Abstract
Apathy is a highly prevalent symptom of dementia. Despite its association with faster cognitive and functional decline, decreased quality of life and increased mortality, no therapies are currently approved to treat apathy. The objective of this review was to summarize the drugs that have been studied for apathy treatment in patients with dementia (specifically Alzheimer’s disease [AD], Huntington’s disease [HD] and Parkinson’s disease [PD] dementia; dementia with Lewy bodies [DLB]; vascular dementia [VaD]; and frontotemporal dementia [FTD]) based on their putative mechanisms of action. A search for relevant studies was performed using ClinicalTrials.gov and PubMed. Eligible studies were randomized controlled trials that were available in English and included at least one drug intervention and an apathy measure scale. A total of 52 studies that included patients with AD (n = 33 studies), PD (n = 5), HD (n = 1), DLB (n = 1), FTD (n = 3), VaD (n = 1), VaD and AD (n = 4), VaD and mixed dementia (n = 1), and AD, VaD and mixed dementia (n = 3) were eligible for inclusion. These studies showed that methylphenidate, olanzapine, cholinesterase inhibitors, choline alphoscerate, citalopram, memantine, and mibampator are the only beneficial drugs in AD-related apathy. For PD-related apathy, only methylphenidate, rotigotine and rivastigmine showed benefits. Regarding FTD- and DLB-related apathy, initial studies with agomelatine and rivastigmine showed benefits, respectively. As for HD- and only-VaD-related apathy, no drugs demonstrated benefits. With regards to mixed populations, memantine, galantamine and gingko biloba showed effects on apathy in the AD plus VaD populations and nimodipine in the VaD plus mixed dementia populations. Of the drugs with positive results, some are already prescribed to patients with dementia to target other symptoms, some have characteristics—such as medical contraindications (e.g., cardiovascular) and adverse effects (e.g., gastrointestinal disturbances)—that limit their clinical use and some require further study. Future studies should investigate apathy as a primary outcome, making use of appropriate sample sizes and study durations to ensure durability of results. There should also be a consensus on using scales with high test/retest and interrater reliabilities to limit the inconsistencies between clinical trials. In conclusion, there are currently no US FDA-approved drugs that target apathy in dementia, so there is an ongoing need for the development of such drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Apathy is defined as a loss of initiative, interest and emotional expression/responsiveness and is commonly found in people with dementia. |
No pharmacological therapies are currently approved to treat apathy. |
This review covers the drugs that have been investigated as options for apathy in patients with Alzheimer’s disease, Huntington’s disease or Parkinson’s disease dementia, Lewy body dementia, vascular dementia and frontotemporal dementia. |
Some agents show promise (particularly methylphenidate for apathy associated with Alzheimer’s disease), but further well-designed studies using apathy as a primary outcome and with appropriate sample sizes and study durations are required. |
1 Introduction
Dementia is a highly prevalent brain disorder characterized by a progressive decline in mental capacity leading to compromised independent living [1]. In 2015, 46 million people worldwide had dementia, and the prevalence is estimated to increase to 132 million by 2050 [1]. Globally, the cost of dementia was $US818 billion in 2015 and is estimated to reach $US2 trillion by 2030 [2]. There are several types of dementias. Alzheimer’s disease (AD) is the most common type, followed by vascular dementia (VaD) [3]. Other common dementias include Parkinson’s disease (PD) dementia, Huntington’s disease (HD) dementia, dementia with Lewy bodies (DLB) and frontotemporal dementia (FTD) [1, 3]. Along with cognitive decline, those with dementia experience behavioral and psychological symptoms, contributing to a poorer quality of life [4]. One such symptom is apathy.
Apathy is one of the most common neuropsychiatric symptoms of dementia and was first characterized by Marin [5] as a lack of motivation. Studies have shown apathy’s association with greater cognitive and functional impairment, increased reliance on caregivers to initiate activities, decreased quality of life and increased mortality [6,7,8,9]. Its prevalence in AD, FTD, DLB, VaD, PD, and HD is up to 95, 100, 50, 65, 62 and 76%, respectively [10,11,12,13]. Apathy as a syndrome is not formally recognized in the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), so its research diagnosis may depend on consensus criteria that are decided upon and widely accepted by experts in the field, such as those for apathy in brain disorders [14] or in dementia [15]. Apathy is now defined based on diminished goal-directed behavior [14, 15], and the latter criteria diagnose apathy based on diminished initiative, diminished interest or diminished emotional expression/responsiveness that causes significant functional impairment and that is not exclusively explained by other etiologies. Apathy has also been measured based on cutoffs from scales measuring severity such as the Apathy Evaluation Scale (AES), the Lille Apathy Rating Scale (LARS) and the apathy subscales of the Neuropsychiatric Inventory (NPI-apathy), and Frontal Systems Behavioral Scale (FrSBe-apathy), among others.
Studies have shown that, across the different neurodegenerative disorders, consistent changes are observed mainly in the fronto-striatal circuits, the dorsal anterior cingulate cortex and the ventral striatum (VS), which includes the nucleus accumbens (NAc) [16]. In these brain regions, apathy has been proposed to be associated with dysfunction of various neurotransmitter systems, such as the cholinergic system and dopaminergic system [17, 18]. The major neurotransmitter systems that have commonly either been associated with apathy or have been hypothesized to have an association with apathy are discussed in this review.
Apathy is of increasing interest as a treatment target because of its negative impact on the quality of life of patients and their caregivers. Currently, behavioral interventions delivered by caregivers are deemed a safe treatment for several neuropsychiatric symptoms in dementia, compared with pharmacological treatment, because of the lack of adverse effects [19]. However, studies on the effects of behavioral interventions on apathy specifically have not been investigated thoroughly [19]. Few drugs are used for the symptomatic management of apathy, and none have been approved by health regulatory agencies for this indication. The purpose of this review was to summarize the candidate drugs for apathy treatment in patients with dementia with a focus on the putative mechanism of action for the drugs studied. Additionally, this review examines the treatments for apathy symptoms found in various types of dementia, which previous review papers rarely discussed.
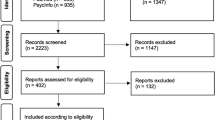
To review the studies on drug treatment for apathy in dementia published up to 31 October 2021, we searched the US National Library of Medicine clinical trials registry (https://www.clinicaltrials.gov/) and the bibliographic database of life sciences PubMed (https://www.ncbi.nlm.nih.gov/). The search criteria of the condition or disease included keywords such as “Alzheimer’s disease,” “dementia,” “frontotemporal dementia,” “Lewy-body dementia,” “vascular dementia,” “Parkinson’s disease,” and “Huntington’s disease,” and included terms such as “AND apathy” OR “emotional bluntness” OR “lack of interest” OR “anergia” in the search. The search was restricted to English only and included placebo- or drug comparator-controlled randomized controlled trials with at least one drug intervention and at least one scale that measures apathy. We performed a quality assessment of the studies that fulfilled the inclusion criteria using the Cochrane Checklist for randomized controlled trials [20].
2 Neurotransmitter Systems and Drugs
Results are organized by the neurotransmitter systems that have been implicated in apathy in neurodegenerative diseases. Within each section, we described the different drugs whose putative mechanism of action (where known) included that system. The tables provided in each section include studies where apathy was a primary or a secondary outcome (Tables 1, 2, 3, 4, 5, 6, 7, 8, 9). For the purposes of this review, we considered apathy as a primary outcome where an apathy scale (e.g., AES) was used or where apathy scores could have been derived from a composite behavioral scale (e.g., NPI). A total of 52 studies fulfilled the inclusion criteria, and the risk of bias for most studies included was low (see the Appendix).
2.1 Dopaminergic System
Dopamine is a central nervous system (CNS) neurotransmitter involved in executive function, motor control, motivation, arousal, reinforcement and reward [21]. A dopamine imbalance is commonly observed among patients with dementia. Studies observed a significantly reduced level of dopamine in AD [22]. Similarly, disruptions of the fronto-striatal and mesocortical dopamine network were observed in PD dementia [23, 24]. Likewise, in HD dementia, substantial losses in dopamine function were observed [25]. In FTD, DLB and VaD, studies observed lower levels of dopamine and dopamine receptors in the basal ganglia [26,27,28]. Studies also showed that disruptions in the brain reward pathway, i.e., neurons projecting from the ventral tegmental area to the NAc/VS and prefrontal cortex, mainly the medial frontal cortex, orbitofrontal cortex and anterior cingulate cortex, were observed in all the above-mentioned dementias and were associated with lack of motivation or apathy [17]. Sections 2.1.1 and 2.1.2 list the studies that explored the effects of treatments targeting the dopaminergic system on apathy.
2.1.1 Drugs Affecting the Dopaminergic System in Alzheimer’s Disease (AD)
Four studies explored the effects of methylphenidate on apathy in AD populations (Table 1). A methylphenidate study by Herrmann et al. [29] measured apathy with AES and NPI-apathy as a primary and a secondary outcome, respectively. Results with AES showed a significant reduction in apathy in the methylphenidate group compared with placebo (Wilcoxon Z = − 2.00; p = 0.045), whereas results with NPI-apathy showed no effects of methylphenidate on apathy [29]. Similarly, Padala et al. [30] showed a significant reduction in apathy at week 12 in the methylphenidate group compared with placebo based on results with the AES-clinician (− 9.9; 95% confidence interval [CI] − 13.6 to − 6.2; p < 0.001) [30]. Rosenberg et al. [31] measured apathy with both the AES and the NPI-apathy, as primary and secondary outcomes, respectively. The other primary outcome was Clinical Global Impression modified for apathy (CGI-apathy). There were no significant effects on apathy based on results with AES, but more people showed a reduction in apathy in the methylphenidate group than with placebo based on results with CGI-apathy (21 vs. 3%; odds ratio 3.7; 95% CI 1.3–10.8; p = 0.02). Results with NPI-apathy also showed a significant reduction in apathy with methylphenidate than with placebo (− 1.8; 95% CI − 3.4 to − 0.3; p = 0.02) [31]. Lastly, Mintzer et al. [32] showed a significant reduction in apathy in the methylphenidate group in comparison with placebo based on results with NPI-apathy as a primary outcome measure (− 1.25; 95% CI − 2.03 to − 0.47; p = 0.002). Of the four methylphenidate studies, Padala et al. [30] and Rosenberg et al. [31] had large effect sizes of 1 and 1.7, respectively, whereas the effect sizes for Herrmann et al. [29] and Mintzer et al. [32] were modest at 0.62 and small at 0.37, respectively.
Frakey et al. [33] explored the effects of modafinil on apathy in patients with AD (Table 1). Apathy was measured as a primary outcome with FrSBe-apathy. The results showed a trend towards a reduction in apathy in the modafinil group at week 8 in comparison with baseline, but the changes were not significant when compared with placebo [33].
Nave et al. [34] explored the effects of sembragiline on apathy in patients with AD (Table 1). Apathy was measured with the AES-clinician as a secondary outcome. The results showed no effects of sembragiline on apathy [34].
Three studies explored the effects of dopamine antagonists on apathy as a secondary outcome (Table 1). A perphenazine study by Pollock et al. [35] observed no effects of perphenazine on apathy based on results with Neurobehavioral Rating Scale (NRS)-apathy. Likewise, an aripiprazole study by De Deyn et al. [36] showed no effects of aripiprazole on apathy, based on results with NPI-apathy. On the other hand, an olanzapine study by De Deyn et al. [37] observed a significant reduction in apathy in the olanzapine 5 mg group in comparison with placebo (− 1.8 ± 3.1; n = 123; p = 0.043), based on results with NPI-nursing home (NPI-NH)-apathy. The calculated effect size was small at 0.23 [37]. It should be noted that olanzapine has opposite effects to methylphenidate and modafinil on dopamine receptors. Methylphenidate acts directly by increasing the normally decreased dopamine levels in AD populations, whereas olanzapine works as an antipsychotic with dopamine D2 receptor antagonism, which generally addresses neuropsychiatric symptoms and probably has a nonspecific effect on apathy. These medications were reviewed together for better interpretation of the potential effects of manipulating the dopaminergic system. Even though it might appear that a stimulant and an antipsychotic drug would have opposing effects, the use of both has shown an increase in tonic dopamine levels [38].
2.1.2 Drugs Affecting the Dopaminergic System in Parkinson’s Disease (PD)
The methylphenidate study by Moreau et al. [39] measured apathy as a secondary outcome with LARS (Table 2). Results showed significant reduction in apathy in a subgroup of seven patients, with moderate apathy in the methylphenidate group in comparison with a subgroup of five patients with moderate apathy in the placebo group (p = 0.03) [39]. When considering all the participants in the study, results with LARS showed no changes in apathy in the methylphenidate group in comparison with placebo [39].
A study by Hauser et al. [40] investigating rotigotine showed no significant difference in apathy in the rotigotine 16 mg group in comparison with placebo, based on results with the patient-rated Apathy Scale (AS-patient) (Table 2). Similarly, the caregiver-rated AS (AS-caregiver) also showed no significant difference in apathy with either rotigotine 8 or 16 mg [40]. On the other hand, Non-Motor Symptom Scale (NMSS)-mood/apathy results showed a significant reduction in apathy only in the rotigotine 16 mg group in comparison with placebo (− 3.88; 95% CI − 7.46 to − 0.30; p = 0.034), with a small effect size of 0.187 [40]. AS-patient was the only primary outcome measure for apathy, whereas NMSS-mood/apathy and AS-caregiver were secondary outcome measures [40]. Castrioto et al. [41] utilized LARS to measure apathy as a primary outcome, with the Starkstein Apathy Scale (SAS) and Ardouin Scale of Behavior in Parkinson’s Disease (ASBPD)-apathy as secondary outcome measures (Table 2). Results with all three apathy outcome measures showed no significant differences with rotigotine versus placebo on apathy [41].
Lastly, Barone et al. [42] showed no effects of rasagiline on apathy, based on results with AS as a secondary outcome measure (Table 2).
2.2 Cholinergic System
Acetylcholine is a neurotransmitter that plays important roles in both the CNS and the peripheral nervous system [46]. It is involved in functions that are highly relevant to dementias, such as attention, learning, memory, and wakefulness and sleep [46]. Cholinergic neuron degeneration has been shown to play a significant role in the cognitive impairment aspect of dementia [47]. In patients with AD, studies observed a severe loss of cholinergic neurons in the basal forebrain and cerebral cortex, especially in the temporal lobes [46]. In comparison with AD, studies in PD dementia observed more severe cholinergic neuron loss occurring at an earlier stage [48, 49]. In early HD dementia, a decrease in acetylcholine cerebrospinal fluid (CSF) levels was observed, whereas a decrease in cholinergic neurons was observed in late HD dementia [50]. Similarly, degeneration of the cholinergic system in the basal forebrain was reported in VaD and DLB [28, 51]. Major cholinergic deficiencies have been shown to disrupt the communication between the limbic system and the neocortical regions, mainly the medial, lateral frontal and anterior temporal regions of the brain [47]. This may play a key role in the development of apathy. Sections 2.2.1 to 2.2.4 list the studies that explored the effects of treatments that modify the cholinergic system on apathy.
2.2.1 Drugs Affecting the Cholinergic System in AD
A post hoc analysis of three clinical trials by Herrmann et al. [52] explored the effects of galantamine on apathy in patients with AD (Table 3). Individual NPI domain scores were one of the primary outcome measures, including NPI-apathy. Results showed no significant reduction in mean changes between groups. However, a significant reduction in mean was noted for one of the priori clusters (cluster 4) of behavioral symptoms, including apathy, in the galantamine group in comparison with placebo (− 0.69; χ2 = 6.87; p < 0.05) [52]. Similarly, a study by Cummings et al. [53] showed a modest but significant reduction in apathy with galantamine 16 mg/day (− 2.5; 95% CI − 4.6 to − 0.3; p < 0.05) and galantamine 24 mg/day (− 2.5; 95% CI − 4.7 to −0.4; p < 0.05) compared with placebo, based on results with NPI-apathy as one of the secondary outcome measures [53].
Five studies in patients with AD explored the effects of metrifonate on apathy (Table 3). Kaufer et al. [54] showed that apathy was significantly reduced with metrifonate compared with placebo (− 0.74; p = 0.03) based on results with NPI-apathy as a secondary outcome. Morris et al. [55] observed a trend towards reduced apathy with metrifonate compared with placebo, but the results were not significant. On the other hand, Dubois et al. [56] showed a significant and modest reduction in apathy with metrifonate 60/80 mg compared with placebo (− 0.70; p = 0.048) based on results with NPI-apathy. Another metrifonate study by Raskind et al. [57] showed a nonsignificant trend towards reduction in apathy in the metrifonate group in comparison with placebo, based on results with NPI-apathy. Lastly, Cummings et al. [58] reported a significant reduction in apathy in the metrifonate group in comparison with placebo, based on results with NPI-apathy. The reported effect size was small at 0.15 [58]. For all metrifonate studies, except the study by Kaufer et al. [54], apathy was not an outcome of interest. The NPI-apathy scores were reported while investigating NPI-total as the secondary outcome measure.
Six studies in patients with AD explored the effects of donepezil on apathy (Table 3). Tariot et al. [59] showed no significant difference in apathy in the donepezil group after 24 weeks in comparison with baseline based on results with NPI-NH-apathy. Gauthier et al. [60] measured apathy in 290 patients with AD with NPI-apathy. Results showed a significant reduction in apathy favoring the donepezil group in comparison with placebo (83 vs. 70% reduction in NPI-apathy; p = 0.0058). Similar results with NPI-apathy were observed in another study by Gauthier et al. [61] with 270 patients with AD. Holmes et al. [62] also showed a significant reduction in apathy in the donepezil group at week 12 in comparison with baseline, based on results with NPI-apathy. NPI-apathy scores for placebo and donepezil groups were not compared; however, NPI-total scores significantly decreased in the donepezil group in comparison with placebo on study week 24 (− 2.9 vs. 3.3; p = 0.02) [62]. Feldman et al. [63] also showed a significant reduction in apathy in the donepezil group in comparison with placebo, based on results with NPI-apathy. None of these donepezil studies identified apathy as an outcome of interest. The NPI-apathy scores were reported while investigating NPI-total as the primary outcome measure (Tariot et al. [59] and Holmes et al. [62]) or as the secondary outcome measure (Gauthier et al. [60, 61] and Feldman et al. [63]). Lastly, the donepezil study by Seltzer et al. [64] explored apathy as a secondary outcome with AS. Results showed no significant difference in apathy in the donepezil group in comparison with placebo [64].
A choline alphoscerate study by Rea et al. [65] measured apathy as a secondary outcome via the NPI-apathy scale (Table 3). Results showed a significant reduction in apathy in the choline alphoscerate group in comparison with placebo [65]. However, apathy was not an outcome of interest for this study. While investigating NPI-total as the secondary outcome measure, the NPI-apathy scores were reported.
Åhlin et al. [66] investigated the effects of tacrine on apathy by utilizing the Nurses' Observation Scale for Inpatient Evaluation (NOSIE) as the secondary outcome measure (Table 3). Results showed no effect of tacrine on apathy [66].
2.2.2 Drugs Affecting the Cholinergic System in PD
A rivastigmine study by Devos et al. [67] measured apathy as a primary outcome using LARS (Table 4). Results showed a significant reduction in apathy in the rivastigmine group in comparison with placebo (F (1,25) = 5.2; p = 0.034), with a large effect size of 0.9 [67].
2.2.3 Drugs Affecting the Cholinergic System in Dementia with Lewy Bodies
A rivastigmine study by McKeith et al. [68] measured apathy using NPI-apathy as a secondary outcome (Table 4). Results showed a significant improvement in apathy in the rivastigmine group in comparison with placebo, with a modest effect size of 0.537 [68].
2.2.4 Drugs Affecting the Cholinergic System in Vascular Dementia (VaD) and AD
A galantamine study by Erkinjuntti et al. [69] measured apathy with NPI-apathy (Table 4). Results showed a significant reduction in apathy in the galantamine group in comparison with placebo. However, apathy was not an outcome of interest for this study. The NPI-apathy scores were reported while investigating NPI-total as the secondary outcome measure.
2.3 Serotonergic System
Serotonin (5HT) is a CNS neurotransmitter that regulates several important physiological processes such as body temperature, sleep, appetite, pain and motor activity [71]. Studies in patients with AD observed a decrease in the number of serotonergic neurons in the raphe nuclei, associated with the hyperphosphorylated tau proteins, a key feature of AD [72]. In PD dementia, a decrease in 5HT2A receptors was observed [73]. In HD mice models, diminished levels of 5HT were noted, particularly in the hippocampus, as being associated with cognitive deficits [74]. In VaD, DLB and FTD, deficiencies in the 5HT system were also observed [26, 28, 75]. Studies showed an overall reduction in serotonergic activity in patients with dementia, so it was earlier hypothesized that antidepressants such as selective serotonin reuptake inhibitors might be effective in reducing apathy. However, subsequent studies suggested that antidepressants could potentially increase apathy because of their inhibitory effects via 5HT2C receptors and stimulatory effects via 5HT1B and 5HT3 receptors on the dopamine system [17]. It is now hypothesized that antagonists at the 5HT receptors might provide some beneficial effects in treating apathy. Sections 2.3.1 to 2.3.3 list the studies that explored the effects of treatments targeting the serotonergic system for apathy.
2.3.1 Drugs Affecting the Serotonergic System in AD
Four studies in patients with AD explored the effects of drugs affecting the serotonergic system for apathy (Table 5). Lawlor et al. [76] studied chlorophenylpiperazine and measured apathy as a secondary outcome with the Brief Psychiatric Rating Scale-anergia. Results showed no significant effects of chlorophenylpiperazine on apathy [76]. A sertraline study by Lanctôt et al. [77] showed no significant effects of sertraline on apathy in agitated participants based on results with NPI-apathy. Zhou et al. [78] reported a significant reduction in apathy in the citalopram group in comparison with placebo, based on results with NPI-apathy. For the studies by Lanctôt et al. [77] and Zhou et al. [78], apathy was not an outcome of interest. The NPI-apathy scores were reported while investigating NPI as one of the primary outcome measures [78] or as a secondary outcome measure [77]. Lastly, Leonpacher et al. [79] reported a trend towards reduced apathy with citalopram based on results with NPI-apathy as a secondary outcome measure, but the effects were not significant.
2.3.2 Drugs Affecting the Serotonergic System in AD and VaD
In a mix of AD and VaD populations, two studies explored the effects of citalopram on apathy as a secondary outcome (Table 5). Pollock et al. [35] showed no effect of citalopram on apathy based on results with NRS-apathy. On the other hand, Nyth et al. [80] showed a significant reduction in apathy in the citalopram group at week 4 in comparison with baseline, based on results with Gottfries–Brane–Steen Scale (GBS)-emotional bluntness. However, the reduction in apathy was not significant when compared with placebo.
2.3.3 Drugs Affecting the Serotonergic System in Frontotemporal Dementia
A trazodone study by Lebert et al. [81] measured apathy with NPI-apathy (Table 5). Results showed a trend towards reduced apathy in the trazodone group in comparison with placebo, but the effects were not significant. Apathy was not an outcome of interest for this study; instead, NPI-apathy scores were reported while investigating NPI-total as the primary outcome measure.
2.4 Noradrenergic System
Norepinephrine is a CNS neurotransmitter mainly involved in attention, perception and memory retrieval [82]. Studies in patients with AD observed a higher norepinephrine degradation, followed by overcompensation via reduced norepinephrine reuptake and increased norepinephrine production, leading to increased norepinephrine concentrations in early AD [83]. However, with progression of AD, the compensatory mechanism appeared inadequate, and the concentrations of norepinephrine dropped to lower than normal [83]. In PD dementia, a substantial reduction of norepinephrine input from locus coeruleus (LC) to cortical areas was observed, i.e., loss of norepinephrine neurons in the LC [84]. In patients with HD dementia, an increase in norepinephrine concentration in the basal ganglia was noted [85]. In VaD, FTD and DLB, limited evidence indicated a reduction in norepinephrine neurons, especially in the LC [26, 28, 86]. However, several studies also showed a relatively unaffected and preserved LC in VaD, FTD and DLB [26, 28, 86]. As noted, methylphenidate studies for AD-related apathy (2.1.1) showed benefit, which was initially thought to be due to its dopamine actions in the striatum and the thalamus. However, it is now believed that a major role is also played by its norepinephrine actions in the prefrontal cortex [87]. The studies listed under Sects. 2.4.1 and 2.4.2 tested the effects on apathy of another norepinephrine, dopamine and 5HT reuptake inhibitor, bupropion, in AD and HD populations.
2.4.1 Drugs Affecting the Norepinephrine System in AD
A bupropion study by Maier et al. [88] measured apathy with AES-clinician and NPI-apathy as primary and secondary outcome measures, respectively (Table 6). No significant effects on apathy were observed in the bupropion group in comparison with placebo, based on results with both apathy outcome measures [88].
2.4.2 Drugs Affecting the Norepinephrine System in Huntington’s Disease
Gelderblom et al. [89] investigated bupropion’s effects on apathy with AES-informant, AES-clinician, AES-self, NPI-apathy, and Unified Huntington’s Disease Rating Scale (UHDRS)-apathy (Table 6). AES-informant was the only primary outcome measure, and the rest were secondary [89]. A trend towards reduced apathy in the bupropion group in comparison with placebo was observed based on results with AES-informant, AES-clinician, AES-self, NPI-apathy, and UHDRS-apathy, but the results were not significant [89].
2.5 GABAergic System
Gamma aminobutyric acid (GABA) is a neurotransmitter with inhibitory actions responsible for attenuating brain signals and activities in the nervous system [90]. Studies in patients with AD observed significantly lower levels of GABA in the CSF [91]. There was also a reduction in GABAergic terminals, especially in the cortical neurons adjacent to amyloid plaques, a key feature of AD [91]. Similarly, in PD dementia, a decrease in CSF GABA levels was observed [92, 93]. Likewise, in HD dementia, a significant decrease in GABA levels was observed, especially in the basal ganglia [94]. Similarly, in FTD, a decrease in GABAergic neurons has been noted [95]. No studies have yet explored the role of GABA system dysfunction in DLB and VaD. One study found that plasma GABA levels positively correlated with apathy scores in patients with severe AD [96]. Section 3.5.1 describes the study that explored the effects on apathy of treatment targeting the GABAergic system.
2.5.1 Drugs Affecting the GABAergic System in AD
A valproate study by Sival et al. [97] measured apathy as a secondary outcome with Behavioral Rating Scale for Geriatric Inpatient-apathetic behavior (Table 7). Results showed no significant effects of valproate on apathy [97].
2.6 Glutamatergic System
Glutamate is the most abundant excitatory neurotransmitter in the CNS [98]. Studies in patients with AD showed a severely disrupted glutamatergic system. A decrease in glutamate reuptake/recycling leads to increased availability of glutamate, causing excitotoxicity and neurodegeneration [99]. Increased concentrations of glutamine in the CSF were also observed [100]. In patients with PD, downregulation of the glutamate transporters, associated with cognitive deficiency/dementia, was observed [101]. In addition, an increase in plasma glutamate levels was noted, reflecting an increase in glutamatergic activity [101]. On the other hand, in HD dementia, a significant decrease in glutamate levels was observed, especially in the basal ganglia [94]. Similarly, in FTD, a decrease in glutamatergic neurons was observed [95]. Also, a dysfunctional metabotropic glutamate receptor has been demonstrated in the development of DLB [102]. Lastly, in VaD, a high level of CSF glutamate was observed [100]. Sections 2.6.1 and 2.6.2 list the studies that explored the effects of treatments targeting the glutamatergic system for apathy.
2.6.1 Drugs Affecting the Glutamatergic System in AD
Two studies in patients with AD explored the effects of memantine on apathy (Table 8). Araki et al. [103] showed a significant reduction in apathy in the memantine group in comparison with placebo, based on results with NPI-apathy (F (1,23) = 22.24; p < 0.01) [103]. The effect size was large at 0.92. On the other hand, Gauthier et al. [104] performed a post hoc analysis of two memantine clinical trials by Reisberg et al. [105] and Tariot et al. [106]. Results with NPI-apathy showed no difference in apathy in the memantine group in comparison with placebo [104]. For both the studies, apathy was not an outcome of interest; instead, the NPI-apathy scores were reported while investigating NPI-total as the secondary outcome measure.
2.6.2 Drugs Affecting the Glutamatergic System in VaD and AD
A memantine study by Winblad et al. [107] measured apathy as a secondary outcome with Ferm’s D-test-hobby/interest (Table 8). Results showed a significant reduction in apathy in the memantine group in comparison with placebo [107].
2.7 Histaminergic System
Histamine is a neurotransmitter in the mammalian brain that is involved in many biological processes, such as temperature regulation, water intake and avoidance behavior [108]. Most importantly, the histaminergic system plays a major role in alertness and memory and learning [109]. Several studies have identified histamine as a potent mediator of blood–brain barrier breakdown, astrocyte activation and neuronal damage response, all key features of AD [110]. Studies also showed alterations of brain histamine concentrations, reduced histamine-releasing factor, decreased number of histamine receptors in the frontal and temporal cortex, and degeneration of histamine neurons in the tuberomammillary nucleus, in patients with AD [111]. Conversely, in patients with PD or HD, significantly increased histamine concentrations and histamine receptor levels were observed, associated with cognitive deficiency/dementia [112, 113]. In FTD, DLB and VaD, a decrease in histaminergic neurons was observed [114,115,116]. A few studies have suggested a link between the histamine system and apathy. One such study examined apathy scores in healthy males and females, where males scored higher [117]. A lower H1R-ligand binding in the limbic system was observed in males, highlighting a potential link between histamine and motivation in humans [118]. Also, it was hypothesized that another mechanism by which methylphenidate reduces apathy (2.1.1) is by increasing histamine levels [119]. It proposed that methylphenidate administration increases D2 receptor activation-enhanced tuberomammillary nucleus neuronal firing, leading to histamine release and wakefulness [119]. Even though these studies provide preliminary information on a potential link between histamine and motivation, better studies to establish the link between histamine and dementia-related apathy are yet to be performed. Because of this lack of research and the fact that prohistaminergic therapy is not generally well-tolerated, no current histamine-related drugs for the treatment of apathy are in clinical trials.
2.8 Orexin System
Orexins, also known as hypocretins, are hypothalamic neuropeptides that have an important role in sleep/arousal states [120]. An increase in orexin levels was observed in patients with AD, correlated with tau protein levels, a key feature of AD [121]. Studies in patients with PD or HD observed significantly reduced numbers of orexinergic neurons in the lateral hypothalamus, associated with cognitive decline/dementia [122, 123]. Reduced orexin levels were also found in FTD, VaD and DLB [124,125,126]. Also, diminished orexin levels in the hypothalamus of a social defeat animal model have been shown to be linked to persistent apathy [127]. However, more studies need to be performed to establish a stronger link between the orexin system and dementia-related apathy.
2.9 Peptide YY and Ghrelin
Peptide YY (PYY) and ghrelin are secreted from the gastrointestinal endocrine cells and act as CNS neurotransmitters [128]. PYY is orexigenic, whereas ghrelin is anti-orexigenic, and both maintain the energy homeostasis [129]. They also play a role in synaptic strength and plasticity and in regulating mood, such as depression and anxiety [128, 130]. In patients with AD, studies observed significantly higher PYY plasma levels and significantly lower levels of ghrelin messenger RNA in the temporal gyrus [131, 132]. In patients with PD dementia, studies observed altered gut microbiota and consequently altered PYY levels and a significant increase in unacylated ghrelin, associated with reduced neurogenesis and neuronal plasticity [133, 134]. PYY levels decreased in early HD mouse models and increased in late HD, and this was associated with non-motor HD symptoms, such as cognitive deficiency/dementia [135]. Also, in patients with HD, studies observed an increase in the level of ghrelin, associated with extreme weight loss and neuronal loss [132, 136]. An animal study performed to explore the potential role of PYY and ghrelin systems in apathy development suggested that apathy was caused by impaired dopamine signaling through D2 receptors, which was induced by peripheral PYY elevation [137]. There was also a compensatory increase in ghrelin levels to increase D2 receptor signaling and to overcome PYY elevation effects, but the compensation was insufficient [137]. Even though this study tried to link PYY, ghrelin and apathy, the effects of ghrelin and PYY on D2 receptors remains controversial and there is a continued need to study this relationship in detail, especially in AD-, PD- and HD-related apathy. This lack of research means no PYY- or ghrelin-related drugs for the treatment of apathy are yet in clinical trials.
2.10 Adenosine System
Adenosine is a neurotransmitter that acts as a CNS depressant, promoting sleep and suppressing arousal [138]. Adenosine and adenosine receptors have increasingly been recognized as important for cognition [139]. Studies in patients with AD observed reduced adenosine receptor levels in the hippocampus and increased levels in the cortex [139]. Also, studies using human neural cell models showed a link between adenosine receptor activation and phosphorylation of tau, a key feature of AD [140]. Studies in patients with PD observed a significant increase in adenosine receptor density, especially in the basal ganglia, which—when targeted with adenosine antagonists—improved cognition [141, 142]. In HD mice models, several studies reported an increase in adenosine receptors [143, 144]. Some studies also observed a hyperactivation of striatal adenosine receptors where the use of a dopamine plus adenosine antagonist reduced the activity of protein kinase A, a protein involved in HD-related cognitive dysfunction [145,146,147]. Increased adenosine receptor expression was observed in FTD and VaD studies, and increased adenosine levels were noted in DLB [148,149,150]. A pharmacological study with istradefylline, a US FDA-approved adenosine receptor antagonist for treatment of PD, showed significantly reduced apathy scores over time in patients with PD [151]. Istradefylline, when tested in AD and HD mice models, showed an enhancement in spatial memory and working memory, respectively, with no focus on apathy [152, 153].
2.11 Others
Three drugs that act via a unique mechanism of action have been explored for their effects on apathy in AD populations (Table 9). Rosenberg et al. [154] investigated semagacestat and apathy and found no effects on apathy based on the results with NPI-apathy as a primary outcome measure. A mibampator study by Trzepacz et al. [155] measured apathy with FrSBe-apathy and NPI-apathy. Results with both apathy outcome measures showed a significant reduction in apathy in the mibampator group in comparison with placebo, with a small effect size of 0.41 [155]. Kim et al. [156] investigated varenicline, where apathy was measured as a secondary outcome with NPI-apathy. Results showed no reduction in apathy in the varenicline group in comparison with placebo [156]. For all three studies [154,155,156], apathy was not an outcome of interest. The NPI-apathy scores were reported while investigating NPI-total as the primary outcome measure [154] or as a secondary outcome measure [155, 156].
In a mix of AD, VaD and mixed dementia populations, two drugs with a unique mechanism of action have been explored for their effects on apathy (Table 9). A nimodipine study by Ban et al. [157] in VaD and mixed dementia populations measured apathy as a secondary outcome via the Sandoz Clinical Assessment Geriatric Scale (SCAG)-withdrawal. Results showed a significant reduction in apathy in the nimodipine group in comparison with placebo, with a modest effect size of 0.69 [157]. Similarly, both studies with gingko biloba by Scripnikov et al. [158] and Bachinskaya et al. [159], showed a significant reduction in apathy in the gingko biloba group in comparison with placebo, based on results with NPI-apathy but not with NPI-caregiver distress-apathy. Neither of the gingko biloba studies explored apathy as an outcome of interest, with NPI-apathy and -caregiver-apathy scores reported while investigating NPI-total and NPI-caregiver distress-total as the secondary outcome measures.
One drug that acts via a unique mechanism of action has been explored for its effects on apathy in VaD populations (Table 9). A pentoxifylline study by Bayer et al. [160] measured apathy as a secondary outcome with GBS-emotional functions and SCAG-apathy. Results with both the apathy outcome measures did not show any effects of pentoxifylline on apathy [160].
In FTD populations, two drugs with a unique mechanism of action have been explored for their effects on apathy. The oxytocin study by Finger et al. [161] measured apathy with Frontal Behavioral Inventory (FBI)-apathy and NPI-apathy. Results with both apathy outcome measures showed no difference in apathy in the oxytocin group in comparison with placebo [161]. Apathy was not an outcome of interest for this study, and the NPI-apathy scores were reported while investigating NPI-total and FBI-total as the secondary outcome measures. Callegari et al. [162] investigated the effects of agomelatine on apathy as a primary outcome with AES-clinician, NPI-apathy, and NPI-apathy-distress caregiver. Results with all three apathy outcome measures showed a significant reduction in apathy in the agomelatine group in comparison with the melatonin group, with a large effect size of 1 [162].
3 Conclusions
Apathy is a highly prevalent neuropsychiatric symptom in dementia populations. Studies have observed its association with decreased cognition, function and quality of life and increased mortality and caregiver burden, making it an important treatment target [6,7,8,9].
Our review of 52 studies showed that for patients with AD, the only drugs with which reduced apathy was observed were cholinesterase inhibitors (seven studies) and choline alphoscerate (one study), methylphenidate (four studies), olanzapine (one study), citalopram (one study), memantine (one study) and mibampator (one study). For methylphenidate, a meta-analysis by Ruthirakuhan et al. [163] also supported its modest benefits in patients with AD, and the recently published positive study by Mintzer et al. [32] further strengthened that conclusion. For PD-related apathy, the only drugs that showed effects on apathy were methylphenidate, cholinesterase inhibitors (one study [rivastigmine]) and rotigotine (one study). For DLB- and FTD-related apathy, only cholinesterase inhibitors (one study [rivastigmine]) and agomelatine (one study) showed benefits. Lastly, no drugs showed any effects on apathy for HD- and only VaD-related apathy populations. For mixed populations, a cholinesterase inhibitor (one study [galantamine]), memantine (one study) and gingko biloba (two studies) showed effects on apathy in the AD plus VaD populations and nimodipine (one study) in the VaD plus MD populations.
An important consideration is that only four methylphenidate studies in AD, one agomelatine study in FTD and one rivastigmine study in PD explored apathy as a primary outcome. This means that all other drug studies that showed benefits were never powered to primarily explore apathy, and some of those populations had very little apathy to begin with.
Regarding the status and use of these drugs, methylphenidate needs to be used cautiously because of potential concerns when used in conditions that are comorbid in older populations, such as hypertension, other cardiovascular conditions and diabetes [164, 165]. As for memantine, even though it is generally safe and well-tolerated, it does not have marked efficacy in treating apathy and may be better at preventing the emergence of apathy [166]. In relation to cholinesterase inhibitors, they are FDA approved for targeting cognitive and general symptoms of dementia, but they have side effects such as gastrointestinal disturbances that can be persistent and intolerable in older populations [167, 168]. Whether choline alphoscerate has any additional benefits over existing drugs, such as cholinesterase inhibitors, remains uncertain and so requires further study. Like cholinesterase inhibitors, olanzapine and citalopram are often prescribed, mostly in end-stage dementia, for agitation/aggression, but they are not FDA approved for this indication [169, 170]. They come with potentially serious side effects, such as drowsiness and confusion, tremors (which can be permanent), pneumonia and stroke with olanzapine and abnormal heart rhythms with citalopram [169,170,171]. Rotigotine is already prescribed for motor symptoms in PD but needs further study for its effects on non-motor symptoms, including apathy [172, 173]. Lastly, mibampator, agomelatine, nimodipine and gingko biloba require further study.
Some other drugs have also shown potential in treating apathy. An open-label study with a Japanese herbal medication, Ninjin’yoeito, showed significant reduction in apathy but needs further study in a randomized controlled trial setting [174]. In addition, other conditions such as traumatic brain injury (TBI) have apathy as one of the prominent symptoms. Amantadine for TBI has shown some success in reducing apathy and could also be of potential benefit for dementia-related apathy [175]. However, adverse effects, such as delirium, associated with amantadine in patients with dementia might outweigh its benefits [176].
Even though several compounds proved beneficial, their effect sizes were small, and they need further investigation. Currently, the best pharmacological options for treating apathy appear to be methylphenidate and cholinesterase inhibitors followed by gingko biloba [177]. There might also be some potential benefit in the coadministration of some of these drugs, such as cholinesterase inhibitors with memantine or memantine with citalopram [78, 177, 178]. However, none of these drugs are yet FDA approved for apathy treatment in dementia.
Some ways to improve the process of finding potential drug treatments for apathy is to better understand its neurobiology. Therefore, future research could focus on subdomains of apathy based on neurobiological, neurochemical and neuroimaging endpoints, which may help in personalizing treatment and identifying new pharmacological targets [179]. As noted in this review, most of the studies examined apathy as a secondary outcome, which means that the populations may have had no or very mild apathy, leading to a reduced likelihood of finding any differences from placebo. Hence, future studies should consider studying apathy as a primary outcome and recruiting patients diagnosed with apathy based on the consensus criteria mentioned earlier [15]. Furthermore, most of the studies used the 12-item NPI as an outcome measure, which is not specific to apathy and, therefore, has a risk of false positives when each domain is analyzed without adjustment for multiple comparisons. Thus, even though there is no gold standard for measuring apathy, there should be a consensus on using scales with high test/retest and interrater reliabilities, such as the AES and NPI-apathy, for future studies to limit the inconsistencies between clinical trials [180].
In addition to including primary outcome measures such as AES that reflect the symptomatic effects, studies should also incorporate the use of secondary outcome measures that will allow the detection of a clinically relevant effect, such as cognitive tests, caregiver burden, activities of daily living and quality of life [181].
For timing, based on previous studies that showed significant changes in apathy, it is recommended that 6–8 weeks is sufficient time to measure changes in the primary measure of apathy without risking confounding from the deterioration of the background condition [181]. However, a continuation period of 3–6 months is needed to detect measurable differences in the secondary outcome measures, such as functionality and activities of daily living [181]. Future studies should make use of these recommendations when designing their trials.
Additionally, even though apathy is more prevalent in the late stages of dementia, we recommend that future studies include only patients with mild-to-moderate dementia to avoid practical challenges, such as including nursing home residents in the trial [181]. Also, future studies should report effect sizes so that their results can reflect their clinical importance.
From a clinical perspective, the following could be proposed for the treatment of apathy associated with AD. Management begins with further investigations to ensure there are no active medical conditions or medications that could be contributing to the onset or worsening of apathy symptoms. Next, using a scale such as the AES [182] or the NPI-apathy subscale [183] to document the severity of apathy symptoms should be considered as part of a measurement-based system of care. The first attempts at treatment should be non-pharmacological interventions such as multisensory stimulation, music therapy, cognitive stimulation and exercise [184]. Given some evidence of benefit for apathy symptoms, and in keeping with many clinical practice guidelines for AD, if the patient has not already been treated with a cholinesterase inhibitor, this could be the first medication initiated. If the patient has not responded adequately to non-pharmacological interventions and a cholinesterase inhibitor, the next medication to consider would be methylphenidate, based on the positive studies described earlier where apathy was the primary outcome measure. Methylphenidate can be initiated at 5 mg in the morning or 5 mg in the morning and at noon and increased to a maximum of 10 mg twice daily. Besides monitoring for benefit clinically and with an apathy rating scale, changes in blood pressure and heart rate should be documented. In general, maximum benefit for methylphenidate appears to be obtained within 4–8 weeks, a time that can be used by the caregiver to re-try non-pharmacological interventions. Should there be no obvious improvements in apathy after 8 weeks, methylphenidate can be discontinued. While this review has suggested potential benefit from other pharmacological interventions, we believe the evidence is not robust enough to provide clinical guidance. Similarly, the evidence does not allow for any clinical recommendations to be made for the pharmacological treatment of apathy in non-AD neurodegenerative disorders.
In conclusion, based on the relatively large number of studies examining the pharmacological management of apathy in neurodegenerative disorders, it appears clear that researchers have recognized the clinical importance of treating apathy. Unfortunately, most of these studies have significant limitations as we have described, which means it is difficult to make definitive recommendations. Therefore, there is still a need for continued exploration of pharmacological agents and new pharmacological targets to treat apathy in neurodegenerative diseases.
References
Chang F, Patel T, Schulz ME. The “Rising Tide” of dementia in Canada: what does it mean for pharmacists and the people they care for? Can Pharm J (Ott). 2015;148(4):193–9.
Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M. World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. 2015.
Duong S, Patel T, Chang F. Dementia: what pharmacists need to know. Can Pharm J (Ott). 2017;150(2):118–29.
Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73.
Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–54.
Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):8.
Devanand DP, Brockington CD, Moody BJ, Brown RP, Mayeux R, Endicott J, et al. Behavioral syndromes in Alzheimer’s disease. Int Psychogeriatr. 1992;4(4):161–84.
Gonzales-Salvador T, Lyketsos C, Baker A, Roques C, Hovanek L, Steele C, et al. Quality of life of patients with dementia in long-term care. Int J Geriatr Psychiatry. 2000;15(2):181–9.
Nijsten JMH, Leontjevas R, Pat-El R, Smalbrugge M, Koopmans R, Gerritsen DL. Apathy: risk factor for mortality in nursing home patients. J Am Geriatr Soc. 2017;65(10):2182–9.
Akyol MA, Küçükgüçlü Ö, Yener G. Investigation of factors affecting apathy in three major types of dementia. Noro Psikiyatr Ars. 2019;57(2):120–5.
Breitve MH, Brønnick K, Chwiszczuk LJ, Hynninen MJ, Aarsland D, Rongve A. Apathy is associated with faster global cognitive decline and early nursing home admission in dementia with Lewy bodies. Alzheimers Res Ther. 2018;10(1):83.
Bonfanti AB, Etcheverry JL, Persi GG, Zezza H, Starkstein S, Gatto EM. Apathy in Parkinson’s disease. Impairment in quality of life. Medicina (B Aires). 2009;69(2):253–8.
Camacho MBR, Mason SL. Apathy in Huntington’s disease: a review of the current conceptualization. J Alzheimers Dis Parkinsonism. 2018;8:431.
Robert P, Lanctôt KL, Agüera-Ortiz L, Aalten P, Bremond F, Defrancesco M, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71–6.
Miller DS, Robert P, Ereshefsky L, Adler L, Bateman D, Cummings J, et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021. https://doi.org/10.1002/alz.12358.
Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118:54–67.
Le Heron C, Holroyd CB, Salamone J, Husain M. Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry. 2019;90(3):302–12.
Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17(5):411–27.
Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: a systematic review. Am J Geriatr Psychiatry. 2012;20(7):549–64.
Sterne JACSJ, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán MG. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid Med Cell Longev. 2016;2016:9730467.
Pan X, Kaminga AC, Wen SW, Wu X, Acheampong K, Liu A. Dopamine and dopamine receptors in Alzheimer’s disease: a systematic review and network meta-analysis. Front Aging Neurosci. 2019;11:175.
Gratwicke J, Jahanshahi M, Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138(6):1454–76.
Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 2013;24(3):267–78.
Bäckman L, Farde L. Dopamine and cognitive functioning: Brain imaging findings in Huntington’s disease and normal aging. Scand J Psychol. 2001;42(3):287–96.
Murley AG, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain. 2018;141(5):1263–85.
Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain. 1999;122(Pt 8):1449–68.
Court JA, Perry EK. Neurotransmitter abnormalities in vascular dementia. Int Psychogeriatr. 2003;15(Suppl 1):81–7.
Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296–301.
Padala PR, Padala KP, Lensing SY, Ramirez D, Monga V, Bopp MM, et al. Methylphenidate for apathy in community-dwelling older veterans with mild Alzheimer’s disease: a double-blind, randomized placebo-controlled trial. Am J Psychiatry. 2018;175(2):159–68.
Rosenberg PB, Lanctôt KL, Drye LT, Herrmann N, Scherer RW, Bachman DL, et al. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J Clin Psychiatry. 2013;74(8):810–6.
Mintzer J, Lanctôt KL, Scherer RW, Rosenberg PB, Herrmann N, van Dyck CH, et al. Effect of methylphenidate on apathy in patients with Alzheimer disease: the ADMET 2 randomized clinical trial. JAMA Neurol. 2021;78(11):1324–1332.
Frakey LL, Salloway S, Buelow M, Malloy P. A randomized, double-blind, placebo-controlled trial of modafinil for the treatment of apathy in individuals with mild-to-moderate Alzheimer’s disease. J Clin Psychiatry. 2012;73(6):796–801.
Nave S, Doody RS, Boada M, Grimmer T, Savola J-M, Delmar P, et al. Sembragiline in moderate Alzheimer’s disease: results of a randomized, double-blind, placebo-controlled phase II Trial (MAyflOwer RoAD). J Alzheimers Dis. 2017;58(4):1217–28.
Pollock BG, Mulsant BH, Rosen J, Sweet RA, Mazumdar S, Bharucha A, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(3):460–5.
De Deyn P, Jeste DV, Swanink R, Kostic D, Breder C, Carson WH, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. J Clin Psychopharmacol. 2005;25(5):463–7.
De Deyn PP, Carrasco MM, Deberdt W, Jeandel C, Hay DP, Feldman PD, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19(2):115–26.
Yanofski J. The dopamine dilemma: using stimulants and antipsychotics concurrently. Psychiatry (Edgmont). 2010;7(6):18–23.
Moreau C, Delval A, Defebvre L, Dujardin K, Duhamel A, Petyt G, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11(7):589–96.
Hauser RA, Slawek J, Barone P, Dohin E, Surmann E, Asgharnejad M, et al. Evaluation of rotigotine transdermal patch for the treatment of apathy and motor symptoms in Parkinson’s disease. BMC Neurol. 2016;16:90.
Castrioto A, Thobois S, Anheim M, Quesada JL, Lhommée E, Klinger H, et al. A randomized controlled double-blind study of rotigotine on neuropsychiatric symptoms in de novo PD. NPJ Parkinsons Dis. 2020;6(1):41.
Barone P, Santangelo G, Morgante L, Onofrj M, Meco G, Abbruzzese G, et al. A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson’s disease patients. Eur J Neurol. 2015;22(8):1184–91.
Scherer RW, Drye L, Mintzer J, Lanctôt K, Rosenberg P, Herrmann N, et al. The Apathy in Dementia Methylphenidate Trial 2 (ADMET 2): study protocol for a randomized controlled trial. Trials. 2018;19(1):46.
Aripiprazole in the treatment of patients with psychosis associated with dementia of Alzheimer's type. https://ClinicalTrials.gov/show/NCT01438060.
Devos D, Krystkowiak P, Clement F, Dujardin K, Cottencin O, Waucquier N, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(5):470–5.
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–15.
Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8(11):1703–18.
Bohnen NI, Albin RL. The cholinergic system and Parkinson disease. Behav Brain Res. 2011;221(2):564–73.
Müller MLTM, Bohnen NI. Cholinergic dysfunction in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(9):377.
D’Souza GX, Waldvogel HJ. Targeting the cholinergic system to develop a novel therapy for Huntington’s disease. J Huntington’s Dis. 2016;5:333–42.
Perry EK, Irving D, Kerwin JM, McKeith IG, Thompson P, Collerton D, et al. Cholinergic transmitter and neurotrophic activities in Lewy body dementia: similarity to Parkinson’s and distinction from Alzheimer disease. Alzheimer Dis Assoc Disord. 1993;7(2):69–79.
Herrmann N, Rabheru K, Wang J, Binder C. Galantamine treatment of problematic behavior in Alzheimer disease: post-hoc analysis of pooled data from three large trials. Am J Geriatr Psychiatry. 2005;13(6):527–34.
Cummings JL, Schneider L, Tariot PN, Kershaw PR, Yuan W. Reduction of behavioral disturbances and caregiver distress by galantamine in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(3):532–8.
Kaufer D. Beyond the cholinergic hypothesis: the effect of metrifonate and other cholinesterase inhibitors on neuropsychiatric symptoms in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9(Suppl 2):8–14.
Morris JC, Cyrus PA, Orazem J, Mas J, Bieber F, Ruzicka BB, et al. Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology. 1998;50(5):1222–30.
Dubois B, McKeith I, Orgogozo JM, Collins O, Meulien D. A multicentre, randomized, double-blind, placebo-controlled study to evaluate the efficacy, tolerability and safety of two doses of metrifonate in patients with mild-to-moderate Alzheimer’s disease: the MALT study. Int J Geriatr Psychiatry. 1999;14(11):973–82.
Raskind MA, Cyrus PA, Ruzicka BB, Gulanski BI. The effects of metrifonate on the cognitive, behavioral, and functional performance of Alzheimer’s disease patients. Metrifonate Study Group. J Clin Psychiatry. 1999;60(5):318–25.
Cummings JL, Nadel A, Masterman D, Cyrus PA. Efficacy of metrifonate in improving the psychiatric and behavioral disturbances of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2001;14(2):101–8.
Tariot PN, Cummings JL, Katz IR, Mintzer J, Perdomo CA, Schwam EM, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc. 2001;49(12):1590–9.
Gauthier S, Feldman H, Hecker J, Vellas B, Ames D, Subbiah P, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr. 2002;14(4):389–404.
Gauthier S, Feldman H, Hecker J, Vellas B, Emir B, Subbiah P. Functional, cognitive and behavioral effects of donepezil in patients with moderate Alzheimer’s disease. Curr Med Res Opin. 2002;18(6):347–54.
Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, Langley A, et al. The efficacy of donepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology. 2004;63(2):214–9.
Feldman H, Gauthier S, Hecker J, Vellas B, Xu Y, Ieni JR, et al. Efficacy and safety of donepezil in patients with more severe Alzheimer’s disease: a subgroup analysis from a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2005;20(6):559–69.
Seltzer B, Zolnouni P, Nunez M, Goldman R, Kumar D, Ieni J, et al. Efficacy of donepezil in early-stage Alzheimer disease: a randomized placebo-controlled trial. Arch Neurol. 2004;61(12):1852–6.
Rea R, Carotenuto A, Traini E, Fasanaro AM, Manzo V, Amenta F. Apathy treatment in Alzheimer’s disease: interim results of the ASCOMALVA trial. J Alzheimers Dis. 2015;48(2):377–83.
Åhlin A, Nybäck H, Junthe T, Öhman G, Nordgren I. Tetrahydroaminoacridine in Alzheimer’s dementia: clinical and biochemical results of a double-blind crossover trial. Hum Psychopharmacol Clin Exp. 1991;6(2):109–18.
Devos D, Moreau C, Maltête D, Lefaucheur R, Kreisler A, Eusebio A, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatry. 2014;85(6):668–74.
McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–6.
Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359(9314):1283–90.
Carotenuto A, Rea R, Traini E, Fasanaro AM, Ricci G, Manzo V, et al. The effect of the association between donepezil and choline alphoscerate on behavioral disturbances in Alzheimer’s disease: interim results of the ASCOMALVA trial. J Alzheimers Dis. 2017;56(2):805–15.
Švob Štrac D, Pivac N, Mück-Šeler D. The serotonergic system and cognitive function. Transl Neurosci. 2016;7(1):35–49.
Šimić G, Babić Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milošević N, et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol. 2017;151:101–38.
Huot P, Fox SH, Brotchie JM. The serotonergic system in Parkinson’s disease. Prog Neurobiol. 2011;95(2):163–212.
Pla P, Orvoen S, Saudou F, David DJ, Humbert S. Mood disorders in Huntington’s disease: from behavior to cellular and molecular mechanisms. Front Behav Neurosci. 2014;8:135.
van der Zande JJ, Joling M, Happach IG, Vriend C, Scheltens P, Booij J, et al. Serotonergic deficits in dementia with Lewy bodies with concomitant Alzheimer’s disease pathology: an (123)I-FP-CIT SPECT study. Neuroimage Clin. 2020;25:102062.
Lawlor BA, Sunderland T, Mellow AM, Hill JL, Molchan SE, Murphy DL. Hyperresponsivity to the serotonin agonist m-chlorophenylpiperazine in Alzheimer’s disease. A controlled study. Arch Gen Psychiatry. 1989;46(6):542–9.
Lanctôt KL, Herrmann N, van Reekum R, Eryavec G, Naranjo CA. Gender, aggression and serotonergic function are associated with response to sertraline for behavioral disturbances in Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17(6):531–41.
Zhou T, Wang J, Xin C, Kong L, Wang C. Effect of memantine combined with citalopram on cognition of BPSD and moderate Alzheimer’s disease: a clinical trial. Exp Ther Med. 2019;17(3):1625–30.
Leonpacher AK, Peters ME, Drye LT, Makino KM, Newell JA, Devanand DP, et al. Effects of citalopram on neuropsychiatric symptoms in Alzheimer’s dementia: evidence from the CitAD study. Am J Psychiatry. 2016;173(5):473–80.
Nyth AL, Gottfries CG. The clinical efficacy of citalopram in treatment of emotional disturbances in dementia disorders. A Nordic multicentre study. Br J Psychiatry. 1990;157:894–901.
Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17(4):355–9.
Ranjbar-Slamloo Y, Fazlali Z. Dopamine and noradrenaline in the brain; overlapping or dissociate functions? Front Mol Neurosci. 2020;12:334.
Gannon M, Wang Q. Complex noradrenergic dysfunction in Alzheimer’s disease: low norepinephrine input is not always to blame. Brain Res. 2019;1702:12–6.
Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C. The noradrenergic system in Parkinson’s disease. Front Pharmacol. 2020;11:435.
Spokes EGS. Neurochemical alterations in Huntington’s chorea: a study of post-mortem brain tissue. Brain. 1980;103(1):179–210.
Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26(2):467–78.
van Dyck CH, Arnsten AFT, Padala PR, Brawman-Mintzer O, Lerner AJ, Porsteinsson AP, et al. Neurobiologic rationale for treatment of apathy in alzheimer’s disease with methylphenidate. Am J Geriatr Psychiatry. 2021;29(1):51–62.
Maier F, Spottke A, Bach JP, Bartels C, Buerger K, Dodel R, et al. Bupropion for the treatment of apathy in Alzheimer disease: a randomized clinical trial. JAMA Netw Open. 2020;3(5):e206027.
Gelderblom H, Wüstenberg T, McLean T, Mütze L, Fischer W, Saft C, et al. Bupropion for the treatment of apathy in Huntington’s disease: a multicenter, randomised, double-blind, placebo-controlled, prospective crossover trial. PLoS ONE. 2017;12(3):e0173872.
Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis. 2015;30(2):367–79.
Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H. Implications of GABAergic neurotransmission in Alzheimer’s disease. Front Aging Neurosci. 2016;8:31.
de Jong PJ, Lakke JP, Teelken AW. CSF GABA levels in Parkinson’s disease. Adv Neurol. 1984;40:427–30.
Tosca P, Canevari L, Di Paolo E, Ferrari R, Verzé S, Zerbi F, et al. Glutamate and GABA levels in CSF from patients affected by dementia and olivo-ponto-cerebellar atrophy. Acta Neurol Scand. 1992;85(6):430–5.
Reynolds GP, Pearson SJ, Heathfield KWG. Dementia in Huntington’s disease is associated with neurochemical deficits in the caudate nucleus, not the cerebral cortex. Neurosci Lett. 1990;113(1):95–100.
Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66(1):17–22.
Lanctôt KL, Herrmann N, Rothenburg L, Eryavec G. Behavioral correlates of GABAergic disruption in Alzheimer’s disease. Int Psychogeriatr. 2007;19(1):151–8.
Sival RC, Haffmans PM, Jansen PA, Duursma SA, Eikelenboom P. Sodium valproate in the treatment of aggressive behavior in patients with dementia—a randomized placebo controlled clinical trial. Int J Geriatr Psychiatry. 2002;17(6):579–85.
Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna). 2014;121(8):799–817.
Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57:1041–8.
Madeira C, Vargas-Lopes C, Brandão CO, Reis T, Laks J, Panizzutti R, et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer’s disease and depression. Front Psychiatry. 2018;9:561.
Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson’s disease: the role of glial cells. J Pharmacol Sci. 2020;144(3):151–64.
Albasanz JL, Dalfó E, Ferrer I, Martín M. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol Dis. 2005;20(3):685–93.
Araki T, Wake R, Miyaoka T, Kawakami K, Nagahama M, Furuya M, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s disease patients and its relationship with cerebral blood flow in the prefrontal area. Int J Geriatr Psychiatry. 2014;29(9):881–9.
Gauthier S, Wirth Y, Möbius HJ. Effects of memantine on behavioural symptoms in Alzheimer’s disease patients: an analysis of the Neuropsychiatric Inventory (NPI) data of two randomised, controlled studies. Int J Geriatr Psychiatry. 2005;20(5):459–64.
Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41.
Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317–24.
Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine). Int J Geriatr Psychiatry. 1999;14(2):135–46.
Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–241.
Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15(1):65–74.
Sedeyn JC, Wu H, Hobbs RD, Levin EC, Nagele RG, Venkataraman V. Histamine induces Alzheimer’s disease-like blood brain barrier breach and local cellular responses in mouse brain organotypic cultures. BioMed Res Int. 2015;2015:937148.
Zlomuzica A, Dere D, Binder S, De Souza Silva MA, Huston JP, Dere E. Neuronal histamine and cognitive symptoms in Alzheimer’s disease. Neuropharmacology. 2016;106:135–45.
Rinne JO, Anichtchik OV, Eriksson KS, Kaslin J, Tuomisto L, Kalimo H, et al. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J Neurochem. 2002;81(5):954–60.
van Wamelen DJ, Shan L, Aziz NA, Anink JJ, Bao AM, Roos RA, et al. Functional increase of brain histaminergic signaling in Huntington’s disease. Brain Pathol. 2011;21(4):419–27.
Naddafi F, Mirshafiey A. The neglected role of histamine in Alzheimer’s disease. Am J Alzheimer’s Dis Other Dement. 2013;28(4):327–36.
Benarroch EE, Schmeichel AM, Parisi JE, Low PA. Histaminergic tuberomammillary neuron loss in multiple system atrophy and dementia with Lewy bodies. Mov Disord. 2015;30(8):1133–9.
Stasiak A, Mussur M, Unzeta M, Łażewska D, Kiec-Kononowicz K, Fogel WA. The central histamine level in rat model of vascular dementia. J Physiol Pharmacol. 2011;62:549–58.
Verdejo-García A, Rivas-Pérez C, López-Torrecillas F, Pérez-García M. Differential impact of severity of drug use on frontal behavioral symptoms. Addict Behav. 2006;31(8):1373–82.
Yoshizawa M, Tashiro M, Fukudo S, Yanai K, Utsumi A, Kano M, et al. Increased brain histamine H1 receptor binding in patients with anorexia nervosa. Biol Psychiatry (1969). 2009;65(4):329–35.
Horner WE, Johnson DE, Schmidt AW, Rollema H. Methylphenidate and atomoxetine increase histamine release in rat prefrontal cortex. Eur J Pharmacol. 2007;558(1):96–7.
Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, Williams AJ. The hypocretin/orexin system. J R Soc Med. 2002;95(5):227–30.
Um YH, Lim HK. Orexin and Alzheimer’s disease: a new perspective. Psychiatry Investig. 2020;17(7):621–6.
Liu C, Xue Y, Liu M-F, Wang Y, Chen L. Orexin and Parkinson’s disease: a protective neuropeptide with therapeutic potential. Neurochem Int. 2020;138:104754.
Petersén Å, Gil J, Maat-Schieman MLC, Björkqvist M, Tanila H, Araújo IM, et al. Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14(1):39–47.
Çoban A, Bilgiç B, Lohmann E, Küçükali Cİ, Benbir G, Karadeniz D, et al. Reduced orexin—a levels in frontotemporal dementia: possible association with sleep disturbance. Am J Alzheimer’s Dis Other Dement. 2013;28(6):606–11.
Song J, Kim E, Kim C-H, Song H-T, Lee JE. The role of orexin in post-stroke inflammation, cognitive decline, and depression. Mol Brain. 2015;8:16.
Lessig S, Ubhi K, Galasko D, Adame A, Pham E, Remidios K, et al. Reduced hypocretin (orexin) levels in dementia with Lewy bodies. NeuroReport. 2010;21(11):756–60.
Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–53.
Holzer P, Reichmann F, Farzi A, Neuropeptide Y. peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46(6):261–74.
Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7(1):37–49.
Serrenho D, Santos SD, Carvalho AL. The role of ghrelin in regulating synaptic function and plasticity of feeding-associated circuits. Front Cell Neurosci. 2019;13:205.
Pedrini S, Gupta VB, Hone E, Doecke J, O’Bryant S, James I, et al. A blood-based biomarker panel indicates IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in an AD cohort. Sci Rep. 2017;7(1):14057.
Shi L, Du X, Jiang H, Xie J. Ghrelin and neurodegenerative disorders—a review. Mol Neurobiol. 2017;54(2):1144–55.
Yang D, Zhao D, Ali Shah SZ, Wu W, Lai M, Zhang X, et al. The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol. 2019;10:1155.
Hornsby AKE, Buntwal L, Carisi MC, Santos VV, Johnston F, Roberts LD, et al. Unacylated-ghrelin impairs hippocampal neurogenesis and memory in mice and is altered in parkinson’s dementia in humans. Cell Rep Med. 2020;1(7):100120.
Cong W, Cai H, Wang R, Daimon CM, Maudsley S, Raber K, et al. Altered hypothalamic protein expression in a rat model of Huntington’s disease. PLoS ONE. 2012;7(10):e47240.
Stoyanova II. Ghrelin: a link between ageing, metabolism and neurodegenerative disorders. Neurobiol Dis. 2014;72:72–83.
Yamada C, Mogami S, Kanno H, Hattori T. Peptide YY causes apathy-like behavior via the dopamine D2 receptor in repeated water-immersed mice. Mol Neurobiol. 2018;55(9):7555–66.
Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7(3):238–45.
Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol. 2014;119:257–307.
Anisur R. The role of adenosine in Alzheimers disease. Curr Neuropharmacol. 2009;7(3):207–16.
Nazario LR, da Silva RS, Bonan CD. Targeting adenosine signaling in Parkinson’s disease: from pharmacological to non-pharmacological approaches. Front Neurosci. 2017;11:658.
Uchida S, Kadowaki-Horita T, Kanda T. Effects of the adenosine A2A receptor antagonist on cognitive dysfunction in Parkinson’s disease. Int Rev Neurobiol. 2014;119:169–89.
Varani K, Rigamonti D, Sipione S, Camurri A, Borea PA, Cattabeni F, et al. Aberrant amplification of A(2A) receptor signaling in striatal cells expressing mutant huntingtin. Faseb j. 2001;15(7):1245–7.
Tarditi A, Camurri A, Varani K, Borea PA, Woodman B, Bates G, et al. Early and transient alteration of adenosine A2A receptor signaling in a mouse model of Huntington disease. Neurobiol Dis. 2006;23(1):44–53.
Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808(5):1380–99.
Martire A, Pepponi R, Domenici MR, Ferrante A, Chiodi V, Popoli P. BDNF prevents NMDA-induced toxicity in models of Huntington’s disease: the effects are genotype specific and adenosine A2A receptor is involved. J Neurochem. 2013;125(2):225–35.
Tyebji S, Saavedra A, Canas PM, Pliassova A, Delgado-García JM, Alberch J, et al. Hyperactivation of D1 and A2A receptors contributes to cognitive dysfunction in Huntington’s disease. Neurobiol Dis. 2015;74:41–57.
Carvalho K, Faivre E, Pietrowski MJ, Marques X, Gomez-Murcia V, Deleau A, et al. Exacerbation of C1q dysregulation, synaptic loss and memory deficits in tau pathology linked to neuronal adenosine A2A receptor. Brain. 2019;142(11):3636–54.
Gussago C, Arosio B, Casati M, Ferri E, Gualandris F, Tedone E, et al. Different adenosine A 2A receptor expression in peripheral cells from elderly patients with vascular dementia and Alzheimer’s disease. J Alzheimers Dis. 2014;40:45–9.
Garcia-Esparcia P, López-González I, Grau-Rivera O, García-Garrido MF, Konetti A, Llorens F, et al. Dementia with lewy bodies: molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front Neurol. 2017;8:89.
Nagayama H, Kano O, Murakami H, Ono K, Hamada M, Toda T, et al. Effect of istradefylline on mood disorders in Parkinson’s disease. J Neurol Sci. 2019;396:78–83.
Orr AG, Lo I, Schumacher H, Ho K, Gill M, Guo W, et al. Istradefylline reduces memory deficits in aging mice with amyloid pathology. Neurobiol Dis. 2018;110:29–36.
Li W, Silva HB, Real J, Wang Y-M, Rial D, Li P, et al. Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol Dis. 2015;79:70–80.
Rosenberg PB, Lanctôt KL, Herrmann N, Mintzer JE, Porsteinsson AP, Sun X, et al. Changes in neuropsychiatric inventory associated with semagacestat treatment of Alzheimer’s disease. J Alzheimers Dis. 2016;54(1):373–81.
Trzepacz PT, Cummings J, Konechnik T, Forrester TD, Chang C, Dennehy EB, et al. Mibampator (LY451395) randomized clinical trial for agitation/aggression in Alzheimer’s disease. Int Psychogeriatr. 2013;25(5):707–19.
Kim SY, Choi SH, Rollema H, Schwam EM, McRae T, Dubrava S, et al. Phase II crossover trial of varenicline in mild-to-moderate Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37(3–4):232–45.
Ban TA, Morey L, Aguglia E, Azzarelli O, Balsano F, Marigliano V, et al. Nimodipine in the treatment of old age dementias. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(4):525–51.
Scripnikov A, Khomenko A, Napryeyenko O. Effects of Ginkgo biloba extract EGb 761 on neuropsychiatric symptoms of dementia: findings from a randomised controlled trial. Wien Med Wochenschr. 2007;157(13–14):295–300.
Bachinskaya N, Hoerr R, Ihl R. Alleviating neuropsychiatric symptoms in dementia: the effects of Ginkgo biloba extract EGb 761. Findings from a randomized controlled trial. Neuropsychiatr Dis Treat. 2011;7:209–15.
Bayer A, Bokonjic R, Booya N. European pentoxifylline multi-infarct dementia study. Eur Neurol. 1996;36(5):315–21.
Finger EC, MacKinley J, Blair M, Oliver LD, Jesso S, Tartaglia MC, et al. Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology. 2015;84(2):174–81.
Callegari I, Mattei C, Benassi F, Krueger F, Grafman J, Yaldizli Ö, et al. Agomelatine Improves Apathy in Frontotemporal Dementia. Neurodegener Dis. 2016;16(5–6):352–6.
Ruthirakuhan MT, Herrmann N, Abraham EH, Chan S, Lanctôt KL. Pharmacological interventions for apathy in Alzheimer's disease. Cochrane Database Syst Rev. 2018;5(5):CD012197.
Charach G, Karniel E, Grosskopf I, Rabinovich A, Charach L. Methylphenidate has mild hyperglycemic and hypokalemia effects and increases leukocyte and neutrophil counts. Medicine (Baltimore). 2020;99(27):e20931-e.
Shin J-Y, Roughead EE, Park B-J, Pratt NL. Cardiovascular safety of methylphenidate among children and young people with attention-deficit/hyperactivity disorder (ADHD): nationwide self controlled case series study. BMJ. 2016;353:i2550.
Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry. 2008;23(5):537–45.
Ohbe H, Jo T, Matsui H, Fushimi K, Yasunaga H. Cholinergic crisis caused by cholinesterase inhibitors: a retrospective nationwide database study. J Med Toxicol. 2018;14(3):237–41.
Barthold D, Joyce G, Ferido P, Drabo EF, Marcum ZA, Gray SL, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimer’s Dis JAD. 2020;76(2):579–89.
Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7(5):229–45.
Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–91.
Qirjazi E, McArthur E, Nash DM, Dixon SN, Weir MA, Vasudev A, et al. Risk of ventricular arrhythmia with citalopram and escitalopram: a population-based study. PLoS ONE. 2016;11(8):e0160768-e.
Chen F, Jin L, Nie Z. Safety and efficacy of rotigotine for treating Parkinson’s disease: a meta-analysis of randomised controlled trials. J Pharm Pharm Sci. 2017;20:285–94.
Carbone F, Djamshidian A, Seppi K, Poewe W. Apomorphine for parkinson’s disease: efficacy and safety of current and new formulations. CNS Drugs. 2019;33(9):905–18.
Ohsawa M, Tanaka Y, Ehara Y, Makita S, Onaka K. A possibility of simultaneous treatment with the multicomponent drug, Ninjin’yoeito, for anorexia, apathy, and cognitive dysfunction in frail Alzheimer’s disease patients: an open-label pilot study. J Alzheimers Dis Rep. 2017;1(1):229–35.
Van Reekum R, Bayley M, Garner S, Burke IM, Fawcett S, Hart A, et al. N of 1 study: amantadine for the amotivational syndrome in a patient with traumatic brain injury. Brain Inj. 1995;9(1):49–53.
Postma JU, Van Tilburg W. Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc. 1975;23(5):212–5.
Theleritis CG, Siarkos KT, Politis AM. Unmet needs in pharmacological treatment of apathy in Alzheimer’s disease: a systematic review. Front Pharmacol. 2019;10:1108.
Matsuzono K, Hishikawa N, Ohta Y, Yamashita T, Deguchi K, Nakano Y, et al. Combination therapy of cholinesterase inhibitor (donepezil or galantamine) plus memantine in the okayama memantine study. J Alzheimers Dis. 2015;45(3):771–80.
Lanctôt KL, Agüera-Ortiz L, Brodaty H, Francis PT, Geda YE, Ismail Z, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement. 2017;13(1):84–100.
Mohammad D, Ellis C, Rau A, Rosenberg PB, Mintzer J, Ruthirakuhan M, et al. Psychometric properties of apathy scales in dementia: a systematic review. J Alzheimers Dis. 2018;66(3):1065–82.
Cummings J, Friedman JH, Garibaldi G, Jones M, Macfadden W, Marsh L, et al. Apathy in neurodegenerative diseases: recommendations on the design of clinical trials. J Geriatr Psychiatry Neurol. 2015;28(3):159–73.
Glenn M. The Apathy Evaluation Scale. The center for outcome measurement in brain injury. 2005. http://www.tbims.org/combi/aes.
Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):S10–6.
Cai Y, Li L, Xu C, Wang Z. The effectiveness of non-pharmacological interventions on apathy in patients with dementia: a systematic review of systematic reviews. Worldviews Evid Based Nurs. 2020;17(4):311–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist with the preparation of this review.
Conflict of interest
Krista L. Lanctôt has received grants from the Alzheimer’s Association, the Alzheimer’s Drug Discovery Foundation, the Canadian Institutes of Health Research, the National Institutes of Aging, and the Weston Brain Institute; consulting fees from BioXcel, Cerevel, Eisai, GW Pharma, ICG Pharma, Kondor Pharma, Novo Nordisk, Otsuka, and Praxis; and stock options from Highmark Interactive outside the submitted work. Nathan Herrmann, Laiba Azhar, Raphael W. Kusumo, and Giovanni Marotta have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent
Not applicable.
Author contributions
The idea for the review was suggested by Nathan Herrmann. Drafts of the manuscript and literature searches were written and performed by Laiba Azhar, Nathan Herrmann, Krista L. Lanctôt, and Raphael W. Kusumo. All authors reviewed and commented on all versions of the manuscript, approved the final manuscript, and agree to be accountable for the manuscript.
Appendix
Appendix
Quality assessment risk of bias summary
Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Binding of participant and personnel (performance bias) | Binding of outcome assessments (detection bias) | Incomplete outcome data (attrition bias) | Selective outcome reporting (reporting bias) | Other bias |
|---|---|---|---|---|---|---|---|
Åhlin et al. [66] | + | + | + | + | + | + | + |
Araki et al. [103] | + | + | − | ? | + | + | + |
Bachinskaya et al. [159] | + | + | + | + | + | + | + |
Ban et al. [157] | + | + | + | + | + | + | + |
Barone et al. [42] | + | + | + | + | + | − | + |
Bayer et al. [160] | + | + | + | + | + | + | + |
Callegari et al. [162] | + | + | + | + | + | + | ? |
Castrioto et al. [41] | + | + | + | + | + | + | + |
Cummings et al. [58] | ? | ? | ? | ? | + | + | + |
Cummings et al. [53] | + | + | + | + | + | + | ? |
De Deyn et al. [37] | + | + | + | + | + | + | ? |
De Deyn et al. [36] | + | + | + | + | + | − | ? |
Devos et al. [67] | + | + | + | + | + | + | + |
Dubois et al. [56] | + | + | + | + | + | + | + |
Erkinjuntti et al. [69] | + | + | + | + | + | + | + |
Feldman et al. [63] | + | + | + | + | + | + | + |
Finger et al. [161] | + | + | + | + | + | + | + |
Frakey et al. [33] | + | + | + | + | + | + | + |
Gauthier et al. [61] | + | + | + | + | + | + | + |
Gauthier et al. [60] | + | + | + | + | + | + | + |
Gauthier et al. [104] | ? | ? | ? | ? | + | + | ? |
Gelderblom et al. [89] | + | + | + | + | + | + | + |
Hauser et al. [40] | + | + | + | + | + | + | + |
Herrmann et al. [52] | + | + | + | + | + | + | ? |
Herrmann et al. [29] | + | + | + | + | + | + | ? |
Holmes et al. [62] | + | + | + | + | + | + | + |
Kaufer et al. [54] | + | + | + | + | + | + | ? |
Kim et al. [156] | + | + | + | + | + | + | + |
Lanctôt et al. [77] | + | + | + | + | + | + | + |
Lawlor et al. [76] | + | + | + | + | + | + | ? |
Lebert et al. [81] | + | + | + | + | + | + | + |
Leonpacher et al. [79] | + | + | + | + | + | − | ? |
Maier et al. [88] | + | + | + | + | + | + | + |
McKeith et al. [68] | + | + | + | + | + | + | + |
Mintzer et al. [32] | + | + | + | + | + | + | + |
Moreau et al. [39] | + | + | + | + | + | + | + |
Morris et al. [55] | + | + | + | + | + | + | + |
Nave et al. [34] | + | + | + | + | + | + | + |
Nyth and Gottfries [80] | + | + | + | + | + | + | + |
Padala et al. [30] | + | + | + | + | + | + | + |
Pollock et al. [35] | + | + | + | + | + | − | ? |
Raskind et al. [57] | + | + | + | + | + | + | + |
Rea et al. [65] | + | + | + | + | − | − | + |
Rosenberg et al. [31] | + | + | + | + | + | + | + |
Rosenberg et al. [154] | + | + | + | + | + | + | + |
Scripnikov et al. [158] | + | + | + | + | − | − | ? |
Seltzer et al. [64] | + | + | + | + | + | + | + |
Sival et al. [97] | + | + | + | + | + | + | + |
Tariot et al. [59] | + | + | + | + | + | + | + |
Trzepacz et al. [155] | + | + | + | + | + | + | + |
Winblad and Poritis [107] | + | + | + | + | + | + | + |
Zhou et al. [78] | + | + | + | + | + | + | + |
Rights and permissions
About this article
Cite this article
Azhar, L., Kusumo, R.W., Marotta, G. et al. Pharmacological Management of Apathy in Dementia. CNS Drugs 36, 143–165 (2022). https://doi.org/10.1007/s40263-021-00883-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00883-0