Abstract
Although migraine symptomatology is well-defined, our understanding of migraine pathophysiology is incomplete. Structural and functional brain imaging can contribute to a greater understanding of migraine pathophysiology. Recent neuroimaging studies demonstrate that migraine is associated with structural and functional alterations of brain regions commonly implicated in pain processing. This review summarizes recent brain structural and functional imaging findings in migraine and highlights those that are associated with characteristics such as the presence or absence of aura, associated cognitive dysfunction, sex-differences (male vs. female migraineurs), age, and disease burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine affects over 12 % (38 million) of the people in the USA and is three times more common in women than in men [1, 2]. Population-based data suggest that migraine is more common than diabetes and asthma combined [3–5]. Migraine is characterized by a constellation of symptoms including moderate to severe headache, hypersensitivity to light, sound, touch, and odor [6–9], gastrointestinal (nausea and emesis), mood, cognitive, autonomic, and constitutional (fatigue, lethargy) symptoms. Many migraine sufferers also describe a range of interictal symptoms between attacks [10–13]. While the migraine phenotype is well characterized [6], there are gaps in our understanding of migraine pathophysiology that may be bridged by structural and functional brain imaging findings.
T1-weighted magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) can be used to study regional gray and white matter structural alterations in migraine, respectively. Brain function in migraine can be measured using functional magnetic resonance imaging (fMRI), a technique based on detecting blood-oxygenation-level dependent (BOLD) signal fluctuations. FMRI can be run during either (i) task-based designs, which measure brain responses to a specific stimulus or action or (ii) resting-state designs, which interrogate the functional connectivity of the brain at rest (rs-fMRI).
There is a growing consensus over the past decade that migraine is related to brain structural and functional alterations in regions that participate in the pain experience. In addition, several functional imaging studies have interrogated brain activation patterns in migraineurs in response to exposure to thermal pain and olfactory or visual stimulation. These studies have implicated common regions that show more activation or hyperactivation between attacks (interictally) in migraineurs relative to healthy controls during sensory processing. These include prefrontal, parietal (postcentral/primary somatosensory gyrus), temporal (temporal pole, fusiform, hippocampus, parahippocampal gyrus) cortex, and subcortical regions (cingulate, thalamic complex) [14–18]. Regions where migraineurs show less interictal activation or hypoactivation relative to healthy controls include areas of the brainstem (pons, medulla) and temporal and parietal (secondary somatosensory) cortex [15, 19–21]. Furthermore, ictal migraineurs show enhanced brain activation during sensory stimulation in temporo-occipital regions (temporal pole, primary visual cortex) as well as subcortical areas (thalamus, insula, amygdala), cerebellum, and brainstem (midbrain, pons, medulla) regions [19, 22, 23]. These regions relate to the cognitive, affective, sensory-discriminative, and modulatory components of pain processing.
Studies that have interrogated resting-state functional connectivity (rs-fc) patterns in migraineurs relative to healthy controls have corroborated the findings of task-based fMRI studies and found altered functional connectivity (fc) amongst various sensory-processing regions [24–34]. DTI and T1-weighted imaging have demonstrated white and gray matter alterations in cortical and subcortical pain-related areas [28, 35–40, 41•, 42, 43] in migraineurs compared to healthy controls. These data indicate that migraine affects gray and white matter integrity as well as functional activation patterns of sensory-processing regions. In addition, migraine also appears to alter how sensory-processing areas are able to communicate with one another. These findings have clarified that migraine is not associated with a dysfunction of a specific brain area or a brain circuit but rather that migraine affects an array of integrated networks that are responsible for the diversity of the symptoms that constitute migraine.
During more recent years, the neuroimaging literature has focused on attaining a better understanding of how alterations in regions that process sensory stimuli and mediate cognitive function relate to specific characteristics of the migraine sufferer (e.g., the sex of the patient) and the nature of the migraine attack (e.g., aura vs. no aura). Several recent neuroimaging studies have compared patients with aura to those without aura, men vs. women, and have interrogated correlations between imaging findings with migraine-associated cognitive dysfunction. Finally, imaging findings in pediatric cases and from longitudinal studies have yielded evidence that migraine can be associated with baseline brain alterations or might change the structure and function of the brain over time.
Here, we review recent neuroimaging findings that have specifically interrogated structural and functional changes associated with (a) aura, (b) sex-specific differences in migraine, (c) cognitive function, and (d) longitudinal brain changes. A PubMed database search was limited to migraine neuroimaging studies published between January 2012 and January 2016, although, due to the scarcity of studies investigating cognitive function deficits, one study published in 2008 was included. The search was limited to group data of migraineurs and healthy control; single-case studies were not included in this review. In order to provide a more focused review, we limited our search to studies using MRI-based techniques, including DTI, T1- and T2-weighted imaging, task-based fMRI, and rs-fc fMRI.
Migraine and Aura
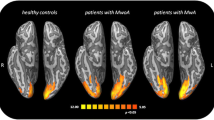
Approximately 30 % of migraine patients have aura and these are visual in the vast majority [1, 44]. In a landmark study, Hadjikhani at al. (2001) showed a wave of heightened visual cortex activation followed by a wave of attenuated visual cortex activation in patients during migraine aura [45], thus suggesting a cortical spreading depression-like event in the visual cortex of humans.
Although a number of studies have compared brain structure and function in migraineurs with aura (MwA) to healthy controls or have included mixed cohorts of MwA and migraineurs without aura (MwoA) compared to healthy controls, these studies are not ideal for isolating the effect of aura on brain integrity as group differences are co-mingled with aura symptoms. Over recent years, several studies have specifically interrogated brain changes associated with aura symptomatology by contrasting groups of MwA, MwoA, and healthy controls (Table 1).
Tedeschi and colleagues (2015) interrogated the functional connectivity of visual cortex and found that interictal MwA had heightened functional connectivity of occipital regions relative to healthy controls and to migraineurs without aura (MwoA) [46]. Rs-fc results by Niddam et al. [47•] showed weaker fc between the anterior insula and extrastriate regions in MwA compared to MwoA and healthy controls. In contrast, Datta et al. (2013) [17] and Tessitore et al. (2015) [30] failed to demonstrate rs-fc differences between MwA and MwoA. As differences in study design and patient characteristics might be a factor in the disparity of findings, future studies using larger sample sizes will be needed to further interrogate brain changes associated with aura.
Several studies using visual stimulation BOLD fMRI paradigms have detected hyper-responsiveness in striate areas during visual stimulation in interictal MwA [17, 48] as well as more brain activation in the visual functional network including the inferior frontal gyrus and the inferior and superior parietal lobule, all regions involved with attention orienting and oculomotor control (Hougaard and colleagues 2014) [18]. Although results of a smaller study by Bridge et al. (2015) [49•] did not find differences in striate cortex activation between MwA and MwoA, the authors demonstrated decreased GABA levels in the occipital cortex and a positive relationship between BOLD-signal change and occipital glutamate/creatine ratios using magnetic resonance spectroscopy (MRS). The combined use of fMRI and MRS is new to the field, and the results are intriguing and support the notion of disordered brain excitatory and inhibitory mechanisms, which might explain the biological susceptibility to spontaneous and/or triggered cortical spreading depression (CSD) events [45].
Several imaging findings have reported no structural changes in MwA. For example, Hougaard et al. (2015) indicated normal cortical thickness and gray matter density despite lateralized aura [50]. Similarly, two other studies demonstrated an absence of gray or white matter changes in striate areas (Tedeschi et al. 2016) and in structural regions underlying the executive function networks (Tessitore et al. 2015) in MwA compared to MwoA [30, 46]. Contrary to these findings, Messina and colleagues (2013) found more cortical surface area (left lingual gyrus and the left pericallosal sulcus) and thicker cortex (bilateral supermarginal gyrus, right insula, right central sulcus) in MwA, along with reduced cortical surface area in the inferior temporal gyrus [39]. A small longitudinal study by Dinia et al. (2013) [47•] detected progression of white matter lesions over a period of 33 months in MwA. Furthermore, results showed there was a positive correlation between aura duration and the number of new white matter lesions that developed, as well as a positive correlation between the number of migraine attacks and new white matter lesions. Although this study lacked a healthy comparison group, this longitudinally designed study by Dinia and colleagues provide important results that suggest a link between the progression of white matter changes and specific migraine characteristics.
In summary, the majority of functional studies indicate that MwA is associated with functional alterations in striate and extrastriate visual processing regions. Two studies in MwA showed a lack of correlation of rs brain connectivity or visually induced brain activation to either headache frequency or to years lived with migraine. This potentially indicates that brain functional activity in the primary visual cortex of MwA is an intrinsic biomarker of susceptibility to aura as opposed to the result of recurrent attacks [46, 48]. Finally, as brain structural data are still limited, it remains inconclusive whether symptoms of aura are related to or cause changes in brain structure.
Sex-Specific Differences in Migraine
Epidemiological studies indicate that migraine typically begins in late childhood or early adulthood and is approximately three times as common in women than it is in men [51, 52]. Whereas the prevalence of migraine is relatively equal between sexes before the onset of puberty, migraine prevalence sharply rises in females after puberty relative to males [2]. In addition, women report more migraine-related symptoms and greater impairment compared to male migraineurs [51]. These data have led some researchers to suggest a putative role of female sex hormones in prevalence and clinical expression of migraine [53]. These sex-specific differences in prevalence and symptomatology are still poorly understood and have driven neuroimaging research to investigate changes in brain structure and function between male and female migraine patients—possibly indicating a sex-specific phenotype (Table 2).
Faria and colleagues (2015) have shown that female pediatric migraine patients who are in mid-puberty have greater gray matter volume relative to age-matched male migraineurs and healthy controls in regions of the thalamus and the putamen [54]. In addition, female pediatric migraineurs had stronger rs-fc between the right precuneus and the left putamen, right caudate, left thalamus, and the left amygdala. Maleki and colleagues (2012) showed thicker posterior insula and precuneus regions in adult female migraineurs compared to adult male migraineurs and healthy controls.
Female migraineurs had stronger rs-fc between the posterior insula and the primary somatosensory cortex, posterior cingulate, precuneus, amygdala, and temporal pole. They also had stronger fc between the precuneus and the amygdala and the primary somatosensory cortex [55]. Another study by the same group (2015) showed that, while thinning of the insula occurred with advancing age in healthy female controls, this was not seen in female migraineurs [56]. A recent meta-analysis by Dai et al. (2015) of nine voxel-based morphological studies (total of 222 migraineurs and 230 healthy controls) indicated that in migraineurs, female sex was associated with less gray matter in the right dorsolateral prefrontal cortex [57•]. Important results provided by this meta-analysis as well as by other smaller studies suggest that there might be sex-specific brain biomarkers for migraine. Furthermore, the regularity with which alterations in brain structure or function have been found for the dorsolateral prefrontal cortex, the precuneus, and the insula suggest a sex-specific role of cognitive-affective pain processing in female migraine patients.
Attention/Cognition Deficits in Migraine: An Ongoing Debate
Migraineurs often self-report attention-related impairment as well as difficulties with processing speed during a migraine attack [58, 59]. These findings are corroborated by neuropsychological studies that show decreased performance on measures of attention, verbal learning, and memory during the attack [60, 61] as well as between attacks [62, 63]. However, other studies have failed to identify neuropsychological function deficits in migraineurs [30, 64, 65].
Many imaging studies to date have demonstrated altered brain structure and function in prefrontal areas of migraine patients [25, 31, 36, 66, 67] in regions associated with cognitive aspects of pain processing. Similarly, a number of recent rs-fc MRI studies have indicated abnormal fc within the salience network, a core resting-state network that includes paralimbic-limbic regions believed to play a role in the detection of stimuli and the allocation of attentional resources. Rs-fc results indicate heightened connectivity within the salience network in migraineurs without aura [68] and reduced functional connectivity between the salience and the visual networks in migraine patients with aura [69].
Recently, several studies have simultaneously interrogated cognitive function and brain structural and functional alterations in cognitive-processing regions in order to better understand whether alterations in brain structure are linked to deficits in cognitive performance in migraine patients (Table 3). In an earlier study, Schmitz and colleagues (2008) [64] found that less frontal and parietal gray matter density in migraine patients negatively correlated with slower response times during an attention-shifting task, thus indicating a potential relationship between cognitive function and gray matter deficits in migraine patients. Using a novel study design, Mathur et al. (2015) investigated brain activation patterns during the performance of a cognitive task while undergoing concurrent thermal-pain stimulation [70•]. Performance of the attention task was no different between healthy controls and migraineurs as reflected by measures of reaction time and error rates. Although both healthy controls and migraineurs had task-related deactivation in regions involved in pain modulation such as the dorsolateral prefrontal cortex, the middle cingulate, and the cerebellum—migraineurs showed significantly less deactivation in these regions. Whereas there was a relationship between deactivation and task-difficulty in healthy controls, this was absent for migraineurs. Results of this study are intriguing and indicate a potential abnormal modulation of pain-cognition circuits in patients with migraine [70•].
Mickleborough et al. (2015) [71] used an fMRI visual attention-orienting task to interrogate brain activation patterns in interictal migraineurs and healthy controls. Interestingly, although migraineurs performed as well as healthy controls on the attention task, migraineurs showed less activation of the ventral fronto-parietal attention network including the right temporal parietal junction, a region relevant for re-orienting attention to salient stimuli [71]. Results by Tessitore et al. (2015) have indicated that, although migraineurs show weaker connectivity in their executive control network relative to healthy controls, there is an absence of performance deficits on a variety of neurocognitive measures [30].
Although the existence of attention deficits in migraine remains controversial, these results could indicate that neurocognitive measures are not as sensitive as fMRI for detecting subtle changes in attention in migraine patients. Furthermore, results show that neurocognitive measures and brain activation patterns do not track each other well and could potentially indicate that fMRI might have better sensitivity than neurocognitive tests for detecting sub-clinical processing deficits in migraine.
Longitudinally Designed Studies and Pediatric Studies in Migraine
Although cross-sectional studies can draw inferences between migraine characteristics and alterations in brain structure and function, these inferences have to be interpreted with caution and have limited prognostic value for predicting disease progression. Repeated imaging of subjects at multiple time-points allows the evaluation of brain changes in individual patients over time. These studies have profound value and enable the actual “tracking” of migraine progression (Table 4).
A recent longitudinal imaging study by Liu et al (2015) [72] in adult patients who were newly diagnosed with migraine demonstrated brain gray matter, but not white matter structural volume loss over a period of one year. Dinia and colleagues (2014) [47•] detected a progression of white matter hyperintensities in migraineurs with aura (33-month follow-up). Neither study showed a correlation between headache characteristics and structural changes. A 3-year follow-up study by Erdélyi-Botor et al. [73] has also showed progression of white matter hyperintensities in migraine patients. These hyperintensities were more common in high-frequency than in low-frequency migraineurs. Lastly, a 9-year follow-up study by Palm-Meinders et al. 2012 [74•] investigated longitudinal MRI-based brain changes in 203 migraineurs and 83 healthy controls. This important large-scale cohort study showed that female migraineurs had more white matter lesions relative to female controls and that female migraineurs showed a higher progression of these white matter lesions over time. However, the authors again showed no association between headache frequency and the progression of white matter lesions. Zhao et al. (2014) [75] found progressive fc changes in pain-processing networks and abnormal regional homogeneity changes in the putamen, thalamus, orbitofrontal cortex, secondary somatosensory cortex, and the brainstem over a period of 6 weeks in migraineurs who were experiencing increasing migraine frequency.
These studies all reveal longitudinal brain structural changes in migraineurs over time though there was variable correlation of headache frequency with the progression of structural alterations. However, as only two studies included a control group, future studies are needed to confirm if structural changes are specific to migraine as opposed to being attributable to aging.
Pediatric Cohorts
The neuroimaging literature has mostly focused on studying adult migraine populations, leaving brain structure and function in children with migraine largely under-investigated. Longitudinal studies that begin during childhood will be especially useful since they offer the unique opportunity to study the migraine disease process at its earliest stage.
Two studies using voxel-based morphometry analysis (VBM) and tract-based spatial statistics (TBSS) found reduced volume of and altered white matter connectivity between regions associated with pain processing in pediatric migraineurs. Rocca and colleagues (2014) [41•] investigated brain volumetric changes in pediatric migraine patients. Results indicate that pediatric migraineurs have greater putamen but reduced gray matter brain volume in fronto-temporal areas including the middle temporal, orbitofrontal, inferior frontal gyrus, and the cingulum relative to age-matched non-migraine controls. Volume in the putamen correlated with years lived with migraine. No changes in white matter brain volume were identified.
A study by Messina and colleagues (2015) [76] that interrogated white matter tract structure in a pediatric migraine cohort showed white matter alterations reflected by lower mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) in widespread cortical (fronto-temporal-occipital), subcortical (thalamic), and brainstem regions as well as increased fractional anisotropy (FA) in the optic radiations. Both studies showed no association between clinical factors (such has headache frequency and years lived with migraine) and cortical volume loss or fiber tract alterations leading the authors to hypothesize that fronto-temporal gray matter volume and white matter structural alterations found in pediatric migraineurs could potentially be trait biomarkers of the disease.
Although white matter lesions identified by high-signal intensities shown on T2-weighted imaging are common in adult migraineurs, recent evidence shows that white matter lesions are also present in 4.4 to 17 % of children and adolescents with migraine [77–80]. Longitudinal data by Bayram and colleagues (2013) detected white matter hyperintensities in pediatric headache patients on baseline MRIs (time-point I), yet, no new white matter lesions in pediatric patients with headache on follow-up imaging after an average of 16 months (time-point II) [79]. This is an interesting finding that will have to be replicated using longitudinally designed studies with longer follow-up intervals in pediatric migraine populations.
An important goal for migraine research is the identification of brain imaging biomarkers that could help identify whether certain characteristics of brain structure or function might differentiate migraine patients from healthy controls and from other headache types. Although results of some longitudinal studies suggest that brain changes are driven by disease factors, other longitudinal data as well as structural findings in pediatric migraine populations suggest that imaging changes reflect a trait biomarker rather than the consequence of repeated attacks. As such, whether migraine-related brain changes exist “from the very beginning” or whether they are modified by the disease is an unresolved issue. Of course, these possibilities are not mutually exclusive. One could hypothesize for example that certain brain alterations are present in migraineurs at the earliest stages and that these regions are further modified as a function of disease burden (e.g., frequency of attacks). Although past cross-sectional studies and current longitudinal studies yield some evidence of a relationship between progressive brain changes and migraine characteristics (such as headache frequency), the literature does not yet provide evidence as to whether these brain changes are reversible with improvements or resolution of migraine attacks.
Conclusion
Neuroimaging research has demonstrated brain structural and functional alterations in migraineurs in a variety of regions associated with sensory and pain processing. More recent findings indicate that these regional alterations might relate to migraine factors such as patient demographics (sex, age), migraine characteristics (presence or absence of aura) or migraine-associated cognitive dysfunctions. Lastly, imaging results from pediatric migraine patients and results of longitudinal studies yield evidence of baseline brain alterations as well as evidence that migraine burden such as migraine frequency might modify brain structure and function over time.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57.
Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–9.
Centers for disease control and prevention. Asthma episodes and current asthma. http://www.cdc.gov/nchs/data/nhis/earlyrelease/.200709_15.pdf.
American Diabetes Association. Total prevalence of diabetes and pre-diabetes. http://.www.diabetes.org/diabetes-statistics/prevalence.jsp.
Schwedt TJ, Shapiro RE. Funding of research on headache disorders by the National Institutes of Health. Headache. 2009;49:162–9.
The international classification of headache disorders. 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
Bigal M, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers a population study. Neurology. 2008;70:1525–33.
Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache. 2004;44:1019–23.
Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–58.
Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–41.
Schwedt TJ, Zuniga L, Chong CD. Low heat pain thresholds in migraineurs between attacks. Cephalalgia. 2015;35:593–9.
Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML. Ictal and interictal phonophobia in migraine-a quantitative controlled study. Cephalalgia. 2009;29:1042–8.
Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700.
Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia. 2013;33:256–65.
Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012;259:1903–12.
Griebe M, Flux F, Wolf ME, Hennerici MG, Szabo K. Multimodal assessment of optokinetic visual stimulation response in migraine with aura. Headache. 2014;54:131–41.
Datta R, Aguirre GK, Hu S, Detre JA, Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013;33:365–74.
Hougaard A, Amin FM, Hoffmann MB, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp. 2014;35:2714–23.
Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb Cortex (New York, NY :1991). 2011;21:435–48.
Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3, e3799.
Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci Off J Soc Neurosci. 2011;31:1937–43.
Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91.
Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–82.
Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70:838–45.
Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012;7, e52927.
Schwedt TJ, Larson-Prior L, Coalson RS, et al. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med (Malden, Mass). 2014;15:154–65.
Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33:1264–8.
Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64.
Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. 2013;53:737–51.
Tessitore A, Russo A, Conte F, et al. Abnormal connectivity within executive resting-state network in migraine with aura. Headache. 2015;55:794–805.
Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14:89.
Xue T, Yuan K, Cheng P, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26:1051–8.
Zhao L, Liu J, Dong X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. 2013;14:85.
Liu J, Zhao L, Li G, et al. Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One. 2012;7, e51250.
Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014;34:1115–24.
Chong CD, Schwedt TJ. Migraine affects white-matter tract integrity: a diffusion-tensor imaging study. Cephalalgia. 2015.
Chong CD, Starling AJ and Schwedt TJ. Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia. 2015
Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015;10, e0116687.
Messina R, Rocca MA, Colombo B, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013;268:170–80.
Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke; a J Cereb Circ. 2006;37:1765–70.
Rocca MA, Messina R, Colombo B, Falini A, Comi G, Filippi M. Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol. 2014;261:350–7. Results yield evidence that suggest brain biomarkers for migraine.
Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4.
Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–55.
Vincent MB, Hadjikhani N. Migraine aura and related phenomena: beyond scotomata and scintillations. Cephalalgia. 2007;27:1368–77.
Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–92.
Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia. 2016;36:139–47.
Dinia L, Bonzano L, Albano B, et al. White matter lesions progression in migraine with aura: a clinical and MRI longitudinal study. J Neuroimaging : Off J Am Soc Neuroimaging. 2013;23:47–52. This longitudinally designed study suggests a link between the progression of white matter changes and specific migraine characteristics.
Cucchiara B, Datta R, Aguirre GK, Idoko KE, Detre J. Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia. 2015;35:585–92.
Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia. 2015;35:1025–30. Results of this study suggest a relationship between brain activation patterns and occipital glutamate/creatine ratios in migraineurs with aura.
Hougaard A, Amin FM, Hoffmann MB, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia. 2015;35:3–9.
Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–99.
Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–8.
Borsook D, Erpelding N, Lebel A, et al. Sex and the migraine brain. Neurobiol Dis. 2014;68:200–14.
Faria V, Erpelding N, Lebel A, et al. The migraine brain in transition: girls vs boys. Pain. 2015;156:2212–21.
Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain :a J Neurol. 2012;135:2546–59.
Maleki N, Barmettler G, Moulton EA, et al. Female migraineurs show lack of insular thinning with age. Pain. 2015;156:1232–9.
Dai Z, Zhong J, Xiao P, et al. Gray matter correlates of migraine and gender effect: an meta-analysis of voxel-based morphometry studies. Neuroscience. 2015;299:88–96. This metaanalysis (of 9 studies) identifies common structural changes in migraine and suggests sex-specific structural differences in the migraine brain.
Gil-Gouveia R, Oliveira AG, Martins IP. Subjective cognitive symptoms during a migraine attack: a prospective study of a clinic-based sample. Pain Phys. 2016;19:E137–50.
Gil-Gouveia R, Oliveira AG, Martins IP. The impact of cognitive symptoms on migraine attack-related disability. Cephalalgia. 2015.
Mazzucchi A, Sinforiani E, Zinelli P, et al. Interhemispheric attentional functioning in classic migraine subjects during paroxysmal and interparoxysmal phases. Headache. 1988;28:488–93.
Kuhajda MC, Thorn BE, Klinger MR, Rubin NJ. The effect of headache pain on attention (encoding) and memory (recognition). Pain. 2002;97:213–21.
Mulder EJ, Linssen WH, Passchier J, Orlebeke JF, de Geus EJ. Interictal and postictal cognitive changes in migraine. Cephalalgia. 1999;19:557–65.
Riva D, Usilla A, Aggio F, Vago C, Treccani C, Bulgheroni S. Attention in children and adolescents with headache. Headache. 2012;52:374–84.
Schmitz N, Arkink EB, Mulder M, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett. 2008;440:92–6.
Pearson AJ, Chronicle EP, Maylor EA, Bruce LA. Cognitive function is not impaired in people with a long history of migraine: a blinded study. Cephalalgia. 2006;26:74–80.
Gao Q, Xu F, Jiang C, et al. Decreased functional connectivity density in pain-related brain regions of female migraine patients without aura. Brain Res 2015.
Lai TH, Chou KH, Fuh JL, et al. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia. 2016.
Tso AR, Trujillo A, Guo CC, Goadsby PJ, Seeley WW. The anterior insula shows heightened interictal intrinsic connectivity in migraine without aura. Neurology. 2015;84:1043–50.
Niddam DM, Lai KL, Fuh JL, Chuang CY, Chen WT, Wang SJ. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2016;36:53–66.
Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. NeuroImage Clin. 2015;7:347–58. Using a novel fMRI study design, authors show results which indicate a potential abnormal modulation of pain-cognition circuits in patients with migraine.
Mickleborough MJ, Ekstrand C, Gould L, et al. Attentional network differences between migraineurs and non-migraine controls: fMRI Evidence. Brain Topogr. 2015.
Liu J, Lan L, Li G, et al. Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. J Pain :Off J Am Pain Soc. 2013;14:1703–8.
Erdelyi-Botor S, Aradi M, Kamson DO, et al. Changes of migraine-related white matter hyperintensities after 3 years: a longitudinal MRI study. Headache. 2015;55:55–70.
Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA. 2012;308:1889–97. Published in 2012, it is the largest longitudinally designed study in the field.
Zhao L, Liu J, Yan X, et al. Abnormal brain activity changes in patients with migraine: a short-term longitudinal study. J Clin Neurol (Seoul, Korea). 2014;10:229–35.
Messina R, Rocca MA, Colombo B, et al. White matter microstructure abnormalities in pediatric migraine patients. Cephalagia. 2015;35(14):1278–86.
Candee MS, McCandless RT, Moore KR, Arrington CB, Minich LL, Bale Jr JF. White matter lesions in children and adolescents with migraine. Pediatr Neurol. 2013;49:393–6.
Eidlitz-Markus T, Zeharia A, Haimi-Cohen Y, Konen O. MRI white matter lesions in pediatric migraine. Cephalalgia. 2013;33:906–13.
Bayram E, Topcu Y, Karaoglu P, Yis U, Cakmakci Guleryuz H, Kurul SH. Incidental white matter lesions in children presenting with headache. Headache. 2013;53:970–6.
Mar S, Kelly JE, Isbell S, Aung WY, Lenox J, Prensky A. Prevalence of white matter lesions and stroke in children with migraine. Neurology. 2013;81:1387–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Catherine D. Chong declares that she has no conflict of interest.
Todd J. Schwedt has received consulting fees from Allergan, Amgen, Dr. Reddy’s, GBS, Supernus, Teva, and Zogenix. He receives royalties from Cambridge University Press and UpToDate .
David W. Dodick, MD, in the past 12 months, has served on advisory boards and has consulted for Allergan, Amgen, Alder, CoLucid, Dr Reddy’s, Merck, ENeura, Eli Lilly & Company, Autonomic Technologies, Teva, Xenen, Tonix, Trigemina, Supernus, ScionNeurostim, and Boston Scientific. He has options in Xalan, Epien, and Second Opinion. He is on the board of directors of the King Devick Test. Within the past 12 months, Dr. Dodick has received royalties, funding for travel, speaking, or editorial activities from the following: Healthlogix, Haymarket Media Group, Ltd., SAGE Publishing, Lippincott Williams & Wilkins, Oxford University Press, and Cambridge University Press. He receives publishing royalties for Wolff’s Headache, 8th edition (Oxford University Press, 2009) and Handbook of Headache (Cambridge University Press, 2010).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Neuroimaging
Rights and permissions
About this article
Cite this article
Chong, C.D., Schwedt, T.J. & Dodick, D.W. Migraine: What Imaging Reveals. Curr Neurol Neurosci Rep 16, 64 (2016). https://doi.org/10.1007/s11910-016-0662-5
Published:
DOI: https://doi.org/10.1007/s11910-016-0662-5