Abstract
Apathy is a neurocognitive syndrome of reduced goal-directed behaviour and is an important cause of disability in neurodegenerative disorders. Frontal–subcortical dysfunction is thought to be important in apathy, but the contribution of individual brain regions to different aspects of the apathy syndrome is poorly understood. We aimed to test the hypotheses that apathy in two distinct neurodegenerative disorders would be associated with frontal lobe atrophy and that reduced initiative and emotional blunting would be associated with distinct patterns of atrophy in functionally relevant brain areas. Seventeen patients with progressive supranuclear palsy (PSP) and 17 patients with Alzheimer’s disease (AD) underwent structural MRI scanning at 3 T to provide data for voxel based morphometric analysis. Apathy was defined using Robert’s 2009 diagnostic criteria and specific symptoms were assessed with the Apathy Inventory. Patients with and without apathy were matched for important demographic and clinical characteristics. Apathy was associated with atrophy of the ventromedial orbitofrontal cortex and left insula in both AD and PSP. Reduced initiative was specifically associated with atrophy of the anterior cingulate and ventrolateral orbitofrontal cortex whilst emotional blunting was specifically associated with atrophy of the left insula. These findings provide further support for the role of medial frontal regions and insular cortex in apathy and suggest that behavioural and emotional aspects of the apathy syndrome may have distinct neuroanatomical bases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apathy is a neurocognitive syndrome of reduced goal-directed behaviour [1]. It is common in many neurological disorders, with rates of approximately 20–50 % in stroke, 50 % after traumatic brain injury, 30 % in Parkinson’s disease (PD), and 50 % or more in Alzheimer’s disease (AD), and progressive supranuclear palsy (PSP) [2]. Apathy causes functional disability, is an important predictor of care-giver distress and results in increased healthcare costs [3, 4]. At present, apathy remains difficult to manage, with limited evidence for the efficacy of proposed psychosocial or pharmacological interventions [5]. Clinical definitions of apathy draw a distinction between behavioural, cognitive and emotional symptom domains, reflecting disruption of distinct aspects of goal directed behaviour [6]. The behavioural aspect refers to reduced initiative, effort or perseverance. The cognitive aspect refers to lack of interest, while the emotional domain refers to reduced emotional responsiveness to positive or negative events. Clues to the possible substrate or the behavioural and emotional aspects of apathy may be sought from the anatomical correlates of volitional and emotional processing in healthy individuals where different brain structures are implicated in these processes [7, 8]. Functional imaging studies of volitional, willed or self-initiated behaviour commonly activate a frontal (or fronto–parietal) network including areas of lateral prefrontal cortex, supplementary motor area and medial frontal cortex [7, 9]. The perception, experience and expression of emotion appear to be served by a widespread network or set of networks involving cortical, basal ganglia, cerebellar and limbic regions, typically dissociable between the different types of basic emotion [8, 10]. The substrate of normal process associated with the apathy cognitive domain (‘interest’) is less clearly defined from previous imaging study in healthy individuals.
Consistent with the evidence from normal brain-behaviour associations, current evidence implicates disruption of frontal–subcortical circuits in the pathophysiology of apathy [11], although the contribution of specific brain regions to individual apathy-related symptoms is poorly understood. The few studies to use structural brain imaging in clinical groups to explore the neuroanatomical correlates of apathy report association with volume loss in regions including the frontal pole, orbitofrontal cortex, anterior cingulate cortex, and dorsolateral prefrontal cortex, e.g., [12–19]. Results are not conclusive, however, as some studies failed to adequately control for multiple comparisons in the analysis [16, 17], to control for important potential confounders such as dementia severity [15, 18], or to ensure that apathy was sufficiently evident in the sample to allow a robust and reliable assessment [14]. To date, there have been no reports of structural brain imaging studies examining specific apathy related symptoms in patients with neurodegenerative disease.
We used voxel-based morphometry (VBM) to study the anatomical basis of apathy in patients with two contrasting neurodegenerative disorders, AD and PSP, chosen as exemplars of diseases in which apathy is common, but with distinct distributions of brain pathology. The aims of the study were two-fold. First, to identify any regions of volume change associated with the presence of syndromal apathy common across AD and PSP, in addition to any apathy-related regional change specific to the individual disorders. Second, to identify any common and/or disease-specific regions of volume change associated with specific apathy related symptoms, independent of the presence or absence of syndromal apathy. VBM is a whole-brain rather than a region-of-interest approach, so we made no detailed predictions beyond general expectations that apathetic patients would show greater frontal lobe volume loss than non-apathetic patients, regardless of primary diagnosis (AD or PSP). In addition, we expected to show that reduced behavioural initiative and emotional blunting would be associated with distinct patterns of atrophy related to the underlying substrate of the networks serving normal function in these domains. The study did not consider the pathological correlates of the ‘cognitive’ symptoms of apathy.
Methods
Sample
The study included 17 patients with PSP and 17 patients with AD. Participants with moderate to severe depressive symptoms (Geriatric Depression Scale score >7), a significant neurological or psychiatric history (other than PSP/AD) or current severe medical illnesses were excluded. Patients with PSP were recruited via referrals from local consultant neurologists. A full clinical history and examination was performed to ensure that all patients conformed to NINDS-SPSP criteria for the diagnosis of possible or probable PSP [20]. Both levels of diagnosis have been shown to have high specificity in pathological validation studies (>93 %) and revised labels ‘clinically probable’ and ‘clinically definite’ have been suggested to reflect this low risk of false positive diagnosis [21, 22]. Patients with AD were recruited from memory clinics at South London and Maudsley NHS Trust and University Hospital Lewisham NHS Trust. All patients conformed to NINCDS-ADRDA criteria for probable Alzheimer’s disease [23].
The study was approved by King’s College Hospital Research Ethics Committee and was therefore performed with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Clinical assessments and assessment of apathy
In addition to a clinical history and examination, standard instruments were used to assess global cognitive function (standardised Mini Mental State Examination (sMMSE); [24]) and depressive symptoms (Geriatric Depression Scale, 15 item version (GDS-15); [25]). For patients with PSP, the severity of motor symptoms was assessed using the Unified Parkinson’s Disease Rating Scale, Part 3 (UPDRS-III) [26].
There is currently no accepted “gold standard” for the diagnosis or measurement of apathy and all existing rating scales have significant limitations [27, 28]. In this study, the presence or absence of apathy was defined using the diagnostic criteria suggested by Robert [29], recently validated [30]. Unlike the original definition offered by Marin [6] which requires the presence of symptoms in all three domains, the Robert criteria require the presence of only two. In the present study, patients displaying no symptoms, or a symptom in a single domain were classed as ‘non-apathetic’ for the between group comparisons. Diagnosis was based on clinical interviews with the patient and caregiver, including the Apathy Evaluation Scale (clinician rated version) [31] and Neuropsychiatric Inventory [32]. In addition, we used the Apathy Inventory, a structured interview with the carer that provided separate clinician-based assessments of the presence or absence of behavioural and emotional aspects of apathy [33].
MRI Scanning
Data were acquired on a 3T HDx scanner (General Electric Medical Systems, Wakashua, USA), equipped with an 8-channel receive coil. A T1 weighted, inversion recovery prepared 3D spoiled grass gradient recalled scan was acquired to allow volumetric and morphological analysis of brain structures. This scan was acquired with an echo time (TE) of 2.8 ms, a repetition time (TR) of 6.7 ms, a preparation time (TI) of 450 ms and a flip angle 20 °. The resulting images had an isotropic voxel size of 1.1 mm. 2D T2-weighted and fluid attenuated inversion recovery (FLAIR) scans were also performed in order to exclude the presence of clinically relevant pathology.
Data analysis
VBM uses voxel-based statistical analysis of structural MRI data to examine differences in the regional composition of brain tissue between groups of subjects.
Pre-processing
Pre-processing was performed using statistical parametric mapping software (SPM5, Wellcome Department of Imaging Neuroscience, University College London). SPM5 uses a generative model to combine normalization and segmentation into a single step (‘unified segmentation’), which also corrects for image intensity non-uniformity [34]. This approach greatly reduces the need for study-specific templates (even for disease groups), because the priors used are matched much more closely to the input image [34]. Modulation was then performed (by multiplying each voxel value by a scaling factor representing its change in size due to normalization) in order to be able to examine regional differences in the volume of tissues rather than differences in ‘tissue concentration’. Spatial smoothing was performed in order to reduce noise and allow for the effect of small residual mis-registrations. The extent of smoothing is an important determinant of the power of the analysis to detect clusters of different sizes [35]. When parametric approaches to statistical analysis are used, smoothing is also required to satisfy the requirements of Gaussian field theory, and smoothing kernels of approximately 8 mm full width half maximum (FWHM) are standard. Non-parametric statistical analysis (as used in the current study) is less reliant on extensive smoothing, so we used a smoothing kernel of 5 mm FWHM with the aim of finding a balance between power and precision of spatial localisation.
Statistical inference
Because structural brain changes usually extend over a number of contiguous voxels, test statistics incorporating spatial information such as 3D cluster mass (the sum of suprathreshold voxel statistics) may be more powerful than test statistics informed only by data at a single voxel [36]. However, as cluster mass is not normally distributed this requires a non-parametric approach to analysis. For this study, permutation testing was used for statistical inference implemented in the BAMM package (brain activation and morphological mapping, a joint development of the Brain Mapping Unit, Department of Psychiatry, University of Cambridge and the Institute of Psychiatry, King’s College London; http:/www.bmu.psychiatry.cam.ac.uk/BAMM/index.html).
Between-group differences in grey matter volume were first estimated by fitting an analysis of covariance (ANCOVA) model at each intracerebral voxel in standard space. This uses the equation:
where T is a vector denoting the image value (tissue volume) at a given voxel for each individual in the cohort, V is the independent variable vector (representing group membership), e models random variation, and the X1-n are vectors representing covariates of no interest. The first stage of analysis used a relatively lenient p value (<0.05) to detect voxels putatively demonstrating differences between the groups. In the second stage, spatial clusters of such voxels were identified and the ‘mass’ of each cluster (the sum of suprathreshold voxel statistics it comprises) was tested for statistical significance. At the cluster level, setting an a priori p value gives little indication of the level of false positive control achieved, as the total number of clusters to which the test will be applied is not known in advance. Using a non-parametric approach, however, the number of clusters expected by chance alone can be directly calculated from the null distribution. Therefore, the number of clusters expected by chance alone was calculated for a range of p values, and for each analysis, the statistical threshold for significance was set such that the number of false positive clusters expected by chance alone would be less than one. In order to identify regionally specific changes in grey matter volume not confounded by global atrophy, total grey matter volume was included as a covariate in the analyses. Montreal Neurological Institute coordinates were transformed to Talairach coordinates for the purposes of cluster identification using the mni2tal algorithm (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). The Talairach Client was used as an automated approach to generating anatomical labels from Talairach coordinates [37].
Results
Subject characteristics
Apathy was diagnosed in 17 patients (50 % of the total sample), eight with PSP (47.1 %) and nine with AD (52.9 %). The remaining 17 patients did not meet criteria for a diagnosis of apathy. The demographic and clinical characteristics of patients with and without apathy in each patient group are shown in Table 1. The patients within each disease group were compared statistically using independent samples t-test for continuous variables and Chi square test for categorical variables. In both disease groups, there was no significant difference between apathetic and non-apathetic patients with respect to any of the demographic and clinical characteristics assessed.
VBM analysis contrasting patients with and without apathy
A 2 × 2 factorial design was used to examine differences in grey matter volume in patients with and without apathy (factor 1 = disease (AD/PSP), factor 2 = apathy (present/absent), covariate = total grey matter volume). Factor 2 and the interaction term were the effects of interest: in the absence of a significant interaction between the two factors, a significant main effect would indicate clusters of volume change associated with apathy across the two diseases.
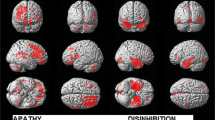
The contrast examining the main effect of the presence or absence of apathy is presented in Fig. 1. At a statistical threshold of p < 0.003 (at which less than one false positive cluster is expected) apathy was associated with reduced frontomedial grey matter volume in a cluster encompassing the anterior cingulate, and another around the left insula. The sagittal view shows the extent of the clusters on the medical cortical surface with significant apathy associated volume loss in the anterior cingulate including the subgenual region. At a more lenient statistical threshold of p < 0.01, an additional cluster was seen in the right insula (not shown in Fig. 1), although at this p value one or more clusters might be expected by chance alone, and this result should interpreted with caution. In addition, patients with apathy showed increased grey matter volume relative to those without apathy in the cerebellum and left lingual gyrus. There was no interaction between disease and apathy, suggesting the absence of significant disease-related variation between AD and PSP in apathy-related change.
Differences in regional grey matter volume between patients with and without apathy (three representative axial slices plus sagittal view). Red clusters indicate reduced volume in apathetic patients. Blue clusters indicate increased volume in apathetic patients. Statistical threshold = p < 0.003 (less than one false positive cluster expected). The table gives precise locations of all significant clusters. See text for detailed description of anatomical locations
VBM analyses contrasting patients with specific apathy-related symptoms
Additional analyses were performed to explore whether specific apathy-related symptoms may be associated with distinct patterns of change in grey matter volume.
Reduced behavioural initiative
Independent of overall apathy classification, patients were divided into sub-groups based on the presence or absence of reduced initiative. Patients with scores of 0 on the Apathy Inventory ‘Behaviour’ domain were classified as having normal initiative and those scoring ≥4 were defined as having reduced initiative (no patients scored between one and three). Reduced initiative was observed in 11 patients with PSP and 13 with AD. A 2 × 2 factorial design was used to examine differences in grey matter volume in patients with and without reduced initiative. The contrast examining the main effect of the presence or absence of reduced initiative is presented in Fig. 2 (statistical threshold p < 0.003; less than one false positive cluster expected by chance alone).
Differences in regional grey matter volume between patients with and without reduced initiative (three representative axial slices plus sagittal view). Red clusters indicate reduced volume in patients with reduced initiative. Blue clusters indicate increased volume in patients with reduced initiative. Statistical threshold = p < 0.003 (less than one false positive cluster expected). The table gives precise locations of all significant clusters. See text for detailed description of anatomical locations
Patients with reduced initiative showed reduced grey matter volume centred in the medial frontal gyrus. The sagittal view identifies this as a region around the paraolfactory sulcus including the subgenual cingulate gyrus and gyrus rectus with a small cluster in the medial olfactory gyrus. Reduced initiative was also associated with increased grey matter volume in the right lingual gyrus and left cuneus. There was no significant interaction between reduced initiative and disease diagnosis suggesting similar patterns in both AD and PSP.
Emotional blunting
Patients were divided into sub-groups with and without emotional blunting on the basis of their scores on the Apathy Inventory ‘Emotion’ domain. Patients with scores of 0 were classified as having normal emotional responses and those scoring ≥4 were defined as showing emotional blunting (no patients scored between one and three). Seven with PSP had emotional blunting and five with AD. A 2 × 2 factorial design was used to examine differences in grey matter volume in patients with and without emotional blunting.
The contrast examining the main effect of the presence or absence of emotional blunting is presented in Fig. 3 (statistical threshold p < 0.003, less than one false positive cluster expected by chance alone). Emotional blunting was associated with reduced grey matter volume in the left insula and increased grey matter volume in the cerebellum, right middle occipital gyrus and left cuneus. There was no significant interaction between disease and emotional blunting in any of these regions, although a significant interaction was observed in the left parahippocampal gyrus. A plot of data from this region showed reduced volume among patients with emotional blunting in the AD group, but not in the PSP group.
Differences in regional grey matter volume between patients with and without emotional blunting (three representative axial slices plus coronal view). Red clusters indicate reduced volume in patients with emotional blunting. Blue clusters indicate increased volume in patients with emotional blunting. Statistical threshold = p < 0.003 (less than one false positive cluster expected). The table gives precise locations of all significant clusters. See text for detailed description of anatomical locations
Discussion
Apathy is associated with reduced grey matter volume in the anterior cingulate, ventromedial orbitofrontal cortex and insula cortex
Across both patient groups, we showed that apathy was associated with reduced grey matter volume in two main regions: a medial frontal area involving anterior cingulate gyrus, ventromedial orbitofrontal cortex and the left insula. The finding of ventromedial orbitofrontal atrophy is broadly consistent with previous structural imaging studies of apathy [12–15, 18, 19] although there is some variability in precise regions from study to study. One rigorous study in AD showed a relationship between apathy and atrophy in a similar region (anterior cingulate cortex and left medial frontal cortex [12]), while another study of 111 AD patients demonstrated associations between apathy and cortical thinning in left caudal anterior cingulate and lateral orbitofrontal and ventrolateral prefrontal cortex [19]. A large study involving six disorder groups found apathy related volume reductions in the ventromedial superior frontal cortex and anterior cingulate cortex [14]. In that study, volume changes were restricted to patient with fronto-temporal dementia/semantic dementia and not observed in patients with AD, PSP or other dementia subtypes. However, apathy was absent in the large majority of these latter patients and mild in the remainder, reducing the ability to identify significant apathy-related volume changes. A recent relevant study using sulcal-based morphometry showed a relationship between orbitofrontal cortical morphology and apathy in CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) [38].
Associations between apathy and volume change in the insula cortex are less consistently reported [16] and so the present finding result represents a novel finding in need of further replication. Nevertheless, it is consistent with a known role of the insula in emotional processing [8] as well as reports of insula activation during volitional behaviour tasks in healthy volunteers [39]. A recent PET study reported apathy-related reductions in cerebral metabolism in patients with PD in many of the prefrontal regions described already but including the right insula [40]. The relatively increased grey matter volume in the cerebellum and left lingual gyrus observed in patients with apathy is more difficult to explain in relation to previously published data. However, using total grey matter volume as a covariate in the analysis will tend to sensitise the analysis to detecting areas of volume increase (or relative preservation) in parallel to areas of volume loss and this can lead to false positive findings. Consequently, the finding of increased volume should be interpreted with caution.
The apathetic and non-apathetic patients in each disease group were well matched with respect to age, gender and disease duration. In addition, there were no differences between these sub-groups with respect to depressive symptoms or global cognitive impairment. The observed absence of significant differences in apathy-related volume changes between the two dementia subtypes supports the hypothesis that apathy, where present, reflects involvement of a common anatomical substrate regardless of the primary disease process. Clinically, variations in the prevalence and severity of apathy, both between and within diseases, may provide a useful indicator of the degree of cingulate, medial frontal and insula cortex involvement when they do not form hallmark pathological features of the disease. For example, in PSP, such pathology is not uniformly seen but can be found in a significant proportion of patients [41, 42] particularly those with Richardson Syndrome variant of PSP [43].
Across disease groups, patients with reduced initiative also showed reduced grey matter volume in the subgenual anterior cingulate cortex and left ventromedial prefrontal cortex (gyrus rectus), while there was no association with insula volume. In contrast, patients with emotional blunting showed reduced grey matter volume around the left insula but not in the cingulate or prefrontal cortex. These findings support a distinction between these two apathy-related symptoms and suggest that they may have distinct neurobiological bases.
Possible roles of the identified regions in relation to apathy
The precise role(s) that the insula cortex might play in apathy is currently unclear. The emotional component of apathy describes a broad clinical construct and includes both a lack of inward emotional experience and a reduced expression of emotion through verbal and non-verbal communication. The methods used to assess emotional blunting in the present study do not allow the separation of these components of emotional processing as they may relate to apathy. However, the involvement of the insula cortex may go beyond the simple experience and/or expression of emotion. This region appears to have a wide range of functions, being implicated in the perceptions of emotions, particularly disgust [10], the experience of pain [44] and emotional expression through language [45]. It is also thought to have higher-level integrative functions linking sensory and particularly salient interoceptive signals with motivational state and emotional experience [46]. More widely still, such integration may play a key role in the metacognitive process of self-awareness and body agency (awareness of initiating and controlling one’s own actions).
Similarly, fronto–medial structures including the anterior cingulate and ventromedial prefrontal cortex have been ascribed a range of function from relatively specific to high level and integrative. They have been implicated in a network controlling attention to action, memory for action and action initiation [9]. Such an action system again may contribute to (and be necessary for) the ability to self-reflect on internal state, emotions and the incentive value of goals, and so drive behaviour. The ventromedial prefrontal area also has an important role in learning and decision making [47].The somatic marker hypothesis ascribes a key role to the area in the learning of associations between behaviours and bio-regulatory states including somatic signals and emotions in complex situations. This is seen as critical for learning the salience of those states and in making decisions about how to act [47].
Such accounts for both insula and medial frontal regions may provide a framework of the metacognitive processes that contribute to apathy beyond the individual cognitive, behavioural and emotional domains. They may also help to explain observations such as the association between apathy and impaired insight and self-awareness [48, 49].
In summary, across two distinct neurodegenerative disorders and using well matched sub-groups of patients, this study supports the importance of medial frontal regions and suggests the involvement of the insula in apathy, with behavioural and emotional apathy symptoms having distinct anatomical bases. However, it may be simplistic to assume that apathy is nothing more than the sum of impairment of independent symptoms. The regions implicated point to the importance of impairment in high level integrative functions in which emotion and other salient internal states are integrated in the control of goal-directed cognition and behaviour, where the medial frontal cortex and insula playing distinct but complementary roles. More widely, metacognitive constructs such as self-reflection and self-awareness may provide a useful framework to investigate apathy beyond its simple clinical phenomenology, and even suggest novel approaches to management.
References
Brown RG, Pluck G (2000) Negative symptoms: the ‘pathology’ of motivation and goal-directed behaviour. Trends Neurosci 23:412–417
Starkstein SE, Leentjens AF (2008) The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry 79:1088–1092
Davis JD, Tremont G (2007) Impact of frontal systems behavioral functioning in dementia on caregiver burden. J Neuropsychiatry Clin Neurosci 19:43–49
Zawacki TM, Grace J, Paul R, Moser DJ, Ott BR, Gordon N, Cohen RA (2002) Behavioral problems as predictors of functional abilities of vascular dementia patients. J Neuropsychiatry Clin Neurosci 14:296–302
Chase TN (2011) Apathy in neuropsychiatric disease: diagnosis, pathophysiology, and treatment. Neurotox Res 19:266–278
Marin RS (1991) Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 3:243–254
Lau HC, Rogers RD, Ramnani N, Passingham RE (2004) Willed action and attention to the selection of action. Neuroimage 21:1407–1415
Murphy FC, Nimmo-Smith I, Lawrence AD (2003) Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci 3:207–233
Passingham RE, Bengtsson SL, Lau HC (2010) Medial frontal cortex: from self-generated action to reflection on one’s own performance. Trends Cogn Sci 14:16–21
Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P (2009) Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432
Cummings JL (1993) Frontal–subcortical circuits and human behavior. Arch Neurol 50:873–880
Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM (2007) Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord 24:91–97
Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A (2007) Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry 15:386–394
Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL (2005) Neuroanatomical correlates of behavioural disorders in dementia. Brain 128:2612–2625
Bruen PD, McGeown WJ, Shanks MF, Venneri A (2008) Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain 131:2455–2463
Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF (2010) Neuroanatomical correlates of apathy in Parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov Disord 25:2318–2325
Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, Libon DJ, Grossman M (2009) Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 27:96–104
Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J (2008) Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology 71:736–742
Tunnard C, Whitehead D, Hurt C, Wahlund LO, Mecocci P, Tsolaki M, Vellas B, Spenger C, Kloszewska I, Soininen H, Lovestone S, Simmons A (2011) Apathy and cortical atrophy in Alzheimer’s disease. Int J Geriatr Psychiatry 26:741–748
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK (2003) Movement Disorders Society Scientific Issues Committee report: sIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18:467–486
Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, Lai EC, Wenning G, D’Olhaberriague L, Verny M, Chaudhuri KR, McKee A, Jellinger K, Bartko JJ, Mangone CA, Pearce RK (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46:922–930
Litvan I (2001) Diagnosis and management of progressive supranuclear palsy. Semin Neurol 21:41–48
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Molloy DW, Standish TI (1997) A guide to the standardized Mini-Mental State Examination. Int Psychogeriatr 9(Suppl 1):87–94
van Marwijk HW, Wallace P, de Bock GH, Hermans J, Kaptein AA, Mulder JD (1995) Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale. Br J Gen Pract 45:195–199
Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F (1994) Unified Parkinson’s Disease Rating Scale characteristics and structure. The cooperative multicentric group. Mov Disord 9:76–83
Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, Weintraub D, Sampaio C, Poewe W, Rascol O, Stebbins GT, Goetz CG (2008) Apathy and anhedonia rating scales in Parkinson’s disease: critique and recommendations. Mov Disord 23:2004–2014
Schrag A (2011) Apathy and depression scales in Parkinson’s disease: are they good enough? J Neurol Sci 310:216–219
Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, Verhey FR, Yessavage J, Clement JP, Drapier D, Bayle F, Benoit M, Boyer P, Lorca PM, Thibaut F, Gauthier S, Grossberg G, Vellas B, Byrne J (2009) Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry 24:98–104
Drijgers RL, Dujardin K, Reijnders JS, Defebvre L, Leentjens AF (2010) Validation of diagnostic criteria for apathy in Parkinson’s disease. Parkinsonism Relat Disord 16:656–660
Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38:143–162
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314
Robert PH, Clairet S, Benoit M, Koutaich J, Bertogliati C, Tible O, Caci H, Borg M, Brocker P, Bedoucha P (2002) The Apathy Inventory: assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int J Geriatr Psychiatry 17:1099–1105
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Jones DK, Symms MR, Cercignani M, Howard RJ (2005) The effect of filter size on VBM analyses of DT-MRI data. Neuroimage 26:546–554
Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ (1999) Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18:32–42
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131
Jouvent E, Reyes S, Mangin JF, Roca P, Perrot M, Thyreau B, Herve D, Dichgans M, Chabriat H (2011) Apathy is related to cortex morphology in CADASIL. A sulcal-based morphometry study. Neurology 76:1472–1477
Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123 (Pt 6):1216–1228
Robert G, Le JF, Lozachmeur C, Drapier S, Dondaine T, Peron J, Travers D, Sauleau P, Millet B, Verin M, Drapier D (2012) Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology 79:1155–1160
Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W (2004) Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 75:246–249
Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C (2005) Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain 128:1259–1266
Schofield EC, Hodges JR, Macdonald V, Cordato NJ, Kril JJ, Halliday GM (2011) Cortical atrophy differentiates Richardson’s syndrome from the parkinsonian form of progressive supranuclear palsy. Mov Disord 26:256–263
Brooks JC, Tracey I (2007) The insula: a multidimensional integration site for pain. Pain 128:1–2
Ihme K, Dannlowski U, Lichev V, Stuhrmann A, Grotegerd D, Rosenberg N, Kugel H, Heindel W, Arolt V, Kersting A, Suslow T (2013) Alexithymia is related to differences in gray matter volume: a voxel-based morphometry study. Brain Res 1491:60–67
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666
Bechara A, Damasio H, Damasio AR (2000) Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307
Aalten P, Van Valen E, Clare L, Kenny G, Verhey F (2005) Awareness in dementia: a review of clinical correlates. Aging Ment Health 9:414–422
Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M (2005) Metacognitive deficits in frontotemporal dementia. J Neurol Neurosurg Psychiatry 76:1630–1635
Acknowledgments
The study was supported by the Medical Research Council through a Clinical Research Training Fellowship (G0500443) (BS). Author RB receives salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Biomedical Research Unit at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanton, B.R., Leigh, P.N., Howard, R.J. et al. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J Neurol 260, 2481–2490 (2013). https://doi.org/10.1007/s00415-013-6989-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-6989-9