Abstract
Neuroimaging techniques fall broadly into two great categories, examining either structure or function, but multiple methods can be employed in either approach. Structural imaging provides static anatomical information whereas functional imaging can be regarded as the method providing dynamic physiological information. However, the division between structural and functional imaging is difficult to make and arbitrary in some measure because structure and function can be often inextricably intertwined in the brain. Recent years have seen rapid growth of neuroimaging methodology which has provided new insights into functional brain organization of migraine patients. In particular, since migraine is regarded as a disorder of the brain, functional neuroimaging offers much in terms of understanding the physiological dysfunction that characterizes migraine. Furthermore, neuroimaging techniques are crucial for clinicians in order to further elucidate pathophysiological mechanisms underlying this complex and often disabling disease and to provide new therapeutical approaches for migraine patients. This chapter aim to focus on the results of structural and functional neuroimaging studies and attempts to synthesize the literature data to provide new pathophysiological concepts for understanding migraine mechanisms.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Neuroimaging techniques fall broadly into two great categories, examining either structure or function, but multiple methods can be employed in either approach. Structural imaging provides static anatomical information whereas functional imaging can be regarded as the method providing dynamic physiological information. However, the division between structural and functional imaging is difficult to make and arbitrary in some measure because structure and function can be often inextricably intertwined in the brain. Furthermore, although some neuroimaging techniques are based on structural high-resolution T1-weighted magnetic resonance imaging (MRI), applied statistical analyses methods are often used also for functional imaging data. The phenomenon of nuclear magnetic resonance (NMR) was first observed in 1945 [1, 2] but the first human in vivo MRI was produced by the end of 70th decade from the past century [3]. Compared with images from previous modalities, brain MRI provided excellent anatomical detail and strong grey matter (GM) and white matter (WM) contrast.
More recently, high-resolution structural MRI methods have been developed such as voxel-based morphometry (VBM) , cortical thickness (CT) and other surface-based (SB) techniques and diffusion tensor imaging (DTI). VBM is a semiautomatic whole-brain method that enables comparisons of GM and WM between groups on a voxel basis, sensitive to subtle macroscopic and mesoscopic structural differences between groups of subjects that can be related to functional correlates and thus further understanding of disease pathophysiology in the brains of migraineurs and non-migraine subjects [4]. CT analysis is a categorical SB technique used in cohort studies, comparing the cortices of patients and healthy controls (HC) in vivo. DTI is specifically employed to assess WM microstructure and can potentially reveal even subtle anatomical abnormalities. Structural connectivity (s-connectivity) analysis is mostly performed on diffusion-derived data, and more recently in combination with volumetric measures.
An early advanced imaging approach for regional cerebral blood flow (rCBF) assessment has been provided by single-photon emission computed tomography (SPECT), using Xenon-133 during migraine attacks [5].
However, positron emission tomography (PET) and more recently functional MRI (fMRI) have superseded the older methods, as they enable the exploration of brain function with greater temporal and spatial resolution and are, today, the most frequently used techniques to attempt to clarify the complexity of migraine mechanisms [6, 7].
Many of the functional imaging studies in migraine research have applied PET to investigate brain activity and metabolism, as well as receptor neurochemistry [8] using different radiotracer such as respectively 15O labelled water (H 152 O), 2-deoxy-2-[18F] fluoro-d-glucose (18FDG) [9] or radioactively labeled ligands [10]. By means of PET it is possible to obtain useful insights into brain activation or functional patterns at rest in migraine [11]. Indeed, in the last few decades, PET studies have been extensively used to clarify the complex pathophysiology of migraine improving our understanding of pain processing [12].
Since the 1990s, the spectacular advent of fMRI revolutionized neuroimaging and improved tremendously our understanding of human brain processes to such an extent that in current practice, the definition of structural MRI seems to have shifted to mean “not functional” MRI.
Because migraine is mainly a disorder of brain function, brain fMRI studies are useful to study the underlying mechanisms of migraine. Since migraine is regarded as a disorder of the brain [13], functional neuroimaging offers much in terms of understanding the physiological dysfunction that characterizes migraine. fMRI is increasingly employed for its non-invasive nature, and by exploiting the so-called blood-oxygen-level dependent (BOLD) effect and the neurovascular coupling it has become a powerful tool.
For completeness of information, spectroscopy and chemical shift imaging could be cited. These techniques aim to measure chemical concentrations, and therefore should be considered separately from other MR techniques.
2 Structural Neuroimaging Changes in Gray Matter
In the past decade, VBM has been widely used in many types of headache conditions. However, in recent years, VBM studies have focused on migraine, but the results showed some contradictions. Although the physiological mechanisms underlying CT are not completely understood, thinning and thickening may reflect cytoarchitectural changes of neuronal density or synaptic pruning as well as the cortical hyperexcitability of the migraine brain.
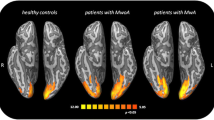
An initial VBM study [14] explored 11 patients suffering from migraine with aura (MwA) and 17 patients with migraine without aura (MwoA), each patient’s group compared with a HC group. The authors found no global or regional macroscopic structural difference in global GM or WM volumes between either patients with migraine (taken as homogenous groups) and HC or between patients with MwA and MwoA. The authors suggested that other methods of phenotyping migraine, such as by genotype or perhaps treatment response could help to better address the issue of subtle structural change in the brain of migraineurs. Rocca et al. [15] followed on this line, according with data from a population-based MRI study [16], and demonstrated that female patients with migraine have a high risk of developing WM hyperintensities (WMH), independently from the presence or the absence of aura. GM density abnormalities were investigated, by using a 3-T MRI scanner and an optimized version of VBM analysis, in seven patients with MwA and nine patients with MwoA (showing visible abnormalities on T2-weighted images) and HC. In these patients, characterized by a peculiar “neuroradiological phenotype”, a reduced GM density, mainly located in the frontal and temporal lobes were observed when compared with HC. Moreover, an increased GM density of both periacqueductal (PAG) and dorsolateral pons (dLP), brain areas strictly related to the pathophysiological substrates of migraine, has been observed in patients with MwA when compared with patients with MwoA. Interestingly, reduced GM density was strongly related to age and disease duration in migraineurs.
A separate VBM study [17] aimed to evaluate the presence of global or focal GM or WM alterations in 27 migraineurs compared to HC and between 16 episodic and 11 chronic migraineurs confirmed that migraineurs are characterized by a significant GM reduction in several cortical areas involved in pain circuitry, independently from the presence of WMH. Episodic and chronic migraineurs (taken as homogenous group) presented a significant focal GM reduction in the right superior temporal gyrus (STG), right inferior frontal gyrus (IFG) and left precentral gyrus (PCG) when compared with HC. Furthermore, chronic migraineurs showed a focal GM decrease in the bilateral anterior cingulate cortex (ACc), left amygdala, left parietal operculum, bilateral insula, left middle frontal gyrus (MFG) and IFG when compared to episodic migraineurs. A significant correlation between GM reduction in ACc and frequency of migraine attacks was found in all the migraineurs. Similarly, Kim et al. [18] demonstrated a significant GM volume reductions in the bilateral insula, motor/premotor and prefrontal cortex (PFc), ACc, right posterior parietal cortex, and orbitofrontal cortex (OFc) in migraineurs (five with MwA and 15 with MwoA) when compared with HC. Observed GM volume changes were related to both increasing headache duration and frequency. A different study [19] has also supported a significantly GM volume reduction in the left medial PFc, dorsal ACc, right visual cortex (Vc), cerebellum and brainstem in 21 patients with MwoA compared with HC. The findings confirm previous observations of a significant correlation between GM reduction in ACc and the frequency of migraine attacks in migraineurs. All together, these VBM studies suggest the concept that migraine may be considered a progressive disorder. Indeed, frequent nociceptive inputs related to repeated migraine attacks in the course of migraineurs life could modify the structural patterns of specific brain regions involved in pain processing.
To clarify the role of repetitive noxious inputs as experienced by migraineurs and underlying GM changes, an elegant experimental paradigm has been conducted in HC receiving repetitive painful stimulation and innocuous thermal stimuli on the right forearm for 11 consecutive working days [20]. Behavioural data demonstrated that 14 HC were “sensitised”, whereas the others 13 HC were “habituated” over the stimulation days. The VBM analysis has revealed in the group of “sensitisers” a significant reduction of GM density in several brain regions involved in pain processing such as the ACc, the insular cortex and the frontal cortex (Fc). By contrast, pain “habituaters” did not show any density changes in the GM. The repetitive application of painful stimuli changed the GM density in pain processing brain regions exclusively in those subjects who were characterized by the lack of habituation. Decrease GM density and increasing pain ratings over time observed in “sensitisers” HC are similar to findings observed in migraineurs and in consequence, an underlying sensitization phenomenon could be suggested also in migraineurs.
On the other hand, the presence of GM abnormalities early in the disease course, and the absence of correlation with patient clinical characteristics suggest that they may represent a phenotypic biomarker of migraine condition more than a consequence of repetitive nociceptive inputs experienced during migraine attacks. Indeed, using a 3.0 T scanner, significant GM atrophy of several regions of the frontal and temporal lobes and an increased volume of the right putamen have been observed in 12 paediatric migraineurs (7 with MwA and 5 with MwoA) when compared with paediatric HC [21]. Moreover, the left fusiform gyrus showed an increased volume in patients with MwA compared to patients with MwoA and HC, whereas it was significantly atrophied in patients with MwoA when compared to the other two groups. Reduced regional GM was not correlated with disease duration and attack frequency, whereas a negative correlation was found between increased volume of the putamen and disease duration.
Nevertheless, in our studies [22,23,24], in which both functional and structural investigations have been conducted, no VBM abnormalities have been found in patients with MwoA compared to both patients with MwA and HC. To identify consistent results of VBM studies in migraineurs a recent meta-analysis [25] has been performed using activation likelihood estimation. A total of five studies were considered, comprising 126 migraineurs (including 23 patients with MwA, 41 patients with MwoA, 11 patients with episodic migraine and 16 with chronic migraine as well as 19 patients with menstrual migraine and 16 with not menstrual migraine) and 134 HC. The included studies have reported GM volume reduction at 84 coordinates as well as GM volume increase at two coordinates in migraine. There were significant reductions in middle Fc and the inferior Fc in migraineurs. However, due to difficulties related to VBM studies including migraineurs with different phenotypes in a single group (specifically both patients with MwoA and patients with MwA) or migraineurs with a single phenotype without comparison with other phenotypes (e.g. only patients with MwA) the authors were not able to perform a subgroup analysis and separate meta-analyses on each migraine phenotype. In consequence, whether VBM abnormalities are strictly related to specific subtypes of migraine or can distinguish the different subtypes of migraine is not defined.
Differences in CT have been reported by Hadjikhani and colleagues in two seminal studies in migraineurs [26, 27]. In the first study, the authors examining the motion-processing network in 24 migraine patients (12 with MwA and 12 MwoA) and HC founded that brain areas involved in motion processing were thickened in all migraineurs. Interestingly, one area of thickening corresponded to the region where previously was found the source of cortical spreading depression (CSD) during migraine aura [28] (area V3A) (see below). This finding raises the question as to whether a “silent” CSD develops as well in MwoA and structural abnormalities in the network of motion-processing areas could account for, or be the result of, the cortical hyperexcitability observed in migraineurs. The second study investigated morphologic changes in the somatosensory cortex (SSc) in 24 migraineurs (12 with MwA, 12 with MwoA) and 12 HC. The authors reported that migraine group had on average thicker SSc than the HC group. The most significant CT changes were observed in the caudal SSc, where the trigeminal area, including head and face, is somatotopically represented.
To delineate possible relationships between CT changes and clinical variables in migraineurs, cortical abnormalities have been investigated in an homogeneous group of 56 patient with MwoA (showing T2-visible WMH) compared with HC [29]. In these patients, cortical thickening in left rostral MFG and bilateral post-central gyri have been observed. The average CT of bilateral post-central gyri positively correlated with disease duration as well as estimated lifetime headache frequency.
Hougaard and colleagues [30] demonstrated difference in CT in the IFG comparing the typical migraine headache side of the patients to the contralateral side in 13 patients (within-subject comparisons) with frequent side-locked MwA (visual aura consistently occurring in the same hemifield). Interestingly, in the same work, the authors found no differences in GM structure with regard to aura, suggesting a structural reorganization of pain inhibitory circuits in response to the repeated intense nociceptive input due to the headache attacks. CT findings have been further elucidated by another study [31] conducted on 46 female migraineurs indicating that these patients show a lack of thinning in the insula by age in contrast to HC.
Recently, Schwedt and colleagues have conducted several investigations on CT changes in migraineurs. In a very interesting study [32], an atypical association between migraine and cortical aging has been demonstrated in 27 migraineurs (18 with MwoA, 9 with MwA) compared with HC. The authors demonstrated that, although both migraineurs and HC have expected age-related thinning in many regions along the cortical regions, migraineurs show structural alterations of temporal and parietal regions that become more pronounced over time. Moreover, CT-to-pain threshold correlations differed between migraineurs and HC for bilateral STG/inferior parietal gyrus (IPG), right PCG, posterior cingulate cortex (PCc)/precuneus, in 31 migraineurs (21 with MwoA, 10 with MwA) compared to HC [33]. In other terms, migraineurs exhibit a non-significant positive correlation between CT in STG/IPG with pain thresholds when compared with HC. Since this region participates in orienting and attention to painful stimuli, absence of the normal correlation might represent a peculiar inability to inhibit pain sensation via shifting attention away from the painful stimulus in migraineurs. Nevertheless, we cannot exclude that individual CT variability could be involved in pain perception as demonstrated by Erpelding and colleagues [34] using a high-resolution structural MRI in HC. In this study, brain GM analysis revealed a strong correlation between greater thermal and pain sensitivity and cortical thickening of the primary SSc. Additionally, greater sensitivity to cold stimuli correlated with CT in the paracentral lobule, and greater warm detection correlated with cortical thinning in the ACc. The authors also found that greater heat pain sensitivity correlated with thickening in the PCc and the OFc.
Furthermore, a study to identify the brain interregional CT correlations that most differed between migraineurs and HC has been conducted [35] on 64 migraineurs compared to HC.
CT was determined for 70 brain regions that cover the cerebral cortex and CT correlations amongst these regions were calculated. A model containing 15 interregional CT correlations differentiated groups of migraineurs from HC with high accuracy. Specifically, the right temporal pole was involved in 13 of the 15 interregional correlations while the right middle temporal cortex was involved in the other two, suggesting that these regions play an important role in migraine pathophysiology.
An alternative strategy to quantify GM morphometric abnormalities involves the use of SB methods, which produce measures of CT and cortical surface (CS). These two measures are thought to reflect different structural characteristics of the human cortex and to be driven by distinct cellular factors [36]. CS area increases dramatically during late foetal development as a consequence of cortical folding, while CT changes dynamically throughout the life span as a consequence of development and diseases [37, 38]. Cortical abnormalities, using the highly sensitive SB morphometry, have been explored in migraineurs (28 with MwA and 28 with MwoA) compared with HC [39]. No significant CT differences in SSc, cingulate gyrus, or V3A/MT+ were found between the groups, including analysis of specific subregions previously reported to be affected in migraineurs [39]. Interestingly, given the sample size, power analyses indicated that even a small difference in CT could have been detected between groups. In another study [40], CT and CS abnormalities have been investigated in patients with migraine to assess their correlation with clinical and radiologic manifestations of the disease. Both CT and CS areas were estimated in 63 migraineurs (31 with MwoA and 32 with MwA) compared with HC. Among patients with MwA, 25 experienced exclusively episodes of MwA, while seven experienced episodes either MwA or MwoA. Migraineurs showed reduced CT and CS area in regions involving in pain processing. Conversely, these two metrics were increased in regions involved in executive functions and visual motion processing. The anatomic overlap of CT and CS area abnormalities was only minimal, with CS area abnormalities being more pronounced and more widely distributed than CT abnormalities. CT and CS area abnormalities were related to aura and WMH but not to disease duration and attack frequency. These results shed a light on cortical abnormalities that could be observed in migraineurs, representing the results of a balance between an intrinsic predisposition, as suggested by CS area abnormalities, and disease-related processes, as indicated by CT abnormalities.
It is well known that the thalamus exerts a critical role in pain processing and cortical excitability control and to investigate thalamic microstructure an innovative multiparametric approach at high-field MRI has been used by Granziera and colleagues [41] in migraineurs (22 with MwoA and 15 patients with MwA) and HC. The authors found that patients with MwA exhibit broad changes in thalamic nuclei when compared with MwoA patients and HC. No structural differences in thalamic nuclei involved in pain processing (such as the ventro-postero-lateral nucleus and ventro-postero-medial nucleus) were observed in both patients with MwA and MwoA. Furthermore, by means of T2* relaxation times evaluation, a relatively higher iron accumulation in the thalamus of patients with MwA compared with patients with MwoA was demonstrated, suggesting a role in pathophysiological mechanisms underlying migraine attacks.
A very interesting functional and morphometric study [42], using high resolution MRI, aimed to investigate hippocampal morphometric changes in migraineurs with different frequency of headache attacks (ten patients suffering from migraine with a low frequency of attacks and ten patients suffering from migraine with a high frequency of attacks) compared with HC. By means of a segmentation approach a significant larger bilateral hippocampal volume was found in low frequency group compared with migraineurs with high frequency and HC. The observed alterations, suggesting an initial adaptive plasticity that may then become dysfunctional with increased frequency, support a hippocampus role in migraine. Indeed, structural (and functional) changes may be the result of repeated stress and, as a consequence, may alter biological responses (including the stress response) over time, as a negative cascade adding to the disease burden through allostatic overload. These responses would appear to be maladaptive, and lead to allostatic overload over time, and have significant implications for disease progression.
In conclusion, it is still not clear whether morphological changes are cause or consequence of abnormal pain processing, but it is well established that disease duration and frequency of migraine attacks correlate highly with such changes. On the other hand, results of several morphologic studies have consistently demonstrated structural abnormalities in brain regions that are part of the network subserving supraspinal nociceptive processing not only in migraine but also in other chronic painful conditions, including facial pain [43], post-traumatic headache [44] and fibromyalgia [45] supporting the notion that chronic stimulation of these areas might cause a loss of GM volume. According with this interpretation, the broad spectrum of individual characteristics in pain perception may be attributable to preceding vulnerabilities. Similarly, an inherited susceptibility for migraine may be responsible for a developmental change that leads to the structural differences in these areas [4]. Similarly, resilience or susceptibility to migraine might also be a consequence of inter-individual variations in brain structure.
3 Structural Neuroimaging Changes in White Matter
Although the clinical definition of migraine requires the brain of a patient to be normal and structural changes to be absent [46], an increasing number of studies support the association of migraine with an increased risk of MRI-detectable WMH [47, 48] probably associated with clinical parameters of disease severity, such as frequency of attacks, migraine duration as well as disease age and family history [49,50,51]. The mechanisms causing WM abnormalities and clinical implications for patients are not yet determined although several causes have been hypothesized [52, 53]. A longitudinal MRI study found clinically silent brain WMH to be predominantly progressive in nature [54], whereas other observations suggest no direct association between clinical features of migraine and WMH progression [55], supporting the hypothesis that this association is stable in older age and may be primarily attributable to changes occurring earlier in life [56].
Microvascular ischemic mechanisms, which in turn may be associated with ischemic stroke, have been suggested [57, 58], independently from aura symptoms [59] and a specifically increased risk of ischemic stroke as well as the risk for cognitive impairment due to WMH in migraineurs should not be assumed [60]. Probably WMH are markers of transient breakdown of the blood–brain resulting from intense but self-limited cerebral hyperperfusion [61] or decreased antioxidant response in migraineurs [62]. Very recently, an MRI study to determine the frequency of WMH and the relationship with both migraine characteristics and cardiovascular risk factors has been conducted in 90 migraineurs (70 with MwoA and 20 with MwA). Silent WMH were observed in 32% of migraineurs and were found more frequently in patients with chronic migraine. The majority of lesions were located in the supratentorial right hemisphere. Migraineurs with and without WMH did not show significant differences in cardiovascular risk factors, such as smoking, serum cholesterol, oral contraceptive pills use and body mass index. These results suggest that the relationship between migraine and WMH may be directly due to the effects of migraine itself (probably via a significant T cell accumulation, sphingomyelinase activation, increased oxidative stress and reduction of both GM and WM triggered by CSD)[63] rather than to cardiovascular risk factors [64]. A different possible explanation may rely on a peculiar vascular vulnerability of migraineurs that may contribute to the pathogenesis of migraine and, in the presence of some other unknown factors may also contribute, over time, to the development of both WMH and cardiovascular disease. At the moment, we can only consider migraine as a risk factor for WMH in the brain [65] but there are no reliable features that may indicate which subjects, across the overall migraine population, will develop vascular events [66], although a link to an increased risk of stroke, especially in patients with MwA, cannot be ruled out. The mechanisms underlying the relationship between migraine and WMH suffer from the lack of conclusive evidence and further research addressing this topic seems essential.
About the relation between migraine and dilated perivascular spaces a large, blinded, population-based study showed no differences in the number of visible perivascular spaces in the basal ganglia and hemispheric WM in both patients with MwA and MwoA compared with non-migrainous headache patients and HC [67].
4 Microstructural Neuroimaging Changes in White Matter
Newer imaging techniques (i.e. DTI) have provided more detailed information about microstructural brain changes in these patients. DTI allows visualization of the orientation and anisotropy of water diffusion characteristics, which are mainly influenced in the brain by tissue features and cellular membranes. It enables the reflection of the integrity of fibre bundles and to detect microstructural alterations in WM, which cannot be visualized on conventional MRI sequences [68]. Altered anisotropy can be the consequence of not only structural WMH, but also of alteration of myelination and axon density. Reduced fractional anisotropy (FA) may result from demyelination, axonal loss, gliosis and inflammation. Axonal diffusivity (AD) may help to detect axonal degeneration, whereas radial diffusivity (RD) may be affected by myelin loss [69]. Previous DTI studies in episodic migraineurs reported several alterations in the interictal phase. To assess the correlation between the extent of macroscopic T2-weighted abnormalities, specifically WMH, and “occult” tissue damage (pathological damage of normal appearing brain tissue), Rocca and colleagues [70] investigated, by means of DTI technique with a histogram-based analysis, 34 migraineurs and 17 HC. Migraineurs showed lower mean diffusivity (MD) histogram peak height of the normal appearing brain tissue compared with HC, whereas no differences were found in FA histogram-derived metrics between migraineurs and HC. Interestingly, no difference was found for any MD and FA histogram-derived metrics between migraineurs with and without brain MRI lesions, and between patients with MwA and MwoA. The authors concluded that although brain damage may extend beyond T2-weighted abnormalities in migraineurs, the severity of these “occult” changes may result to have a mild impact. DTI approach [26] has been used also to explore motion-processing network, involving brain areas known as a CSD source involved in visual aura in patients with MwA and probably in ‘‘silent’’ CSD in MwoA, in 24 migraineurs (12 with MwA; 12 with MwoA) and HC. WM abnormalities in the areas subjacent visual motion-processing (MT+ and V3A) in superior colliculus and the lateral geniculate nucleus were found in migraineurs compared with HC. Another investigation [71] in 24 migraineurs (12 with MwoA; 12 with MwA) compared with HC demonstrated permanent interictal areas of lower FA in the ventrolateral PAG in patients with MwoA and in the ventral trigeminothalamic tract in patients with MwA, pointing to an effect of migraine on the trigeminal SSc and modulatory pain system.
Microstructural abnormalities have been investigated by means of a region-of-interest (ROI) approach, in the corpus callosum (CC) in 24 patients with MwoA (12 without depressive/anxious disorder; 12 with depressive/anxious disorder) compared with HC [72]. Significant differences in FA values at all locations of the CC among the three groups were observed. The FA values from both migraine groups were significantly lower than those from HC. The FA values from migraineurs with depressive/anxious disorder were significantly lower than those of the migraineurs without depressive/anxious disorder. There were negative correlations between FA value of genu of the CC and disease course as well as FA value of genu and body of the CC and headache frequency. However, negative correlations were also found between FA values at all locations of the CC and anxiety and depression severity, suggesting that microstructural changes in the CC could be a possible neuroanatomical basis of migraine complicated with depressive and anxious disorder.
Abnormalities in CC and microstructural WM changes related to depressive disorder have been independently explored also by means of a novel approach to detect microstructural WM integrity alterations using a diffusion-weighted imaging with a fine-tuned nonlinear registration and nonparametric permutation testing in an alignment-invariant tract representation (tract-based spatial statistics [TBSS]) in migraineurs [73]. Indeed, reduced FA values of the genu of CC has been demonstrated in 21 patients with MwoA compared with HC [74]. Furthermore, WM microstructural abnormalities seems to be correlated with interhemispheric functional connectivity (f-connectivity) changes in of ACc in these patients, suggesting the possibility that WM changes of the CC modulate the resting-state (RS) f-connectivity between defined and highly pain-related brain areas such as ACc.
Yu and colleagues (who had already previously demonstrated significant lower FA, MD and AD in multiple brain regions in 20 patients [75] with MwoA), investigated WM integrity in 40 patients with MwoA (20 with depressive symptoms and 20 without depressive symptoms) compared with HC. Patients with MwoA as a group showed several WM tracts abnormalities compared with HC. However, migraineurs with depression symptoms showed decreased FA and increased MD and RD, with conserved AD, in WM tracts including the genu, body and splenium of the CC, bilateral superior longitudinal fasciculi and the right anterior corona radiate compared with migraineurs without depression symptoms. These WM tracts changes correlated significantly with depressive severity. The results suggested that both depression symptoms (more sensitive as RD) and migraine (more sensitive as AD) could affected WM integrity. FA and apparent diffusion coefficient (ADC) values of red nuclei, PAG, thalami, posterior limbs of internal capsules and subcortical WM were explored, by [76] means of a ROI approach, in 14 patients with MwoA during a migraine attack compared with HC. WM abnormalities were found only in the red nuclei, where ADC showed higher values than in HC, without correlation with age, duration of disease, frequency of attacks and localization of pain in migraineurs.
Recently, in a DTI study [77] a comparison was made of FA and MD obtained from the analysis of migraine-recurrence-induced changes in the thalamus of 24 patients with MwoA both during (10 patients) and between (14 patients) attacks compared with HC. During the ictal phase (but not during interictal period) patients with MwoA showed a significantly higher FA and slightly lower MD values in bilateral thalami compared with HC. Furthermore, right thalamic FA was positively correlated with the number of days since the last attack in migraineurs. These findings support previous neurophysiological evidence of altered interictal thalamic activity in migraine probably related to plastic peri-ictal modifications in regional branching and crossing of fibres.
Obviously, it is not possible to confirm the exact underlying mechanism for the above mentioned observation and in particular whether WM microstructural changes are responsible for triggering an attack or if they are the consequents of the attack itself. In order to evaluate whether WM abnormalities in the first period of migraineurs’ life, TBSS and DT probabilistic tractography analysis have been used in 15 paediatric migraineurs [78] and HC. A significant lower MD, AD and RD diffusivity of WM tracts located in the brainstem, thalamus and fronto-temporo-occipital lobes bilaterally has been shown in paediatric migraineurs compared to HC. Patients also exhibited increased FA of the optic radiations. No correlation was found between WM tract abnormalities and disease duration and attack frequency, suggesting that WM abnormalities could be interpreted as microstructural features of migraineurs from the earliest stages of life and independently from clinical parameters of disease severity.
Global probabilistic tractography was used to investigated the integrity of WM tracts that underlie regions of the “pain matrix” and to assess putative correlation with disease duration in 23 migraineurs and HC [21]. Migraineurs showed greater MD in the left and right anterior thalamic radiations, left corticospinal tract, and right inferior longitudinal fasciculus tract. Migraineurs also showed greater RD in the left anterior thalamic radiations, left corticospinal tract as well as left and right inferior longitudinal fasciculus tracts. A positive correlation between migraine duration and MD in the right anterior thalamic radiations and left corticospinal tract has been observed in these patients. By means of DT tractography structural changes in optic radiation were quantified in seven patients with MwA, eight patients with MwA (experiencing visual aura) and HC [79]. WM changes located in optic radiation and their relation to clinical manifestations and T2-visible hyperintensities were investigated. No difference was found for any of the WM fibre bundles metrics between patients with MwoA and HC, whereas patients with MwA were characterized by a reduced average FA of both optic radiations compared with HC and reduced average FA of the right optic radiation compared with patients with MwoA. They also showed higher right optic radiation MD than HC. In this study, optic radiation metrics were not correlated with clinical parameters. More recently, DTI [80] data were analyzed using a TBSS approach and FA, MD, RD and AD were compared between 39 chronic migraineurs, 34 patients with episodic MwoA and HC as well as between migraineurs as a group and HC. In contrast to previous studies, the authors did not find alterations in DTI-derived metrics in episodic migraineurs compared with HC. Furthermore, no statistically significant differences in chronic migraineurs when compared with episodic migraineurs and HC were found. These data revolutionized previous insights about microstructural changes in both patients with episodic or chronic migraine. However, accordingly with Neeb and colleagues investigations, in our previous multiparametric studies [22,23,24] we have never observed DTI abnormalities (with both a whole-brain and ROI approach) in patients with episodic MwoA and MwA. This suggests that patients’ sample homogeneity could be a critical factor to justify the different microstructural findings reported by different researchers in the same disease.
5 Functional Neuroimaging During Spontaneous Migraine Attacks
Because migraine is mainly a disorder of brain function, fMRI has been considered an appropriate tool to investigate the underlying mechanisms of migraine activation. In this context, the headache attack might be considered an obvious and “specific” stimulation paradigm, and BOLD changes during the headache attack could be contrasted to a baseline condition observed during the interictal period. Weiller and colleagues in a pioneering H 152 O PET study have shown significantly higher regional rCBF values in cingulate, Vc, auditory cortex and brainstem, specifically in the dorsal pons (dP) during a spontaneous attack [81] in nine patients with MwoA. The observed activations were abolished, after a therapeutic dose of sumatriptan, in cortical areas but not in the brainstem, suggesting that brainstem activation was unlikely to be the result of pain perception nor an increased activity of anti-nociceptive systems (because a persistent activation was present also after sumatriptan-related pain relief). Another H 152 O PET study, with a high-resolution PET, was conducted to test the hypothesis of brainstem activation during migraine attacks and to refine the anatomic brainstem localization. For this aim, five migraineurs (two patients with MwA and three patients with MwoA) underwent imaging both during spontaneous migraine attacks and interictal periods. A significant activation in the dP, lateralized to the left, was observed comparing the ictal with interictal states. The activation was also demonstrated in the right ACc, PCc, cerebellum, thalamus, insula, PFc and temporal lobes. Contextually, an area of deactivation during migraine phase was located in the pons, lateralized to the right [82].
A very interesting H 152 O PET study [83] was conducted in seven patients with MwoA in the early migraine phase, after headache relief by sumatriptan and during an attack-free period. The authors observed, during the headache phase, significant activations not only in the midbrain and pons but also in the hypothalamus and the activations were persistent even after successful treatment by sumatriptan. These findings support the concept that hypothalamic involvement may be not strictly related to trigemino-autonomic cephalalgias [83, 84] and corroborate clinical observation on the key role of the hypothalamus in the pathophysiological aspects of migraine attacks [85, 86] such as the trigger factors and the premonitory features. Premonitory phase of migraine and related neuronal correlates have been more recently explored by means of H 152 O PET imaging. Glyceryl trinitrate (nitroglycerin) has been used to trigger premonitory symptoms and migraine headache in eight patients with MwoA who habitually experienced premonitory symptoms during spontaneous attacks. In this case, the premonitory phase has been considered as the period following when the nitroglycerin-induced non-specific headache phase had completely ceased and patients started to experience symptoms warning them of an impending headache. Activations in the posterolateral hypothalamus, midbrain tegmental area, PAG, dP and Vc, temporal cortex and PFc have been found comparing the first premonitory scans to baseline scans in all migraineurs. In particular, hypothalamic activation observed in the premonitory phase of glyceryl trinitrate-triggered migraine attacks can explain many of the premonitory symptoms and provide insight into the migraine activation due to homeostasis changes [87]. Among premonitory symptoms nausea occurs in about a quarter of migraineurs, suggesting primary brain alterations unrelated to the experience of headache. To explore the neural correlates of nausea, a H 152 O PET study has been performed in the premonitory phase of nitroglycerin-induced migraine in ten patients with MwoA and then patients with and without nausea were compared (three patients had nausea and seven did not have nausea in the premonitory phase during the scanning session). The results showed activation in brain circuits mediating nausea such as rostral dorsal medulla (including the nucleus tractus solitarius, the dorsal motor nucleus of the vagus nerve and the nucleus ambiguous) and PAG only in the patients experiencing nausea. These structures were involved independently from pain and trigeminal activation, suggesting that nausea is a centrally driven symptom in migraine [88].
Recently, a 18FDG-PET study [89] has been conducted to assess altered brain metabolism in vestibular migraine (VM), a disabling neurological disorder characterized by vestibular symptoms, such as vertigo, dizziness, or imbalance in at least 50% of migraine episodes in patients with MwoA or with aura MwA [46]. Two patients with VM were investigated during and between VM attacks in addition to detailed neurotological evaluation. During the attacks, both patients showed an activation of the bilateral cerebellum and frontal cortices, and deactivation of the bilateral posterior parietal and occipito-temporal areas. One patient also showed hypermetabolism in the dorsal pons and midbrain, right posterior insula,and right temporal cortex while the other patient had an additional activation of the left temporal cortex. Compared with interictal images, ictal PET showed increased metabolism in the bilateral cerebellum, frontal cortices, temporal cortex, posterior insula, and thalami. The findings of contemporary activation of the vestibulo-thalamo-cortical pathway and decreased metabolism in the Vc may represent a reciprocal inhibition between the visual and vestibular systems in patients with VM.
Experimental investigation by PET imaging has been further improved by the availability of suitable radiotracers targeting different neurochemical systems [90]. Since 5-hydroxytryptamine (5-HT)CA receptors were thought to be implicated in migraine pathogenesis, PET studies with specific radioligands have been conducted to investigate serotoninergic function in migraineurs. In an early study [91], PET with 18F-fluorosetoperone (a 5-HT2-specific radioligand) did not reveal differences of cortical 5-HT2 receptors’ distribution volumes in migraineurs (five patients with both MwA and MwoA and four patients with MwoA) when compared with HC. Another PET study using an α-[11C]methyl-l-tryptophan tracer was conducted to measure brain serotonin synthesis in 11 patients with MwoA during attacks, reporting an increased rate of brain serotonin synthesis in the acute phase [92]. These data have been recently confirmed using specific antagonist of serotonin receptors [93], and the authors advocate that increased 5-HT1A receptor availability is present early during migraine attacks in the pontine raphe of migraineurs [84, 91].
PET investigations during migraine attacks were also employed to investigate the effects of molecules known to be clinically effective. A H 152 O PET study has been conducted to investigate the effect on brain circulation of a 5-HT1B/1D receptor agonist (rizatriptan), which caused a 13% CBF and blood volume decrease possibly related to the effect of triptans on the large cerebral arteries or on arterioles [94]. In the same period, an interesting PET study using radioactive [carbonyl-11C] zolmitriptan [95] evaluated the uptake and distribution of triptans into the CNS supporting their central mode of action. Although PET imaging has offered much in terms of understanding the neural correlates of migraine and associated symptoms, and functional changes depending on pharmacological modulation, many questions about cerebral network functions in migraine are still open and the pending solution is dependent also on the refinement of technology. Today, there is an increasing interest in developing PET-radiotracers for specific receptors thought to be implicated in pain and headache pathogenesis such as glutamate and opioid receptors [12]. However, we still lack information regarding the impact of migraine attacks and its relief on the function of μ-opioid receptor (μOR) mediated neurotransmission, the primary target of opioid medications. This line of enquiry is of particular importance as this neurotransmitter system is arguably the endogenous brain mechanism most centrally involved in pain regulation, as well as in the effectiveness of opioid medications. Recently, a PET study using the selective l-opioid receptor (lOR) radiotracer [11C]carfentanil [96] has elucidate the allodynic response of the central l-opioid system during spontaneous migraine attack following a sustained pain threshold challenge on the trigeminal ophthalmic region. Six migraineurs showed ictal cutaneous allodynia (CA) during the thermal challenge that was concurrent and positively correlated with lOR activation in the midbrain, extending from red nucleus to ventrolateral PAG. These findings demonstrated for the first time in vivo the high lOR activation in the migraineurs’ brains in response to their allodynic experience. The same research group using the same technique evaluated in vivo the μ-opioid system during spontaneous migraine attacks in seven migraineurs [97]. In the ictal phase, there was μOR activation in the medial PFc, which was strongly associated with the μOR availability level during the interictal phase. Furthermore, μ-opioid binding changes showed moderate negative correlation with the combined extension and severity of the attacks. These results indicated for the first time that there is high μOR activation in the brain during migraine attacks in response to pain. Similar PET data have been used to investigate, using a novel 3D immersive and interactive neuronavigation (3D-IIN) approach, the endogenous µ-opioid transmission in the ictal migraine phases in a patient with MwA who has been suffering with migraine for 10 years [98]. During the ictal PET session (spontaneous headache attack) there was a reduction in µOR BPND in the pain-modulatory regions of the endogenous µ-opioid system during the ictal phase, including the cingulate cortex, nucleus accumbens (NAcc), thalamus and PAG, indicating that µORs were already occupied by endogenous opioids released in response to the ongoing pain [98].
In MwA patients, early imaging studies have been performed to explore the theory suggesting that CSD was the electrophysiological correlate of visual aura [99, 100]. The seminal Olesen’s SPECT study has shown that unilateral occipitoparietal oligemia during the aura was preceded by hyperemia, that oligemia may spread anteriorly and that severe headache could occur during this oligemic phase [101]. However, the work of Hadjikhani and colleagues [28] could be considered the most important fMRI study to better understand the pathophysiological mechanism underlying the aura phenomenon. The authors, using high-field fMRI with near-continuous recording during visual aura in three subjects, have initially observed a focal increase in BOLD signal (possibly reflecting vasodilation), developed within extrastriate cortex (area V3A). This BOLD changes progressed contiguously and slowly over the occipital cortex, congruent with the retinotopy of the visual percept. Following the same retinotopic progression, the BOLD signal then diminished (possibly reflecting vasoconstriction after the initial vasodilation), as did the BOLD response to visual activation. During periods with no visual stimulation, but while the subject was experiencing scintillations, BOLD signal followed the retinotopic progression of the visual percept. These data strongly suggest that an electrophysiological event such as CSD generates the aura in human visual cortex. Today, converging evidence suggests that the oligemia persists well into the pain phase supporting the concept that vasodilatation could not explain the pain during migraine attack [28, 102]. The role of CSD in MwA is well established, but its contribution to the pathophysiology of MwoA, which involves the new concept of a clinically silent CSD, is still an intriguing issue [103].
6 Functional Neuroimaging During Painful Stimuli
We previously wrote that migraine attack might be considered such an obvious and “specific” stimulation paradigm to investigate the underlying mechanisms of migraine activation. Nevertheless, the main limitation of this experimental approach lies in the capture of spontaneous and unpredictable attacks of relatively short duration, such as migraine, while imaging techniques require considerable planning [6, 104]. In the last few years, these factors have determined the selection of studies dominated by noxious stimulation paradigms designed to better explore abnormalities in sensory, adaptive, and affective components of pain processing network in migraineurs and HC. Since pioneer studies using nitroglycerine or capsaicin to elicit cranial pain in migraineurs, various noxious stimuli have been used in imaging studies. Among these, May and colleagues, to test the hypothesis that brainstem activation may represent the so-called ‘migraine generator’, performed a PET study [105] in seven HC. In these subjects, a small amount of capsaicin was administered subcutaneously in the right forehead to evoke a burning painful sensation in the first division of the trigeminal nerve. The authors found an increased rCBF bilaterally in the insula, in the ACc, the cavernous sinus and the cerebellum. Interestingly, using the same stereotactic space limits as in Weiller’s study, no brainstem activation was observed in the acute pain state compared to the pain-free state. However, an increased activation was found in the region of the cavernous sinus, suggesting that this structure may be involved in trigeminal pain. In the last years, pain-inducing heat is applied with an MRI-compatible contact thermode with a predefined or individualized temperature to each patient to elicit pain of moderate or severe intensity. In particular, the contact thermode can offers an easy approach to explore trigeminal system using a painful stimulation. Indeed, the regions innervated by the three branches of the trigeminal nerve can be easily distinct and stimuli to activate the trigeminal system are well-identified. Moreover, the trigeminal system reflects a somatotopic brain representation, and functional changes in trigeminal system can be detected at multiple levels (from trigeminal ganglion to the trigeminal nucleus and even in higher brain centres). For these peculiar characteristics, experimental trigeminal pathway activation has been extensively used to explore neural mechanisms underlying migraine during both headache attack and interictal period.
The elegant study of Moulton and colleagues [106] could be considered one of the landmarks of migraine fMRI research in the course of a painful stimulation using the contact thermode. The authors determined the heat pain threshold as the average of three different evaluations in 12 migraineurs and HC. During BOLD-fMRI sessions, a non-painful stimulation (41 °C) and a noxious heat stimulus (pain threshold +1 °C) were applied to the side of the forehead involved during migraine attacks. Assuming a brainstem region of interest, during non-painful stimulation there was a significantly greater BOLD response in the dLP in HC than in migraineurs. Conversely, during the painful stimulation a significant activation of the nucleus cuneiformis (NCF), a dLP structure involved in descending pain modulation, was observed. Interestingly, perception of painful stimuli did not show differences between patients with migraine and HC. Clinical and fMRI findings suggested that a central sensitization during attacks may be related to NCF “hypo-function” in patients with migraine experiencing CA. The same research group has lately conducted a BOLD-fMRI study using a painful trigeminal stimulation [107] in 11 migraineurs during the interictal period and HC. Moreover, eight migraineurs were tested by means of the same experimental stimulation during both the ictal and interictal periods. The authors demonstrated, using a ROI-based approach, an increased BOLD response to trigeminal painful stimulation in temporal pole (TP) and parahippocampal gyrus, centred on the entorhinal cortex (EC) in migraineurs, during the interictal period compared with HC and during migraine attack compared with the interictal period. Microstructural connectivity analysis, by means of DTI, revealed that TP and EC showed an enhanced connectivity with different brain structures involved in pain processing. These findings shed some light on migraine mechanisms, suggesting that hyperexcitability of associative multisensory areas, such as TP and EC (during both migraine attack and the interictal period), may be related to pain circuits.
Our group has explored the functional reorganization of pain-related pathways during trigeminal painful stimulation, using a whole-brain analysis approach, in 16 drug-naïve patients with MwoA during the interictal period [108]. By means of the contact thermode, a severe noxious (53 °C), a moderate noxious (51 °C) and a control (41 °C) stimulus were applied randomly to the maxillary skin. During the control trigeminal stimulus no differences in activation were observed between patients with MwoA and HC, whereas a significantly greater activation to the moderately painful heat stimulus was observed in the perigenual part of the ACc, and a significantly decreased activation to the severe painful heat stimulus was observed bilaterally in the secondary SSc. A group-by-stimulus whole-brain interaction analysis revealed a significant BOLD response in the pons which was associated with higher headache-related disability, intensity of pain in the course of a migraine attack and frequency of migraine. Similarly to the behavioural findings observed in the Moulton’s study [106], patients and HC did not show any significant difference in perception at any level of experimental stimulation. In our opinion, the functional reorganization of pain-related cortical areas in patients with MwoA could represent a compensatory or adaptive mechanism to reduce painful input to the cortex by increasing cerebral anti-nociceptive activity.
A new experimental stimulation has been developed by Stankewitz et a. [109] based on the intranasal administration of low concentration of gaseous ammonia, producing a trigeminal nerve irritation, which can be well-implemented within an event-related BOLD-fMRI study. The authors, for the first time, have explored processing, perception and modulation of pain by means of BOLD-fMRI in the course of repeated trigeminal painful stimulation over several days in 15 migraineurs [110] compared with HC. Migraineurs and HC were stimulated for eight consecutive days. BOLD-fMRI was assessed in the course of trigemino-nociceptive stimuli (ammonia) and no-noxious control stimuli (air puffs) on days 1, 8 and 90 in migraineurs. PFc, ACc, red nucleus and ventral medulla exhibited an increased activity in HC and a decreased response in migraineurs, from the first to the eighth day. These divergent BOLD responses did not correlate with pain perception (i.e. migraineurs and HC showed a gradual decrease of pain ratings from day 1 to day 8, which only marginally increased again on day 90). The findings suggested that altered pain processing networks may explain the dysfunctional neuronal filters of sensory input in migraineurs, likely due to repetitive migraine attacks.
The role of recurring headache attacks in migraineurs has been further explored in association to the migraine cycle in 20 migraineurs [111] (ten patients experienced a migraine attack in the next 72 h after scanning and were therefore in the preictal phase and 13 patients were scanned during acute headache attacks). During painful trigeminal stimulation using ammonia gas, the authors observed a robust activation in cortical and subcortical areas involved in pain processing in migraine patient exclusively within the interictal period and in HC. However, a lower activation in a brainstem area corresponding to the spinal trigeminal nucleus was detected in migraineurs compared with HC. Interestingly, the BOLD response increased during the pain-free migraine cycle toward the migraine attack, and it was down-regulated just before or immediately at the beginning of a migraine attack. In our opinion, beyond the putative role of spinal trigeminal nucleus as “migraine modulator”, this event-related BOLD-fMRI study highlights two important concepts. The first is a phenomenological concept, which is necessary to better understand the neurobiological significance of periodic functional changes of migraineous brain. Migraine cycle spans over several days during different phases (prodromic, aura , headache, resolution and recovery), and trigeminal activity in migraineurs is not constant but strongly variable. The second is a methodological concept, which underlines the importance of taking the time to the next attack into account when investigating migraineurs. Another recent study [112] has investigated the “migraine cycle” and its relation with pain-induced activation of specific brain regions in 24 adult migraineurs (who were at least 48 h pain free) and HC. There were no significant correlations between brain activation and time to next migraine attack. However, a greater pain-induced activation of lentiform nucleus, fusiform gyrus, subthalamic nucleus, hippocampus, middle cingulate cortex (MCc), premotor cortex, SSc, dorsolateral PFc, and a reduced activation in PCG and STG have been observed in migraineurs compared to HC. Moreover, there were significant correlations between BOLD response and headache frequency for MCc, right dorsolateral PFc, left fusiform gyrus, left PCG and left hippocampus and with disease duration for left fusiform gyrus. It is evident that the majority of regions with enhanced pain-induced activation in headache-free migraineurs participate in cognitive aspects of pain perception such as expectation of pain and pain memory. Enhanced cognitive pain processing by migraineurs might reflect cerebral hypersensitivity related to high expectations and hypervigilance for pain. Indeed, pain perception is a complex sensory experience that is processed in a network of distributed cortical areas and within this network (the so-called “pain matrix” or, more recently, “neurolimbic pain network”) the encoding and evaluation of painful events depend crucially on the functional interplay of these regions [113].
7 Functional Neuroimaging During Visual Stimuli
Around 45% of migraineurs report symptoms of light hypersensitivity in the interictal state, and about 90% during a migraine attack [114,115,116]. Significant evidences to understand migraine mechanisms were provided by H 152 O studies using luminous stimulations, which demonstrated a multisensory integration between light perception and trigeminal nociception.
Cao and colleagues [117] investigated Vc activation in the early phase of the visually triggered migraine attack in 12 migraineurs (10 with MwA and visual symptoms, 2 with MwoA). Visually triggered headache and visual change in migraineurs were correlated with spreading suppression of the initial neuronal activation and increased Vc oxygenation. The authors suggest that this spreading suppression may be associated with initial activation of a migraine attack, independent of whether there are associated aura symptoms. Some years later, Boulloche and colleagues [118] have demonstrated that luminous stimulations activated the Vc bilaterally in seven migraineurs also during interictal period. A concomitant heat pain stimulation (applied in the territory of the ophthalmic branch of the right trigeminal nerve) potentiated cortical activation in these patients and induced Vc activation in HC. The authors hypothesized that Vc hyperexcitability could be related to brainstem modulation of cortical excitability characterized by integration mechanisms with trigeminal structures.
Brainstem activation has been explored in 26 migraineurs (23 with MwA; 3 with MwoA) during repeated visual stimulation [119]. Repetitive visual stimulation triggered migraine symptoms in 12 patients: four with MwA developed both visual symptoms and headaches, and six with MwA and two with MwoA experienced headaches only. Four patients who had MwA experienced the onset of their usual aura or onset of their typical headache either during the experiment or immediately after. In the remaining 10 migraineurs, and all HC, visual stimulation failed to trigger symptoms at any time. A significant BOLD response has been observed in red nucleus and substantia nigra in association with visually triggered symptoms of migraine, suggesting that these brainstem structures are a part of a neuronal network activated during an attack. Denuelle and colleagues [120] have investigated photophobic mechanism during spontaneous migraine attacks, after headache relief by sumatriptan and during attack-free interval in eight migraineurs. The authors found that low luminous stimulation could activate the Vc during migraine attacks and after headache relief but not during the attack-free interval. The Vc activation was statistically stronger during migraine headache than after pain relief.
By 1H-magnetic resonance spectroscopy (MRS) [121] changes in brain metabolites due to Vc activation during visual stimulus have been investigated in 22 patients with MwA and 22 patients with MwoA. In the Vc, photic stimulation is linked with a consistent decrease of the N-acetylaspartate (NAA) signal and a parallel increase of the lactate peak in patients with MwA when compared with MwoA and HC. NAA loss might result from a decrease in NAA formation subsequent to ATP depletion, and these data could be related to a transient dynamic uncoupling following a rapid recoupling of oxidative metabolism after stimulation, due to a less efficient mitochondrial functioning in patients with MwA. In view of these associations, several studies have used fMRI to investigate responses to visual stimuli in migraineurs. Some of these studies specifically investigated patients with MwA, because Vc hyperexcitability might predispose the brain to visual hypersensitivity and visual aura .
Using fMRI-BOLD approach [122], light sensitivity and photophobia have been assessed exploring the response of the Vc to light stimuli in 19 patients with migraine (7 with MwA; 12 with MwoA) compared to HC. This study showed a significant hyperexcitability of the Vc with a wider photoresponsive area in migraineurs during interictal period. The authors suggested that the underlying mechanism of cortical reactivity in migraineurs is probably dual and may be part of a constitutional (defensive) mechanism or represents an acquired (sensitization) phenomenon.
Beyond primary visual areas, the dynamics of the basic interictal state with regard to extrastriate, motion-responsive middle temporal area (MT-complex) has been explored with BOLD-fMRI at 3 T using coherent/incoherent moving dot stimuli in 24 migraineurs (12 with MwA, 12 with MwoA) in the interictal period [123]. A weaker bilateral activation has been found in the MT-complex in both patients with MwA and MwoA compared with HC, whereas a significant stronger activation mainly at the left side in response to visual stimulation in the MT-complex was found in patients with MwoA and MwA compared with HC.
Cortical response to a visual stimulus during the interictal period has been compared also in another study investigating 25 patients with MWA and 25 patients with MwoA [124]. Despite similar interictal symptoms of visual discomfort, BOLD-fMRI response to visual stimulation within primary Vc and lateral geniculate nuclei were greater in patients with MwA compared to patients with MwoA and HC suggesting a direct connection between cortical hyperresponsiveness and migraine aura . Based on both altered visual motion processing in striate and extrastriate areas and optokinetic stimulation inducing symptoms associated with migraine in migraineurs, activation patterns and the hemodynamic response to optokinetic stimulation have been explored in 18 patients with MwA [125] using a novel approach based on a structural (by fMRI approach) and temporal (by functional transcranial Doppler) resolution. In this way, the activation pattern of the Vc (V1–V5) as well as the vasomotor reactivity of the posterior cerebral artery have been investigated. The authors found attenuation of the physiological right lateralization with a significantly increased activation in the left V5 complex, the left area V3, and the right V5 complex. Furthermore, the analysis of the visually evoked flow response of the rCBF in the posterior cerebral artery showed a larger side-difference of the offset latency and a reduced steepness of the decreasing slope on the left side, supporting the concept of an interictal motion-processing deficit in migraine. Recently [126], functional interhemispheric differences in responses to visual stimulation between symptomatic and asymptomatic hemispheres during the interictal phase has been evaluated in 20 patients with frequent side-fixed visual aura attacks (≥90% of auras occurring in the same visual hemifield). BOLD responses were selectively increased in the symptomatic hemispheres in the IPG, the IFG and the superior parietal lobule. The migraineurs also showed a significantly increased response in the same cortical areas when compared to HC. These findings suggest a hyperexcitability of the visual network (involved in oculomotor control, guidance of movement, motion perception, visual attention and visual spatial memory system) in the interictal phase of migraine with visual aura .
All together the reported data confirm that migraineurs, during visually stimulating patterns, have high activation in the primary and extrastriate Vc likely correlated to a cortex hyperexcitability that could not be explained only by trigeminal nociception because it persisted also during interictal period.
8 Functional Neuroimaging During Olfactory Stimuli
It is well known that, although the osmophobia is not reported in IHS classification criteria, migraineurs are hypersensitive to odours during and between migraine attacks. Furthermore, half of migraineurs report that certain odours can trigger migraine attacks [127]. PET studies also provided important insights into the neural mechanisms underlying associated migraine symptoms, such as photophobia, phonophobia and osmophobia, the latter being very specific to this form of headache [128]. During olfactory stimulation, migraineurs subjects exhibited a significantly higher activation in piriform and temporal cortices when compared with HC. Demarquay and colleagues [129], using voxel-based and ROI analyses, evaluated olfactory processing in 11 migraineurs experiencing olfactory hypersensitivity and investigated whether rCBF associated with olfactory stimulation was modified in patients compared with HC. During both olfactory and non-olfactory conditions, a higher rCBF in the left piriform cortex and antero-STG in has been found in migraineurs. During odour stimulation, migraineurs also showed significantly higher activation in the left temporal pole and significantly lower activation in the frontal (left IFG as well as left and right MFG) and temporo-parietal (left and right angular, and right posterior-STG) regions, PCc and right locus coeruleus. These results could reflect a particular role of both the piriform cortex and antero-STG in migraineurs experiencing olfactory hypersensitivity and odour-triggered migraine. The abnormal cerebral activation patterns during olfactory stimulation might reflect altered cerebrovascular response to olfactory stimulation due to the migraine disease, or an abnormal top-down regulation process related to olfactory hypersensitivity. More recently, migraine neuronal processing in response to olfactory stimulation (rose odour) has been investigated during interictal (in 20 migraineurs) and ictal period (13 of the 20 patients were scanned within 6 h after the onset of a spontaneous migraine attack) [130]. Imaging data showed that migraineurs during interictal period did not differ from control subjects. However, during spontaneous and untreated attacks, migraineurs showed significantly higher BOLD response in brain areas including limbic structures (amygdala and insular cortices). Interestingly, in response to olfactory stimulation, a significant activation has been observed also in the rostral pons (RP). The findings suggest that the activity level of this structure can be triggered by olfactory input and thus points to the strong physiologic relationship between the olfactory and the trigemino-nociceptive pathway in the migraine pathophysiology. Specifically, odour-induced activation of the RP might be a mechanism by which could odours trigger migraine attacks.
9 Functional Neuroimaging During Vestibular Stimuli
Recently our group has conducted a BOLD-fMRI study [131] in patients with VM (according to ICHD-III, beta version) [46] during the interictal period. The functional response of vestibular neural pathways during caloric vestibular stimulation in 12 patients with VM, 12 patients with MwoA and HC has been explored. Electronystagmography evaluation was performed to exclude vestibular disorders and to verify that caloric stimulus induced vestibular nystagmus. In all subjects, caloric vestibular stimulation elicited a statistically significant activation in bilateral insular cortex, thalamus, cerebellum and brainstem. Interestingly, a discrete PAG activation was observed, suggesting a peculiar relationship between vestibular stimulation and activation of a brain area which plays a key role in pain processing [132]. This finding could suggest that reciprocal connections between brainstem vestibular nuclei and structures involved in modulation of trigeminal nociceptive inputs may have some role in VM pathophysiology [133]. Furthermore, the analysis of difference between groups showed a significant divergent response in the mediodorsal thalamus in patients with VM relative to both patients with MwoA and HC. It is noteworthy that the thalamus represents a key structure in transmitting sensory input from the brainstem to the cortex, exerting a pivotal function in pain processing and cortical excitability control. This observation could clarify the VM pathophysiological mechanism, suggesting a dys-modulation in the multimodal sensory integration and processing of both vestibular and nociceptive information, resulting in a vestibulo-thalamo-cortical dysfunction. Furthermore, thalamic functional abnormalities exhibited a positive correlation with the frequency of VM attacks. Nevertheless, it is not possible to establish whether thalamic findings are a primary phenomenon due to the hereditary liability resulting in VM attacks or a secondary phenomenon as a result of repetitive VM attacks.
10 Resting Brain in Migraine
18FDG-PET is widely used to measure glucose uptake into tissue including the brain and in the last decades, several 18FDG-PET studies have been conducted to compare brain metabolism between migraineurs and HC, demonstrating substantial differences in brain metabolism between the two subject groups. Among these, Kassab and colleagues have explored resting glucose uptake in posterior supratentorial and infratentorial WM in migraineurs during the interictal period in 11 migraineurs compared with HC. The authors identified two regions of significant increase in glucose uptake mapped predominantly to the posterior WM of the cerebrum and cerebellum in migraineurs relative to the HC. These findings suggested a primary metabolic disturbance in the posterior WM of the brain in migraineurs. This point of view has been supported by Montagna and colleagues that investigated 22 patients with MwoA in headache-free periods by means of 31P-magnetic resonance spectroscopy (MRS) of brain and muscle [134]. Brain 31P-MRS showed significantly low phosphocreatine, increased adenosine diphosphate, and decreased phosphorylation potential demonstrating an abnormal energy metabolism in MwoA, as previously demonstrated in patients with migraine stroke and MwA [135]. To compare metabolism in the brain of migraineurs during headache-free periods with those obtained from HC a recent 18FDG-PET study [136] has been conducted to evaluate interictal metabolic differences between 20 episodic migraineurs (four with MwA; 16 with MwoA) and HC. A significant hypometabolism in several regions known to be involved in central pain processing, such as bilateral insula, bilateral ACc and PCc, left premotor and PFc and left primary SSc has been found. Moreover, regional metabolism of both the insula and the ACc showed significant negative correlations with disease duration and lifetime headache frequency, suggesting that repeated migraine attacks over time could lead to metabolic abnormalities of selective brain regions belonging to the central pain matrix. These findings may be interpreted as a primary metabolic brain deficit related to migraine disorder or, alternatively, could suggest that a phenotypic trait could play a role in secondary metabolic abnormalities of brain regions involved in pain processing.
Recently, a novel tool which explores connectivity between functionally linked, but anatomically separated, brain regions has been developed. The use of this technique, called RS-fMRI, has allowed the identification, at rest, of the main brain functional networks without requiring subjects to perform specific active tasks. Methodologically, [137] several approaches can be applied for the analysis of RS-fMRI, including seed-based, independent component analysis based and/or cluster-based methods. The seed approach is the simplest to investigate spatial patterns, based on the direct correlations with time courses of signal change from a seed measurement. This technique is widely used in f-connectivity mainly due to its ease of interpretation and good sensitivity, however, its main limitation is the dependence on the a priori definition of a seed region, which prevents the method from studying multiple systems simultaneously. To overcome this limitation, blind source separation algorithms, such as independent component analysis (ICA), have become popular in f-connectivity analysis of BOLD-fMRI data. Indeed, ICA transforms individual patient RS-fMRI data sets into series of networks maps, allowing for a voxel-based population analysis of whole-brain f-connectivity without the need to specify the ROI constituting the layout of the neural network. RS-fMRI allows to identify a set of biologically meaningful spatial maps of independent components that are topographically organized in highly reproducible functional networks with biological relevance, called RS networks (RSN). Analysis of f-connectivity investigates the functional organization of the brain based on temporal correlations in BOLD signal fluctuations in different brain regions. Most f-connectivity analyses are done when the brain is at rest, whit the person being studied is not performing any task and is not being stimulated. In the RS there is continuous low frequency fluctuation in the BOLD signal throughout the brain. Brain regions with temporal correlations in BOLD signal are deemed to be functionally connected or functionally communicating. The most commonly reported RSN are the default mode network (DMN), the FPN (or executive network), the sensorimotor network and the visual and auditory networks. The presence of functional connections and the strength of such functional connections can be atypical in the presence of neurological diseases including migraine. Using RS-fMRI, several studies have identified f-connectivity abnormalities in migraineurs, mainly located at the level of the pain processing network. Along this research line, Mainero and colleagues [138] have analyzed the baseline functional interaction within the networks of PAG and a subset of brain areas involved in nociceptive and somatosensory processing and as well as in pain modulation. The study, conducted in 17 migraineurs (eight with MwA; nine with MwoA) during the interictal period compared with HC. The authors demonstrated a stronger f-connectivity between the PAG and several brain areas within the nociceptive and somatosensory processing pathways in migraineurs compared to HC. In addition, as the monthly frequency of migraine attacks worsens, the strength of the f-connectivity in some areas within these pathways increased, whereas a significant decrease in RS f-connectivity between the PAG and brain regions with a predominant role in pain modulation (PFc, ACc, amygdala) was evidenced. Interestingly, migraineurs with a history of CA exhibited significantly reduced f-connectivity between PAG, PFc, and ACc compared to migraineurs without CA. These data revealed on interictal dysfunctional dynamics within pain pathways in migraine manifested as an impairment of the descending pain modulatory circuits, likely leading to loss of pain inhibition, and hyperexcitability primarily in nociceptive areas. Yu and colleagues [139] have applied regional homogeneity (ReHo) method to analyze local temporal homogeneity of intrinsic fluctuation, and investigated the f-connectivity alterations of regions showing morphometric deficits during rest condition in 26 patients with MwoA compared with HC. Migraineurs showed a significant decrease in interval ReHo values in the right rostral ACc, PFc, OFc and the supplementary motor area (SMA) when compared with HC. In addition, ReHo values were negatively correlated with the duration of disease in the right ACc and PFc. The results suggested that the RS abnormalities of these regions may be associated with functional impairments in pain processing in patients with MwoA.
Our group in a series of RS-fMRI studies, has demonstrated f-connectivity abnormalities in several RSN. To explore DMN f-connectivity in patients with MwoA and investigate its clinical significance, the RS f-connectivity of the DMN in 20 patients with MwoA, during the interictal period, and 20 HC have been compared [22]. Patients with MwoA showed decreased f-connectivity in prefrontal and temporal regions of the DMN when compared to HC. Observed functional abnormalities were unrelated to detectable structural abnormalities or clinical and neuropsychological features of migraineurs. Similarly, based on converging neuropsychological evidence suggesting executive difficulties in migraine during interictal periods, we have evaluated the f-connectivity of the fronto-parietal networks (FPN) known to be associated with executive functions (EF), in 14 patients with MwoA, in the interictal period, in comparison to HC [140]. Our data showed that MwoA patients, compared to HC, had significant f-connectivity reduction within the right FPN and specifically in the MFG and the dorsal ACc. In addition, we found that MFG reduced f-connectivity was negatively correlated with the pain intensity of migraine attacks. There were no structural differences between the two groups. Surprisingly, neuropsychological data revealed no significant executive dysfunction in MwoA patients. This observation has been supported by a recent study confirming a disrupted executive control network f-connectivity not only in patients with MwoA but also in patients with MwA, in the interictal period. Although f-connectivity abnormalities are present in the absence of clinically relevant executive deficits, they may reflect a vulnerability to high-demanding conditions of daily living activities in patients with migraine. Indeed, a putative explanation of these clinical, neuropsychological and functional findings is that the observed connectivity dysfunction in DMN and FPN could underlie or be related to a maladaptive brain response to repeated stress which seems to characterize patients with migraine [141, 142]. Indeed, according to recent studies, recurrent migraine attacks alter both f-connectivity and s-connectivity [4], and these changes may disrupt mechanisms of stress response [141, 142]. When behavioural or physiological stressors are frequent or severe, allostatic responses can become maladaptive, leading, in a vicious cycle, to further allostatic load. Moreover, due to a high energetic demand, the observed DMN dysfunction may be associated with an impaired brain energy metabolism which has been demonstrated in previous MR spectroscopy studies in migraineurs, likely due to an imbalance between ATP production and ATP use [134, 135].
In the last decades, based on the prominent role played by the Vc in migraine aura pathophysiology, visual pathways have been extensively explored in patients with MwA both during the aura phase and the interictal period. Visual evoked potentials studies have revealed, although with conflicting results, abnormal visual information processing in migraineurs, subtending abnormalities in habituation and sensitization mechanisms [143]. Similarly, conflicting results has been observed in f-connectivity findings related to visual network in patients with MwA. More recently, the RS-fMRI approach has been used to evaluate the f-connectivity in 20 patients with MwoA and 20 MwoA during the interictal period, compared with HC [24]. A significant increased f-connectivity in the right lingual gyrus within the RS visual network has been demonstrated in patients with MwA, compared to both patients with MwoA and HC. These abnormalities were present in the absence of structural or microstructural abnormalities and not related to migraine severity. The results support the hypothesis of an extrastriate cortex involvement, centred in the lingual gyrus, a brain region related to mechanisms underlying the initiation and propagation of the migraine aura . This RS-fMRI finding may represent a functional biomarker that could differentiate patients experiencing the aura phenomenon from patients with MwoA, even between migraine attacks. Niddam and colleagues [144], have recently investigated f-connectivity abnormalities in intrinsic cognitive networks in 26 patients with MWA and 26 patients with MwoA during interictal period and HC. The authors used a whole-brain approach with seeds placed in the anterior insula and the MFG, key nodes of the salience and dorsal attention networks, respectively. In opposite to our observations, a reduced f-connectivity has been observed Vc (area V3A) in patients with MwA during interictal period when compared with patients with MwoA and HC. Another RS-fMRI study exploring RS f-connectivity in patients with MwA outside of attacks showed different results. Indeed, no differences of f-connectivity were found between 40 patients with MwA and HC, suggesting an abnormal f-connectivity, during interictal period, only during exposure to external stimuli, as reported by previous studies demonstrating increased cortical hyperresponsivity in the interictal phase of MwA [145].
The possible mechanisms underlying the disrupted f-connectivity in both patients with MwA and MwoA are currently unknown and it is not possible to answer the question whether observed functional abnormalities are acquired through a genetic predisposition or as a result of migraine burden. Nevertheless, longitudinal changes in brain activity between repeated observations within a short time ReHo and interregional f-connectivity were assessed in 19 female patients with MwoA and 20 HC [146]. All patients reported that their headache activity increased over time. Abnormal ReHo changes in the patient group relative to the HC were found in the putamen, OFc, secondary SSc, brainstem, and thalamus. Moreover, these brain regions exhibited longitudinal ReHo changes at the 6-week follow-up examination. These headache activity changes were accompanied by disproportionately dysfunctional connectivity in the putamen in the migraineurs, as revealed by f-connectivity analysis, suggesting that the putamen plays an important role in integrating diverse information among other migraine-related brain regions. The results obtained in this study suggest that progressive brain aberrations in migraine progress as a result of increased headache attacks. The same research group [147], examined RS abnormalities in patients with MwoA to explore the relationship between neuroimaging markers and disease duration (long-term and short-term) in 40 patients with MwoA compared with HC. MwoA patients with long-term disease duration showed comprehensive neuronal dysfunction than patients with short-term disease duration when compared with HC, In addition, increased average ReHo values in the thalamus, brain stem, and temporal pole showed significantly positive correlations with the disease duration. On the contrary, ReHo values were negatively correlated with the duration of disease in the ACc, insula, PCc and superior occipital gyrus. These findings of progressive brain damage in relation to increasing disease duration suggest that MwoA is a progressive central nervous disease, and the length of the disease duration was one of the key reasons to cause brain dysfunction in migraineurs. The repeated migraine attacks over time result in RS abnormalities of selective brain regions belonging to the pain processing and cognition.
Based on the hypothalamus implications in the autonomic symptoms of migraine as well as neuroimaging evidence of hypothalamic activation during attacks, RS-fMRI techniques have been used to explore, using a seed approach, f-connectivity changes between the hypothalamus and the rest of the brain in in 12 patients with MwoA compared to HC [148]. Patients with MwoA showed an increased hypothalamic FC with a number of brain regions involved in regulation of autonomic functions, including the locus coeruleus, caudate, parahippocampal gyrus, cerebellum, and the temporal pole. Stronger functional connections between the hypothalamus and brain areas that regulate sympathetic and parasympathetic functions may explain some of the hypothalamic-mediated autonomic symptoms that accompany or precede migraine attacks. A peculiar f-connectivity between pain-modulating circuits and the limbic system have been also investigated comparing f-connectivity between the amygdala and the cortex in 11 patients with MwA and 11 with MwoA as well as in HC and in two other chronic pain conditions not associated with CSD: trigeminal neuralgia (nine patients) and carpal tunnel syndrome (11 patients) [149]. The authors evidenced that amygdala f-connectivity to the visceroceptive insula was increased in both patients with MwA and MwoA when compared to all other patient groups. These data reinforce the evidence of a neurolimbic pain network dysfunction and may likely reflect repetitive episodes of CSD leading to the development of migraine pain.
RSN of the BG in patients with MWoA has been investigate in 40 patients with MwoA [150]. Increased f-connectivity between the BG and several brain regions within nociceptive and somatosensory processing pathways was observed in migraineurs compared with HC. Correlation analysis revealed significant correlations between the volume of the bilateral caudate and right NAcc and disease duration. In addition, an increased monthly frequency of migraine attack was associated with increased f-connectivity between the bilateral caudate and left insula, and longer disease duration was correlated with increased f-connectivity between the right NAcc and bilateral ACc. BG dysfunctional dynamics during interictal RS-fMRI within pain pathways have been also correlated with reduced volume of the BG in MwoA. The findings support the hypothesis that impaired pain processing and modulatory processes in MwoA may be associated with abnormal structure and function within the pain-related pathways of the BG.
The conflicting data we are facing probably reflect the low numbers and clinical heterogeneity of subjects involved in the available neuroimaging studies (i.e. inclusion of patients suffering from MwA and MwoA; very different frequency or intensity of attack; different current pharmacological treatments) as well as the modality of imaging used. We believe that combining functional and structural techniques will prompt a new and more effective way of looking into migraneous brain organization and function. Recruiting patients for studies aimed at exploring the acute or prodromal phase of migraine is certainly not easy, but more specifically designed studies, looking at different stages of the migraine attack, will clarify the involvement of several brain structures, especially the real role of brainstem. It is noteworthy that the main difficulty with neuroimaging studies, in an episodic disorder such as migraine, is to capture spontaneous attacks when the imaging techniques require considerable planning. We are aware that experimental pain is absolutely different from spontaneous migraine and that by doing so we cannot draw any firm conclusions about real migraine mechanisms. However, we believe that further refinements of this kind of approach will improve our capabilities of exploring pain processing-related cerebral activity in migrainous subjects. Functional techniques suitable to study increasingly smaller brain structures and to detect even subtle abnormalities are rapidly evolving and will take us closer to the key structures and mechanisms of migraine. However, the interpretation of the biological significance of these various functional changes could remain incomplete without a combination of expanding genomic information about neurochemical pathways and genetic polymorphisms linked to specific migraine phenotypes or subtypes. Finally, there is no doubt that the clinicians’ expertise is of pivotal importance, as only an headache expert may have the basic and clinical clues to plan a specific functional neuroimaging protocol to address a still rather complicated open issues.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- NMR:
-
Nuclear magnetic resonance
- GM:
-
Grey matter
- WM:
-
White matter
- VBM:
-
Voxel-based morphometry
- CT:
-
Cortical thickness
- SB:
-
Surface-based
- DTI:
-
Diffusion tensor imaging
- HC:
-
Healthy control
- s-connectivity:
-
Structural connectivity
- rCBF:
-
Regional cerebral blood flow
- SPECT:
-
Single photon emission computed tomography
- PET:
-
Positron emission tomography
- fMRI:
-
Functional magnetic resonance imaging
- 18FDG:
-
2-deoxy-2-[18F] flour-d-glucose
- BOLD:
-
Blood oxygen level dependent
- MwoA:
-
Migraine without aura
- MwA:
-
Migraine with aura
- WMH:
-
White matter hyperintensities
- PAG:
-
Periacqueductal grey matter
- dLP:
-
Dorso lateral pons
- IFG:
-
Inferior frontal gyrus
- PCG:
-
Precentral gyrus
- ACc:
-
Anterior cingulated cortex
- MFG:
-
Middle frontal gyrus
- PFc:
-
Prefrontal cortex
- OFc:
-
Orbito frontal cortex
- Vc:
-
Visual cortex
- Fc:
-
Frontal cortex
- SSc:
-
Somatosensory cortex
- IPG:
-
Inferior parietal gyrus
- PCc:
-
Posterior cingulate cortex
- CSD:
-
Cortical spreading depression
- CS:
-
Cortical surface
- AD:
-
Axonal diffusivity
- RD:
-
Radial diffusivity
- MD:
-
Mean diffusivity
- ROI:
-
Region of interest
- CC:
-
Corpus callosum
- TBSS:
-
Tract-based spatial statistics
- RS:
-
Resting-state
- f-connectivity:
-
Functional connectivity
- ADC:
-
Apparent diffusion coefficient
- dP:
-
Dorsal pons
- 5-HT:
-
5-hydroxytryptamine
- µOR:
-
µ-opioid receptor
- lOR:
-
l-opioid receptor
- CA:
-
Cutaneous allodynia
- 3D-IIN:
-
3D immersive and interactive neuronavigation
- NAcc:
-
Nucleus accumbens
- NCF:
-
Nucleus cuneiformis
- TP:
-
Temporal pole
- EC:
-
Entorhinal cortex
- MCc:
-
Middle cingulated cortex
- NAA:
-
N–acetylaspartate
- RP:
-
Rostral pons
- VM:
-
Vestibular migraine
- MRS:
-
31P-magnetic resonance spectroscopy
- RSN:
-
Resting-state networks
- DMN:
-
Default mode network
- ReHo:
-
Regional homogeneity
- SMA:
-
Supplementary motor area
- FPN:
-
Fronto-parietal networks
- EF:
-
Executive functions
References
Bloch F. Nuclear induction. Phys Rev. 1946;70:460–74.
Purcell EM, Torrey HC, Pound RV. Resonance absorption by nuclear magnetic moments in a solid. Phys Rev. 1946;69:37–8.
Symms M, Jäger HR, Schmierer K, Yousry TA. A review of structural magnetic resonance neuroimaging. J Neurol Neurosurg Psychiatry. 2004;75(9):1235–44.
Ellerbrock I, Engel AK, May A. Microstructural and network abnormalities in headache. Curr Opin Neurol. 2013;26(4):353–9.
Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9(4):344–52.
Tedeschi G, Russo A, Tessitore A. Functional neuroimaging in migraine: usefulness for the clinical neurologist. Neurol Sci. 2012;33(Suppl 1):S91–4.
May A. A review of diagnostic and functional imaging in headache. J Headache Pain. 2006;7(4):174–84.
Sprenger T, Henriksen G, Valet M, et al. Positron emission tomography in pain research. From the structure to the activity of the opiate receptor system. Schmerz. 2007;21:503–13.
Sprenger T, Ruether KV, Boecker H, et al. Altered metabolism in frontal brain circuits in cluster headache. Cephalalgia. 2007;27:1033–42.
Sprenger T, Willoch F, Miederer M, et al. Opioidergic changes in the pineal gland and hypothalamus in cluster headache: a ligand PET study. Neurology. 2006;66:1108–10.
Grupen C, Buvat I. PET imaging: basics and new trends. Handbook of particle detection and imaging. Springer; 2012. p. 935–971.
Tedeschi G, Russo A, Tessitore A. Relevance of functional neuroimaging studies for understanding migraine mechanisms. Expert Rev Neurother. 2013;13(3):275–85.
Goadsby PJ, Lipton RB, Ferrari MD. Migraine–current understanding and treatment. N Engl J Med. 2002;346:257–70.
Matharu MS, Good CD, May A, et al. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol. 2003;10(1):53–7.
Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–70.
Kruit MC, Van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. J Am Med Assoc. 2004;291:427–34.
Valfrè W, Rainero I, Bergui M, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–17.
Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604.
Jin C, Yuan K, Zhao L, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26(1):58–64.
Stankewitz A, Valet M, Schulz E. Pain sensitizers exhibit grey matter changes after repetitive pain exposure: a longitudinal voxel-based morphometry study. Pain. 2013;154(9):1732–7.
Messina R, Rocca MA, Colombo B, et al. White matter microstructure abnormalities in pediatric migraine patients. Cephalalgia. 2015.
Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;8(14):89.
Tessitore A, Russo A, Conte F, et al. Abnormal connectivity within executive resting-state network in migraine with Aura. Headache. 2015;55(6):794–805.
Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia. 2015.
Hu W, Guo J, Chen N, et al. A meta-analysis of voxel-based morphometric studies on migraine. Int J Clin Exp Med. 2015;8(3):4311–9.
Granziera C, DaSilva AF, Snyder J, et al. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006;3(10):e402.
DaSilva AF, Granziera C, Snyder J, et al. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–5.
Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687–92.
Kim JH, Kim JB, Suh SI, et al. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia. 2014;34(14):1125–33.
Hougaard A, Amin FM, Hoffmann MB, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia. 2015;35(1):3–9.
Maleki N, Barmettler G, Moulton EA, et al. Female migraineurs show lack of insular thinning with age. Pain. 2015;156(7):1232–9.
Chong CD, Dodick DW, Schlaggar BL, et al. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. 2014;34(14):1115–24.
Schwedt TJ, Chong CD. Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PLoS One. 2014;9(6):e99791.
Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153:1602–9.
Schwedt TJ, Berisha V, Chong CD. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS One. 2015;10(2):e0116687.
Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–6.
Frye RE, Liederman J, Malmberg B, et al. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb Cortex. 2010;20(11):2625–35.
Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265.
Datta R, Detre JA, Aguirre GK, et al. Absence of changes in cortical thickness in patients with migraine. Cephalalgia. 2011;31(14):1452–8.
Messina R, Rocca MA, Colombo B, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013;268(1):170–80.
Granziera C, Daducci A, Romascano D, et al. Structural abnormalities in the thalamus of migraineurs with aura: a multiparametric study at 3 T. Hum Brain Mapp. 2014;35(4):1461–8.
Maleki N, Becerra L, Brawn J, et al. Common hippocampal structural and functional changes in migraine. Brain Struct Funct. 2013;218(4):903–12.
Schmidt-Wilcke T, Hierlmeier S, Leinisch E. Altered regional brain morphology in patients with chronic facial pain. Headache. 2010;50(8):1278–85.
Obermann M, Nebel K, Schumann C, et al. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73(12):978–83.
Burgmer M, Gaubitz M, Konrad C, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71(5):566–73.
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. 3rd edn (beta version). Cephalalgia. 2013;33(9):629–808.
Swartz RH, Kern RZ. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol. 2004;61:1366–8.
Honningsvåg LM, Hagen K, Håberg A, et al. Intracranial abnormalities and headache: a population-based imaging study (HUNT MRI). Cephalalgia. 2015.
Seneviratne U, Chong W, Billimoria PH. Brain white matter hyperintensities in migraine: clinical and radiological correlates. Clin Neurol Neurosurg. 2013;115(7):1040–3.
Dinia L, Bonzano L, Albano B, et al. White matter lesions progression in migraine with aura: a clinical and MRI longitudinal study. J Neuroimag. 2013;23:47–52.
Trauninger A, Leé l-Ossy E, Kamson DO, et al. Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain. 2011;12:97–103.
Kruit MC, Van Buchem MA, Launer LJ, et al. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia. 2010;30:129–36.
Evans RW. Clinical neuroimaging of migraine. In: Borsook D, May A, Goadsby P, Hargreaves R, editors. The migraine brain. New York: Oxford University Press; 2012. p. 85–95.
Erdélyi-Bótor S, Aradi M, Kamson DO, et al. Changes of migraine-related white matter hyperintensities after 3 years: a longitudinal MRI study. Headache. 2015;55(1):55–70.
Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA. 2012;308:1889–97.
Hamedani AG, Rose KM, Peterlin BL, et al. Migraine and white matter hyperintensities: the ARIC MRI study. Neurology. 2013;81(15):1308–13.
Aradi M, Schwarcz A, Perlaki G, et al. Quantitative MRI studies of chronic brain white matter hyperintensities in migraine patients. Headache. 2013;53(5):752–63.
Kurth T, Diener H-C. Migraine and stroke: perspectives for stroke physicians. Stroke. 2012;43:3421–6.
Bashir A, Lipton RB, Ashina S, et al. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013;81(14):1260–8.
Friedman DI, Dodick DW. White matter hyperintensities in migraine: reason for optimism. JAMA. 2012;308:1920–1.
Gupta VK. White matter hyperintensities: pearls and pitfalls in interpretation of MRI abnormalities. Stroke. 2004;35(10):2239–41.
Aytaç B, Coşkun Ö, Alioğlu B, et al. Decreased antioxidant status in migraine patients with brain white matter hyperintensities. Neurol Sci. 2014;35(12):1925–9.
Pusic AD, Mitchell HM, Kunkler PE, et al. Spreading depression transiently disrupts myelin via interferon-gamma signaling. Exp Neurol. 2015;264:43–54.
Toghae M, Rahimian E, Abdollahi M, et al. The prevalence of magnetic resonance imaging hyperintensity in migraine patients and its association with migraine headache characteristics and cardiovascular risk factors. Oman Med J. 2015;30(3):203–7.
Hougaard A, Amin FM, Ashina M. Migraine and structural abnormalities in the brain. Curr Opin Neurol. 2014;27(3):309–14.
Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep. 2014;16(9):524.
Husøy AK, Indergaard MK, Honningsvåg LM, et al. Perivascular spaces and headache: a population-based imaging study (HUNT-MRI). Cephalalgia. 2015.
Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–29.
Rocca MA, Colombo B, Inglese M, et al. A diffusion tensor magnetic resonance imaging study of brain tissue from patients with migraine. J Neurol Neurosurg Psychiatry. 2003;74(4):501–3.
DaSilva AF, Granziera C, Tuch DS, et al. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. NeuroReport. 2007;18(4):301–5.
Li XL, Fang YN, Gao QC, et al. A diffusion tensor magnetic resonance imaging study of corpus callosum from adult patients with migraine complicated with depressive/anxious disorder. Headache. 2011;51(2):237–45.
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55.
Yuan K, Qin W, Liu P, et al. Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS One. 2012;7(9):e45476.
Yu D, Yuan K, Qin W, et al. Axonal loss of white matter in migraine without aura: a tract-based spatial statistics study. Cephalalgia. 2013;33(1):34–42.
Kara B, Kiyat Atamer A, Onat L, et al. DTI findings during spontaneous migraine attacks. Clin Neuroradiol. 2013;23(1):31–6.
Coppola G, Tinelli E, Lepre C, et al. Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. Eur J Neurol. 2014;21(2):287-e13.
Chong CD, Schwedt TJ. Migraine affects white-matter tract integrity: a diffusion-tensor imaging study. Cephalalgia. 2015.
Rocca MA, Pagani E, Colombo B, et al. Selective diffusion changes of the visual pathways in patients with migraine: a 3-T tractography study. Cephalalgia. 2008;28(10):1061–8.
Neeb L, Bastian K, Villringer K, et al. No microstructural white matter alterations in chronic and episodic migraineurs: a case-control diffusion tensor magnetic resonance imaging study. Headache. 2015;55(2):241–51.
Weiller C, May A, Limmroth V, et al. Brainstem activation in spontaneous human migraine attacks. Nat Med. 1995;1(7):658–60.
Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol. 2005;62(8):1270–5.
Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418–26.
Russo A, Tessitore A, Giordano A, et al. The pain in migraine beyond the pain of migraine. Neurol. Sci. 2012;33(Suppl 1):S103–6.
Aurora SK, Barrodale PM, Tipton RL, et al. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47(7):996–1003; discussion 1004.
Pavlovic JM, Buse DC, Sollars CM, et al. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache. 2014;54(10):1670–9.
Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137(Pt 1):232–41.
Maniyar FH, Sprenger T, Schankin C, et al. The origin of nausea in migraine-a PET study. J Headache Pain. 2014;3(15):84.
Shin JH, Kim YK, Kim HJ, Kim JS. Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia. 2014;34(1):58–67.
Aurora SK, Barrodale PM, Tipton RL, et al. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47(7):996–1003.
Chabriat H, Tehindrazanarivelo A, Vera P, et al. 5HT2 receptors in cerebral cortex of migraineurs studied using PET and 18F-fluorosetoperone. Cephalalgia. 1995;15(2):104–8.
Chugani DC, Niimura K, Chaturvedi S, et al. Increased brain serotonin synthesis in migraine. Neurology. 1999;53(7):1473–9.
Demarquay G, Lothe A, Royet JP, et al. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia. 2011;31(1):84–94.
Okazawa H, Tsuchida T, Pagani M, et al. Effects of 5-HT1B/1D receptor agonist rizatriptan on cerebral blood low and blood volume in normal circulation. J Cereb Blood Flow Metab. 2006;26(1):92–8.
Wall A, Kågedal M, Bergström M, et al. Distribution of zolmitriptan into the CNS in healthy volunteers: a positron emission tomography study. Drugs R&D. 2005;6(3):139–47.
Nascimento TD, DosSantos MF, Lucas S, et al. μ-Opioid activation in the midbrain during migraine allodynia—brief report II. Ann Clin Transl Neurol. 2014; 1(6):445–50.
DaSilva AF, Nascimento TD, DosSantos MF, et al. Association of μ-opioid activation in the prefrontal cortex with spontaneous migraine attacks—brief report I. Ann Clin Transl Neurol. 2014;1(6):439–44.
DaSilva AF, Nascimento TD, Love T, et al. 3D-neuronavigation in vivo through a patient’s brain during a spontaneous migraine headache. J Vis Exp. 2014;(88).
Leao AAP. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–90.
Smith JM, Bradley DP, James MF, Huang CL. Physiological studies of cortical spreading depression. Biol Rev Camb Philos Soc. 2006;81(4):457–81.
Olesen J, Friberg L, Olsen TS, et al. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann Neurol. 1990;28(6):791–8.
Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache. 2010;50(4):725–30.
Sprenger T, Goadsby PJ. What has functional neuroimaging done for primary headache and for the clinical neurologist? J Clin Neurosci. 2010;17(5):547–53.
Russo A, Tessitore A, Tedeschi G. Migraine and trigeminal system-I can feel it coming…. Curr Pain Headache Rep. 2013;17(10):367.
May A, Kaube H, Büchel C, et al. Experimental cranial pain elicited by capsaicin: a PET study. Pain. 1998;74(1):61–6.
Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799.
Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb Cortex. 2011;21:435–48.
Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012;259:1903–12.
Stankewitz A, Voit HL, Bingel U, Peschke C, May A. A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia. 2010;30:475–85.
Aderjan D, Stankewitz A, May A. Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain. 2010;151:97–103.
Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011;31:1937–43.
Schwedt TJ, Chong CD, Chiang CC, et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34(12):947–58.
Maizels M, Aurora S, Heinricher M. Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache. 2012;52(10):1553–65.
Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–5.
Vanagaite J, Pareja JA, Storen O, et al. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–41.
Wober-Bingol C, Wober C, Karwautz A, et al. Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia. 2004;24:12–7.
Cao Y, Welch KM, Aurora S, et al. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999;56(5):548–54.
Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Géraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81(9):978–84.
Cao Y, Aurora SK, Nagesh V, et al. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59(1):72–8.
Denuelle M, Boulloche N, Payoux P, et al. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011;76(3):213–8.
Sarchielli P, Tarducci R, Presciutti O, et al. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage. 2005;24(4):1025–31.
Martín H, Sánchez del Río M, de Silanes CL, et al. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache. 2011;51(10):1520–8.
Antal A, Polania R, Saller K, et al. Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalalgia. 2011;31(3):338–45.
Datta R, Aguirre GK, Hu S, et al. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013;33:365–74.
Griebe M, Flux F, Wolf ME, et al. Multimodal assessment of optokinetic visual stimulation response in migraine with aura. Headache. 2014;54(1):131–41.
Hougaard A, Amin FM, Hoffmann MB, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp. 2014;35(6):2714–23.
Zanchin G, Dainese F, Trucco M, et al. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007;27(9):1061–8.
De Carlo D, Toldo I, Dal Zotto L, et al. Osmophobia as an early marker of migraine: a follow-up study in juvenile patients. Cephalalgia. 2012;32(5):401–6.
Demarquay G, Royet JP, Mick G, et al. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia. 2008;28(10):1069–80.
Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77(5):476–82.
Russo A, Marcelli V, Esposito F, et al. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014;82(23):2120–6.
Borsook D, Burstein R. The enigma of the dorsolateral pons as a migraine generator. Cephalalgia. 2012;32(11):803–12.
Espinosa-Sanchez JM, Lopez-Escamez JA. New insights into pathophysiology of vestibular migraine. Front Neurol. 2015;6(6):12.
Montagna P, Cortelli P, Monari L, et al. 31P-magnetic resonance spectroscopy in migraine without aura. Neurology. 1994;44(4):666–9.
Barbiroli B, Montagna P, Cortelli P, et al. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology. 1992;42(6):1209–14.
Kim JH, Kim S, Suh SI, et al. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia. 2010;30(1):53–61.
Colombo B, Rocca MA, Messina R, et al. Resting-state fMRI functional connectivity: a new perspective to evaluate pain modulation in migraine? Neurol Sci. 2015;36(Suppl 1):41–5.
Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70(5):838–45.
Yu DH, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012;25(5):806–12.
Russo A, Tessitore A, Giordano A, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia. 2012;32(14):1041–8.
Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012;52(Suppl 2):102–6.
Borsook D, Maleki N, Becerra L, et al. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–34.
Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442–53.
Niddam DM, Lai KL, Fuh JL, et al. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2015.
Hougaard A, Amin FM, Magon S, et al. No abnormalities of intrinsic brain connectivity in the interictal phase of migraine with aura. Eur J Neurol. 2015;22(4):702-e46.
Zhao L, Liu J, Yan X, et al. Abnormal brain activity changes in patients with migraine: a short-term longitudinal study. J Clin Neurol. 2014;10(3):229–35.
Zhao L, Liu J, Dong X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. 2013;17(14):85.
Moulton EA, Becerra L, Johnson A, et al. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One. 2014;9(4):e95508.
Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013;33(15):1264–8.
Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013;14(8):836–44.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Russo, A., Tessitore, A., Tedeschi, G. (2017). Neuroimaging in Migraines. In: Saba, L. (eds) Neuroimaging of Pain. Springer, Cham. https://doi.org/10.1007/978-3-319-48046-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-48046-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48044-2
Online ISBN: 978-3-319-48046-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)