make meaningful comparisons, but in the future it will be instructive to look at overlaps in treatment strategies and disease-specific drug effects. As insight into neuropathology, neurochemistry, and genetics of apathy improves, better patient stratification may improve treatment development as well.
These limitations in trial design and apathy measurement affect current study outcomes, with the majority yielding inconclusive results, for example due to effects that are inconsistent across time or across scales, even within the same study. To maximize what we can learn from such trials, we endorse recently published recommendations for a more rigorous approach in the evaluation of apathy treatments in dementia [84••]. These include better documentation of the study population, in terms of (i) neurological disease and/or dementia type, (ii) baseline apathy diagnosis and apathy severity, and (iii) the presence of confounding factors, including concurrent medications and other behavioral or mood-related symptoms. With regard to study outcomes, more attention should be placed on clinically as well as statistically significant effect sizes. Apathy-specific measurement scales, caregiver input, and sufficient study duration to determine sustainability of effects are required to detect meaningful improvements.
Conclusions
We conclude that convincing pharmacological strategies for managing apathy are yet to be developed. Previously reported benefits of AChEIs and memantine were not replicated in recent studies. Antidepressants had mixed results with positive effects found only for agomelatine, while psychostimulants, analgesics, and oxytocin have potential treatment effects that require more substantive evidence. For some pharmacotherapies, including antipsychotic review, improvement of apathy was found only in combination with nonpharmacological approaches. Ultimately, the synergistic effect between pharmacological and nonpharmacological approaches or a stepped approach starting with psychosocial interventions may prove to be effective in the treatment of apathy, as suggested by a few studies discussed here [37•, 62]. The clinical use of pharmacotherapies requires a more substantive evidence base.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance, •• Of major importance
Selbæk G, Engedal K, Bergh S. The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: a systematic review. J Am Med Dir Assoc. 2013;14:161–9.
Ishii S, Weintraub N, Mervis JR. Apathy: A Common Psychiatric Syndrome in the Elderly. J Am Med Dir Assoc. 2009;10:381–93.
Borsje P, Wetzels RB, Lucassen PL, Pot AM, Koopmans RT. The course of neuropsychiatric symptoms in community-dwelling patients with dementia: a systematic review. Int Psychogeriatr. 2015;27:385–405.
Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3:243–54.
Robert P, Onyike CU, Leentjens AFG, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24:98–104.
Mulin E, Leone E, Dujardin K, Delliaux M, Leentjens A, Nobili F, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26:158–65.
Lanctôt KL, Agüera-Ortiz L, Brodaty H, Francis PT, Geda YE, Ismail Z, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement. 2016
Guercio BJ, Donovan NJ, Munro CE, Aghjayan SL, Wigman SE, Locascio JJ, et al. The Apathy Evaluation Scale: A Comparison of Subject, Informant, and Clinician Report in Cognitively Normal Elderly and Mild Cognitive Impairment. J Alzheimers Dis. 2015;47:421–32.
Huang SS, Lee MC, Liao YC, Wang WF, Lai TJ. Caregiver burden associated with behavioral and psychological symptoms of dementia (BPSD) in Taiwanese elderly. Arch Gerontol Geriatr Elsevier Ireland Ltd. 2012;55:55–9.
Lee DR, McKeith I, Mosimann U, Ghosh-Nodyal A, Thomas AJ. Examining carer stress in dementia: The role of subtype diagnosis and neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2013;28:135–41.
Rosdinom R, Zarina MZN, Zanariah MS, Marhani M, Suzaily W. Behavioural and psychological symptoms of dementia, cognitive impairment and caregiver burden in patients with dementia. Prev Med (Baltim) Elsevier Inc. 2013;57:S67–9.
Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters HV, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49:355–61.
Förstl H, Burns A, Levy R, Cairns N, Luthert P, Lantos P. Neuropathological correlates of behavioural disturbance in confirmed Alzheimer’s disease. Br J Psychiatry. 1993;163:364–8.
Skogseth R, Mulugeta E, Jones E, Ballard C, Rongve A, Nore S, et al. Neuropsychiatric correlates of cerebrospinal fluid biomarkers in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25:559–63.
Lanctôt KL, Agüera-Ortiz L, Brodaty H, Francis PT, Geda YE, Ismail Z, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement. 2016
Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s Disease. J Am Geriatr Soc Blackwell Sci Inc. 2001;49:1700–7.
Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–28.
Cipriani G, Lucetti C, Danti S, Nuti A. Apathy and dementia. Nosology, assessment and management. J Nerv Ment Dis. 2014;202:718–24.
Levy R. Apathy: A pathology of goal-directed behaviour. A new concept of the clinic and pathophysiology of apathy. Rev Neurol (Paris). 2012;168:585–97.
Stanton B, Leigh P, Howard R. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J Neurol. 2013;260:2481–90.
Agüera-Ortiz L, Hernandez-Tamames JA, Martinez-Martin P, Cruz-Orduña I, Pajares G, López-Alvarez J, et al. Structural correlates of apathy in Alzheimer’s disease: a multimodal MRI study. Int J Geriatr Psychiatry. 2016
Ruthirakuhan M, Herrmann N, Abraham E, Lanctôt K. Pharmacological interventions for apathy in Alzheimer’s disease (Protocol). Cochrane Databse Syst Rev. 2016
Kaufer D. Beyond the cholinergic hypothesis: the effect of metrifonate and other cholinesterase inhibitors on neuropsychiatric symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9 Suppl 2:8–14.
Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17:411–27.
Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10:196–9.
Lanctôt KL, Herrmann N, Rothenburg L, Eryavec G. Behavioral correlates of GABAergic disruption in Alzheimer’s disease. Int Psychogeriatr. 2007;19:151–8.
Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38:143–62.
Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–9.
Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res NIH Public Access. 2011;70:73–97.
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14.
Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–6.
Berman K, Brodaty H, Withall A, Seeher K. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry. 2012;20:104–22.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Br Med J Publishing Group. 2011;343:d5928.
Cumbo E, Ligori LD. Differential effects of current specific treatments on behavioral and psychological symptoms in patients with alzheimer’s disease: A 12-month, randomized, open-label trial. J Alzheimer’s Dis. 2014;39:477–85.
Oh Y-S, Kim J-S, Lee PH. Effect of Rivastigmine on Behavioral and Psychiatric Symptoms of Parkinson’s Disease Dementia. J Mov Disord. 2015;8:98–102. This study found that rivastigmine improved caregiver distress due to apathy, although actual apathy scores on the NPI did not significantly improve.
Freund-Levi Y, Jedenius E, Tysen-Bäckström AC, Lärksäter M, Wahlund L-O, Eriksdotter M. Galantamine Versus Risperidone Treatment of Neuropsychiatric Symptoms in Patients with Probable Dementia: An Open Randomized Trial. Am J Geriatr Psychiatry. 2014;22:341–8.
Tokuchi R, Hishikawa N, Matsuzono K, Takao Y, Wakutani Y, Sato K, et al. Cognitive and affective benefits of combination therapy with galantamine plus cognitive rehabilitation for Alzheimer’s disease. Geriatr Gerontol Int. 2015. Study demonstrating improved apathy outcomes when galantamine was combined with rehabilitation in individuals with AD.
Rea R, Carotenuto A, Traini E, Fasanaro AM, Manzo V, Amenta F. Apathy Treatment in Alzheimer’s Disease: Interim Results of the ASCOMALVA Trial. J Alzheimer’s Dis. 2015;48:377–83. This study highlights the potential of combining AChEIs with cholinergic precursors. Apathy and related caregiver distress were significantly lower for up to 2 years in participants receiving combination donepezil/precursor rather than donepezil alone.
Perry EK, Haroutunian V, Davis KL, Levy R, Lantos P, Eagger S, et al. Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer’s disease. Neuroreport. 1994;5:747–9.
Mori E, Ikeda M, Kosaka K, Donepezil-DLB Study Investigators. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72:41–52.
Ikeda M, Mori E, Kosaka K, Iseki E, Hashimoto M, Matsukawa N, et al. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord. 2013;36:229–41.
Mori E, Ikeda M, Nagai R, Matsuo K, Nakagawa M, Kosaka K. Long-term donepezil use for dementia with Lewy bodies: results from an open-label extension of Phase III trial. Alzheimers Res Ther. 2015;7:5.
Kimura T, Takamatsu J. Pilot study of pharmacological treatment for frontotemporal dementia: Risk of donepezil treatment for behavioral and psychological symptoms. Geriatr Gerontol Int. 2013;13:506–7.
Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary Findings: Behavioral Worsening on Donepezil in Patients With Frontotemporal Dementia. Am J Geriatr Psychiatry. 2007;15:84–7.
Spalletta G, Caltagirone C, Padovani A, Sorbi S, Attar M, Colombo D, et al. Cognitive and affective changes in mild to moderate Alzheimer’s disease patients undergoing switch of cholinesterase inhibitors: a 6-month observational study. PLoS One. 2014;9:e89216.
Matsuzono K, Sato K, Kono S, Hishikawa N, Ohta Y, Yamashita T, et al. Clinical Benefits of Rivastigmine in the Real World Dementia Clinics of the Okayama Rivastigmine Study (ORS). J Alzheimer’s Dis. 2015;48:757–63.
Shimizu S, Kanetaka H, Hirose D, Sakurai H, Hanyu H. Differential effects of acetylcholinesterase inhibitors on clinical responses and cerebral blood flow changes in patients with Alzheimer’s disease: A 12-month, randomized, and open-label trial. Dement Geriatr Cogn Dis Extra. 2015;5:135–46.
Matsuzono K, Hishikawa N, Ohta Y, Yamashita T, Deguchi K, Nakano Y, et al. Combination Therapy of Cholinesterase Inhibitor (Donepezil or Galantamine) plus Memantine in the Okayama Memantine Study. J Alzheimers Dis. 2015;45:771–80.
Zhang N, Wei C, Du H, Shi FD, Cheng Y. The Effect of Memantine on Cognitive Function and Behavioral and Psychological Symptoms in Mild-to-Moderate Alzheimer’s Disease Patients. Dement Geriatr Cogn Disord. 2015;40:85–93.
David R, Koulibaly M, Benoit M, Garcia R, Caci H, Darcourt J, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases: A SPECT study with partial volume effect correction. Clin Neurol Neurosurg. 2008;110:19–24.
Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28:296–301.
Rosenberg PB, Lanctôt KL, Drye LT, Herrmann N, Scherer RW, Bachman DL, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74:810–6. Trial designed specifically for apathy, with promising effects of methylphenidate on one of two apathy outcomes in AD, however not in the one considered primary.
Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–55.
Ferré S. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl). 2016;233:1963–79.
Kromhout MA, Jongerling J, Achterberg WP. Relation between caffeine and behavioral symptoms in elderly patients with dementia: an observational study. J Nutr Health Aging. 2014;18:407–10.
Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: Same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2009;49:246–9.
Settle EJ. Antidepressant drugs: disturbing and potentially dangerous adverse effects. J Clin Psychiatry. 1998;59:25–30.
Leontjevas R, Teerenstra S, Smalbrugge M, Vernooij-Dassen MJFJ, Bohlmeijer ET, Gerritsen DL, et al. More insight into the concept of apathy: a multidisciplinary depression management program has different effects on depressive symptoms and apathy in nursing homes. Int Psychogeriatr. 2013;25:1941–52.
Callegari I, Mattei C, Benassi F, Krueger F, Grafman J, Yaldizli Ö, et al. Agomelatine Improves Apathy in Frontotemporal Dementia. Neurodegener Dis. 2016;16:352–6. The only recent pharmacological trial showing significant improvement of apathy, using agomelatine in individuals with bvFTD or semantic dementia.
Racagni G, Riva MA, Molteni R, Musazzi L, Calabrese F, Popoli M, et al. Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J Biol Psychiatry. 2011;12:574–87.
Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 2014;15:15924–50.
Rajkumar AP, Ballard C, Fossey J, Corbett A, Woods B, Orrell M, et al. Apathy and Its Response to Antipsychotic Review and Nonpharmacological Interventions in People With Dementia Living in Nursing Homes: WHELD, a Factorial Cluster Randomized Controlled Trial. J Am Med Dir Assoc. 2016;17:741–7.
Kimura T, Takamatsu J. Pilot study of pharmacological treatment for frontotemporal lobar degeneration: effect of lavender aroma therapy on behavioral and psychological symptoms. Geriatr Gerontol Int. 2013;13:516–7.
Kung F-C, Ishii R, Liu H-C, Takeda M. New possibility of traditional Chinese and Japanese medicine as treatment for behavioral and psychiatric symptoms in dementia. Clin Interv Aging Dove Press. 2012;7:393–6.
Teranishi M, Kurita M, Nishino S, Takeyoshi K, Numata Y, Sato T, et al. Efficacy and tolerability of risperidone, yokukansan, and fluvoxamine for the treatment of behavioral and psychological symptoms of dementia: a blinded, randomized trial. J Clin Psychopharmacol. 2013;33:600–7.
Ballard C, Smith J, Husebo B, Aarsland D, Corbett A. The role of pain treatment in managing the behavioural and psychological symptoms of dementia (BPSD). Int J Palliat Nurs MA Healthcare London. 2011;17:420, 422, 424.
Husebo BS, Ballard C, Fritze F, Sandvik RK, Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry. 2014;29:828–36.
Marsh AA, Yu HH, Pine DS, Blair RJR. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl). 2010;209:225–32.
Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007.
Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6.
Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: A critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 2016;79:234–42.
MacDonald K, Feifel D. Oxytocin in schizophrenia: A review of evidence for its therapeutic effects. Acta Neuropsychiatr Blackwell Publishing Ltd. 2012;24:130–46.
Jesso S, Morlog D, Ross S, Pell MD, Pasternak SH, Mitchell DGV, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain. 2011;134:2493–501.
Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, et al. Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology. 2015;84:174–81. Safety and tolerability study of oxytocin demonstrated, with preliminary outcomes suggesting potential benefits for apathy treatment in bvFTD.
Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al. Safety and Efficacy of Medical Cannabis Oil for Behavioral and Psychological Symptoms of Dementia: An-Open Label, Add-On. Pilot Study J Alzheimers Dis. 2016;51:15–9.
Dykstra Goris E, Ansel KN, Schutte DL. Quantitative Systematic Review of the effects of Non-pharmacological Interventions on Reducing Apathy in Persons with Dementia. J Adv Nurs. 2016;1–17.
Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: a systematic review. Am J Geriatr Psychiatry. 2012;20:549–64.
Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. SAGE Publications; 2016;2040622316658463.
Ligthart SA, Richard E, Fransen NL, Eurelings LSM, Beem L, Eikelenboom P, et al. Association of Vascular Factors With Apathy in Community-Dwelling Elderly Individuals. Arch Gen Psychiatry Am Med Assoc. 2012;69:243–54.
Groeneweg-Koolhoven I, Comijs HC, Naarding P, de Waal MWM, van der Mast RC. Apathy in Older Persons With Depression: Course and Predictors: The NESDO Study. J Geriatr Psychiatry Neurol SAGE Publ. 2016;29:178–86.
Brodaty H, Liu Z, Withall A, Sachdev PS. The Longitudinal Course of Post-Stroke Apathy Over Five Years. J Neuropsychiatry Clin Neurosci. [Internet]. American Psychiatric AssociationArlington, VA; 2013 [cited 2016 Aug 15];25:283–91. Available from: http://psychiatryonline.org/doi/abs/10.1176/appi.neuropsych.12040080
Yao H, Takashima Y, Mori T, Uchino A, Hashimoto M, Yuzuriha T, et al. Hypertension and white matter lesions are independently associated with apathetic behavior in healthy elderly subjects: the Sefuri brain MRI study. Hypertens Res Nat Publishing Group. 2009;32:586–90.
Eurelings LSM, Jaccard J, van Charante EP M, Eikelenboom P, Ligthart SA, van Gool WA, et al. The mediating role of cardiovascular risk factors in the relationship between symptoms of apathy and incident cardiovascular disease in community-dwelling older individuals. Int. Psychogeriatrics. Camb Univ Press. 2016;28:669–79.
Cummings J, Friedman JH, Garibaldi G, Jones M, Macfadden W, Marsh L, et al. Apathy in Neurodegenerative Diseases: Recommendations on the Design of Clinical Trials. J Geriatr Psychiatry Neurol. 2015;28:159–73. Important review with recommendations on how to address current challenges in trial design for apathy treatment in neurological disease.
Arciniegas DB, Anderson CA. Donepezil-Induced Confusional State in a Patient With Autopsy-Proven Behavioral-Variant Frontotemporal Dementia. J Neuropsychiatry Clin Neurosci. 2013;25:E25–6.
Links KA, Black SE, Graff-Guerrero A, Wilson AA, Houle S, Pollock BG, et al. A case of apathy due to frontotemporal dementia responsive to memantine. Neurocase. 2013;19:256–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
Fleur Harrison and Liesbeth Aerts declare that they have no conflict of interests. Henry Brodaty is on the advisory board and acted as a consultant for Nutricia and his department has received payment to participate in drug trials for AD from Sanofi, Servier, and Tau Therapeutics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Geriatric Disorders
Rights and permissions
About this article
Cite this article
Harrison, F., Aerts, L. & Brodaty, H. Apathy in Dementia: Systematic Review of Recent Evidence on Pharmacological Treatments. Curr Psychiatry Rep 18, 103 (2016). https://doi.org/10.1007/s11920-016-0737-7
Published:
DOI: https://doi.org/10.1007/s11920-016-0737-7