Abstract
Morphometric MRI studies in adult patients with migraine have consistently demonstrated atrophy of several gray matter (GM) regions involved in pain processing. We explored the regional distribution of GM and white matter (WM) abnormalities in pediatric patients with episodic migraine and their correlations with disease clinical manifestations. Using a 3.0 T scanner, brain T2-weighted and 3D T1-weighted scans were acquired from 12 pediatric migraine patients and 15 age-matched healthy controls. GM and WM volumetric abnormalities were estimated using voxel-based morphometry (p < 0.05, family-wise error corrected). Compared to controls, pediatric migraine patients experienced a significant GM atrophy of several regions of the frontal and temporal lobes which are part of the pain-processing network. They also had an increased volume of the right putamen. The left fusiform gyrus had an increased volume in patients with aura compared to patients without aura and controls, whereas it was significantly atrophied in patients without aura when compared to the other two groups. No abnormalities of WM volume were detected. In migraine patients, regional GM atrophy was not correlated with disease duration and attack frequency, whereas a negative correlation was found between increased volume of the putamen and disease duration (r = −0.95, p < 0.05). These results show that GM morphometric abnormalities do occur in pediatric patients with migraine. The presence of such abnormalities early in the disease course, and the absence of correlation with patient clinical characteristics suggest that they may represent a phenotypic biomarker of this condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Migraine is the most common acute and recurrent headache syndrome in children, with a prevalence ranging from 3.2 to 14.5 %, which increases from preschool age to mid-adolescence [30]. In children, the clinical manifestations of migraine may differ from those of adult patients, with a more common localization to the frontal and temporal brain regions, bilaterally, a duration of the attacks between 1 and 72 h and the frequent concomitant presence of abdominal pain, nausea, or vomiting, plus two of five other associated symptoms (photophobia, phonophobia, difficulty of thinking, light headedness, or fatigue). As children move through adolescence, the characteristics of the attacks tend to become similar to those seen in adults with migraine [21, 30]. Both in adult and pediatric patients, the diagnosis of migraine is mainly based on clinical criteria: a typical history of paroxysmal headaches with a normal clinical setting between episodes. Nevertheless, neuroimaging studies in children and adolescents with migraine are frequently performed in clinical practice because of fear of missing serious underlying diseases and increasing parental demands [32].
In adult patients with migraine, structural and functional MRI techniques have been applied extensively to provide additional insights into the pathophysiology of the disease and to identify possible regions triggering the attacks, which might become the targets of specific treatments. A consistent finding of these investigations has been the demonstration that, similarly to other chronic painful conditions, including chronic back pain [36], fibromyalgia [38], and cluster headache [1], migraine patients experience a distributed pattern of gray matter (GM) volume abnormalities to regions that are part of the network subserving supraspinal nociceptive processing [9, 23, 33]. Of note, such abnormalities, are not limited to the pain network, but extend to several other regions involved in visual motion-processing (such as area MT/V5 and V3A) and sensorimotor processing [15]. Using diffusion tensor MRI, subtle modifications of diffusivity indexes of several white matter (WM) areas have also been demonstrated in adult migraine patients [10, 34].
Despite having contributed to broaden the understanding of migraine pathophysiology, there are several aspects from previous research that need further investigations. In this perspective, two unsolved issues are the following: (a) How early do these morphological modifications occur? (b) Do they represent the consequence of attack repetition over time or they are rather a phenotypic biomarker of the disease? A valuable strategy to address these questions is to study pediatric patients with migraine, in whom, by definition, the interval between the biological and the clinical onset of the disease is shorter than that of adult patients. To this aim, we applied voxel-based morphometry (VBM) in pediatric patients with episodic migraine to assess the presence and distribution of GM and WM abnormalities as well as their correlation with attack frequency and disease duration.
Materials and methods
Subjects
Patients were recruited consecutively from the migraine population attending the Outpatient Clinic, Department of Neurology, Scientific Institute and University Ospedale San Raffaele. All patients met the criteria of the international classification of headache disorders for the diagnosis of migraine [21]. We enrolled 15 right-handed patients with episodic migraine in headache-free state for at least 1 month prior to the MRI scan and 15 right-handed, gender and age-matched pediatric healthy controls, without a familial history of migraine, no history of neurological dysfunction (including migraine) and a normal neurological exam. Patients with hypertension, hypercholesteremia, diabetes mellitus, vascular/heart diseases and other major systemic, neurological or psychiatric conditions were excluded. Images of three patients were discarded during the analysis due to motion artifacts (the demographic and clinical characteristics of these patients did not differ from those of the remaining group). The main demographic and clinical characteristics of patients with migraine and healthy controls are summarized in Table 1. Seven patients had a diagnosis of migraine with visual aura (MWA), and five a diagnosis of migraine without aura (MWoA). Apart from two patients who suffered from a right-sided migraine, all the remaining patients had bilateral migraine. At the time of MRI, three patients were on prophylactic medication for migraine. Nine patients had a familial history of migraine. All subjects were assessed clinically by a single neurologist, who was unaware of the MRI results.
Standard protocol approvals and patient consents
Local ethical standards committee on human experimentation approved the study protocol and all subjects’ parents provided written informed consent prior to study participation.
MRI acquisition
Using a 3.0 T Intera scanner (Philips Medical Systems, Best, The Netherlands), the following sequences of the brain were obtained from all the subjects: (1) axial T2-weighted turbo-spin echo [repetition time (TR)/echo time (TE) = 3,000/120 ms, flip angle (FA) = 90º, matrix size = 512 × 512, field of view (FOV) = 230 mm2, 28, 4 mm thick, contiguous slices]; (2) axial fluid attenuated inversion recovery (FLAIR) (TR/TE = 11,000/120 ms, inversion time = 2,800 ms, FA = 90°, matrix size = 256 × 256, FOV = 230 mm2, 28, 4 mm thick, contiguous slices); (3) coronal FLAIR (TR/TE = 11,000/120 ms, inversion time = 2,800 ms, FA = 90°, matrix size = 320 × 295, FOV = 220 mm2, 40, 3 mm thick slices); (4) axial 3D T1-weighted fast field echo (FFE) (TR/TE = 25/4.6 ms; flip angle = 30°; matrix size = 256 × 256; FOV = 230 × 230 mm2; 220 contiguous slices with voxel size = 0.89 × 0.89 × 0.8 mm).
MRI analysis
T2-weighted scans were analysed for the presence of lesions and FLAIR scans were always used to increase confidence in their identification. Lesion volumes (LV) were measured using a local thresholding segmentation technique (Jim 5.0, Xinapse System, Leicester, UK).
On 3D FFE images, normalized brain volumes were calculated using the cross-sectional version of the structural imaging evaluation of normalized atrophy (SIENAx) software [41]. VBM and the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm) were used to assess differences of GM and WM volumes between migraine patients and controls. First, 3D FFE images were segmented in GM, WM and CSF by using the standard unified segmentation model in SPM8. Then, GM and WM segmented images of all subjects, in the closest possible rigid-body alignment with each other, were used to produce GM and WM templates and to drive the deformation to the templates. At each iteration, the deformations, calculated using the diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) registration method [2], were applied to GM and WM, with an increasingly good alignment of subject morphology to produce templates. Spatially normalized images were then modulated to ensure that the overall amount of each tissue class was not altered by the spatial normalization procedure. To better align the final template with the Montreal Neurologic Institute (MNI) space, an affine registration between the customized GM template and the SPM GM template (in the MNI space) was also calculated and added to the header of each image as a new orientation, in order to have all images in a standard space. The same transformation was applied to the WM customized template. The images were then smoothed with an 8 mm full width at half maximum (FWHM) Gaussian kernel.
Statistical analysis
Demographic, clinical and conventional MRI characteristics were compared between groups using the Mann–Whitney U test for continuous variables and the Fisher exact test for categorical variables (SPSS software, version 13.0).
Between-group comparisons of smoothed GM and WM maps were assessed using analyses of covariance, including age, gender and the normalization factor derived from SIENAx (which can be considered as a measure of head size) as covariates. The correlation between regional GM/WM abnormalities and clinical data (disease duration and attack frequency) was assessed using SPM8 and a linear regression analysis. For all analyses run with SPM, results were assessed at a threshold of p < 0.05 corrected for multiple comparisons (FWE). In regions which are part of the pain processing network, where an a priori hypothesis was available [9, 23, 33], results were also tested at a p < 0.001, uncorrected for multiple comparisons (cluster extent = 10 voxels).
Results
Age (p = 0.3) and gender (p = 0.6) did not differ between migraine patients and controls or between MWA and MWoA patients. Patients with MWoA had a higher attack frequency (p = 0.03) than those with MWA, whereas disease duration did not differ significantly between the two groups (p = 0.8) (Table 1).
No WM hyperintense lesions were found in healthy controls and 8 (67 %) of the 12 patients with migraine. Four patients with MWoA had few small, punctate T2 hyperintense lesions in deep and subcortical WM (Fig. 1). Mean T2 LV in these patients was 0.10 ml (SD = 0.06 ml). NBV did not differ between migraine patients and healthy controls (p = 0.08), neither between MWA and MWoA patients (p = 0.1).
The regional analysis of the topography of WM abnormalities showed no difference between healthy subjects and pediatric migraine patients.
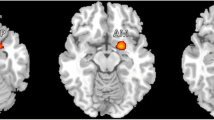
The results of the regional analysis of GM atrophy are summarized in Table 2 and shown in Fig. 2. Compared with pediatric controls, pediatric migraine patients had significant GM atrophy of the left middle temporal gyrus (p < 0.05, FWE corrected), right orbitofrontal gyrus (p < 0.001, uncorrected), left inferior frontal gyrus (p < 0.001, uncorrected) and subgenual cingulum (p < 0.001, uncorrected). The opposite comparison showed an increased GM volume of the right putamen in migraine patients vs. controls (MNI coordinates 27, 1, 3; cluster extent = 150 voxels; t value = 4.69, p < 0.05 FWE corrected). Such an increased right putaminal volume was also found in the analysis of the two groups of migraine patients (MWA and MWoA) taken separately vs healthy controls (data not shown). The analysis of regional GM abnormalities in the two groups of migraine patients also identified reduced volume of the left fusiform gyrus (BA 19) (MNI coordinates −27, −55, −11) in MWoA patients when compared to healthy controls and MWA patients. This structure had an increased volume in MWA patients compared to the other two groups (Fig. 3) (p < 0.05, FWE corrected).
Areas showing significant gray matter (GM) volume differences (p < 0.001, uncorrected for display) between pediatric migraine patients and healthy controls, superimposed on a high-resolution T1-weighted template: a areas with reduced GM volume in migraine patients vs controls (blue-green scale according to t values); b areas with increased GM volume in migraine patients vs controls (red-yellow scale according to t values). MTG middle temporal gyrus, IFG inferior frontal gyrus, OFG orbitofrontal gyrus, L left, R right
a Axial and sagittal views on a high-resolution T1-weighted template showing the results of the comparison between patients with migraine with aura (MWA) vs healthy controls and patients without aura (MWoA). An increased volume of the left fusiform gyrus was found in the first group. This region was significantly atrophied in MWoA. b Graphical representation showing plots of GM volume of the left fusiform gyrus in the three groups of subjects (red blobs single-subject data, gray line fitted data). MWA migraine patients with aura, MWoA migraine patients without aura, L left, R right
In pediatric migraine patients, regional GM atrophy was not correlated with disease duration and attack frequency, whereas a negative correlation was found between increased volume of the putamen and disease duration (Fig. 4) (p < 0.05, FWE corrected, r = −0.95).
Discussion
MRI studies in adult patients with migraine have demonstrated consistently a distributed pattern of morphological brain abnormalities characterized by the coexistence of regions of decreased and increased GM volume [9, 23, 29, 33]. The role of such abnormalities in the pathogenesis of this condition is still a matter of debate especially when considering the discrepant results of the analysis of correlations between structural abnormalities and disease clinical manifestations [9, 22, 26, 29, 33, 39]. Indeed, some studies found a relation between GM atrophy and disease duration [22, 33, 39] or attack frequency [22, 26, 39], suggesting that morphological brain abnormalities may be the consequence of repetition of the attacks over time. However, several other investigations found no correlation between volumetric modifications and disease clinical manifestations [9, 29], suggesting that the former may be a phenotypic biomarker of the disease.
Other unsolved issues include the definition of how early brain structural abnormalities occur in migraineurs and whether these alterations may progress over time. Recent conventional MRI studies have shown that WM hyperintense lesions occur early in migraineurs [5, 20]. Studies which have assessed the progression of WM lesions over time have provided conflicting results since some studies found an increased lesion burden over time in these patients [11, 31], whilst others did not [20]. At present, longitudinal studies using advanced MRI techniques are lacking. Therefore, the performance of longitudinal VBM studies, or the evaluation of patients at the beginning of the disease (that is, pediatric subjects) are worthwhile strategies to try to untie such controversies.
The first important result of our study is the demonstration that, similarly to what has been observed in adult patients with migraine [22, 33, 39] children affected by migraine experience atrophy of several GM regions of the temporal and frontal lobes, including the cingulum, which are involved in processing painful stimuli [27]. Regions of the frontal lobes have been proposed to play an important role in controlling the functional interactions among key nociceptive processing brain regions, by driving endogenous pain-inhibitory circuits [25]. Numerous studies have found that placebo analgesia can modulate pain perception by increasing prefrontal activity during anticipation and perception of pain [43]. Specifically, the orbitofrontal cortex (OFC) is believed to be involved in affective pain modulation [8] and it belongs to the modulatory circuits related to attention. A recent resting state functional MRI study has shown that reduced functional connectivity of the OFC is negatively correlated with disease duration in adult MWoA patients [44], suggesting an impairment of affective pain modulation in patients with migraine that may worsen with disease progression. At present, despite several studies have disclosed atrophy of different regions of the temporal lobes in patients with migraine [22, 33, 39] as well as in those with chronic painful conditions [37], the role of these areas in pain processing is not fully understood, albeit it is known that these areas play a role in assigning emotional valence to short-term memories related to pain [13]. Notably, the temporal region that was significantly atrophied in our pediatric migraine patients (the MTG) is involved in visual motion processing, another function typically affected in this condition [12, 28].
The second intriguing finding of this study is the increased volume of the right putamen we found in the whole group of migraine patients as well as in MWA and MWoA separately in comparison to healthy controls. Several clinical, electrophysiological and neuroimaging studies have documented a role of the basal ganglia in pain and analgesia processing [3], because these nuclei are involved in sensory-discriminative, emotional/affective, cognitive dimension of pain and pain modulation [6]. Previous studies in patients with chronic pain, including back pain [36], fibromyalgia [38], and chronic vulvar pain [40] have shown an increased GM volume of the basal ganglia, which has been correlated to patients’ clinical symptomatology [40]. A recent study in patients with migraine [26] has reported an increased volume and reduced activity and functional connectivity of the basal ganglia in patients with high-frequency vs those with low frequency attacks, suggesting that such abnormalities may be a dynamic consequence of repetitive stimulation of sensory pathways. Noteworthy, we found no correlation between attack frequency and putaminal volume in pediatric migraine patients, whereas a strong negative correlation was found with disease duration. Our data suggest that, whatever is the mechanism leading to an increased putaminal volume in these patients (repetitive stimulation or congenital abnormality), it likely occurs early in migraine patients with a pediatric-onset of the disease.
Consistently with previous findings from morphometric studies in adult patients with chronic pain syndromes [4, 9], we found no correlation between regional GM atrophy and clinical data (disease duration and attack frequency), thus supporting the notion that such abnormalities are not likely the mere consequence of chronic pain, but they may rather represent a phenotypic biomarker of the disease. This hypothesis is further supported by the positive familial history for migraine in the majority of our patients. Clearly, we can not exclude that the absence of such a relationship might be due to the fact that we are assessing a dynamic phenomenon. Indeed, several recent morphological studies in adult migraine patients have demonstrated that a given brain region may experience both increased and decreased volume according to the stage of the disease (ictal vs. iterictal), time to the subsequent attack, and response to treatment [16, 42]. A normalization of these structural abnormalities has also been shown after pain relief [17, 35].
The third important finding of this study was the demonstration of a selectively increased volume of the left fusiform gyrus in pediatric migraine patients with visual aura; on the contrary this structure had a reduced volume in migraineours without aura. Functionally, the fusiform gyrus contributes to high-order visual processing, including colour and form perception and stereopsis [14, 24]. Clinically, visual aura can be characterized by bright or multicolours flashes, scintillating scotoma, and distortion of the size or shape of objects [7], which can be attributed to a disturbed neuronal activity in the fusiform gyrus. Previous functional imaging studies in adult patients [18, 19] have shown a link between visual disturbances during visual aura and function of extrastriate and occipito-temporal areas, which may correspond to the neurophysiologic event of cortical spreading depression. Several processes, which may render the cortex more excitable and prone to elicit and propagate visual aura are likely to contribute to such a structural modification of the fusiform gyrus, including an increased neuronal and/or synaptic density, an increased number of glial cells, reactive gliosis, and plastic structural modifications following repetitive stimuli propagation.
This study is not without limitations. First, the number of pediatric migraine patients was relatively small. This is why, to reinforce our findings, we decided to report and describe almost exclusively results corrected for multiple comparisons. Clearly, because of such a limitation, we did not perform an analysis of correlation between GM modifications and clinical measures in MWA and MWoA patients, separately. Second, we did not have reliable information concerning the time elapsed from the last attack, which would have allowed us to better interpret our findings. Further studies in larger cohort of patients and with a longitudinal design are now warranted to confirm our findings and to clarify their role in the pathophysiology of migraine.
References
Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M (2012) Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 32:109–115
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Borsook D, Upadhyay J, Chudler EH, Becerra L (2010) A key role of the basal ganglia in pain and analgesia—insights gained through human functional imaging. Mol Pain 6:27
Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B (2009) Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med 71:566–573
Candee MS, McCandless RT, Moore KR, Arrington CB, Minich LL, Bale JF Jr. (2013) White matter lesions in children and adolescents with migraine. Pediatr Neurol 49:393–396
Chudler EH, Dong WK (1995) The role of the basal ganglia in nociception and pain. Pain 60:3–38
Cutrer FM, Huerter K (2007) Migraine aura. Neurologist 13:118–125
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–589
DaSilva AF, Granziera C, Snyder J, Hadjikhani N (2007) Thickening in the somatosensory cortex of patients with migraine. Neurology 69:1990–1995
DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N (2007) Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. NeuroReport 18:301–305
Dinia L, Bonzano L, Albano B, Finocchi C, Del Sette M, Saitta L, Castellan L, Gandolfo C, Roccatagliata L (2013) White matter lesions progression in migraine with aura: a clinical and MRI longitudinal study. J Neuroimaging 23:47–52
Ditchfield JA, McKendrick AM, Badcock DR (2006) Processing of global form and motion in migraineurs. Vision Res 46:141–148
Godinho F, Magnin M, Frot M, Perchet C, Garcia-Larrea L (2006) Emotional modulation of pain: is it the sensation or what we recall? J Neurosci 26:11454–11461
Gonzalez F, Relova JL, Prieto A, Peleteiro M (2005) Evidence of basal temporo-occipital cortex involvement in stereoscopic vision in humans: a study with subdural electrode recordings. Cereb Cortex 15:117–122
Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N (2006) Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med 3:e402
Grazzi L, Chiapparini L, Ferraro S, Usai S, Andrasik F, Mandelli ML, Bruzzone MG, Bussone G (2010) Chronic migraine with medication overuse pre-post withdrawal of symptomatic medication: clinical results and FMRI correlations. Headache 50:998–1004
Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I (2010) Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthr Rheum 62:2930–2940
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98:4687–4692
Hall SD, Barnes GR, Hillebrand A, Furlong PL, Singh KD, Holliday IE (2004) Spatio-temporal imaging of cortical desynchronization in migraine visual aura: a magnetoencephalography case study. Headache 44:204–208
Hamedani AG, Rose KM, Peterlin BL, Mosley TH, Coker LH, Jack CR, Knopman DS, Alonso A, Gottesman RF (2013) Migraine and white matter hyperintensities: the ARIC MRI study. Neurology 81:1308–1313
Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorders: 2nd edition. Cephalalgia 24 Suppl 1:9–160
Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB (2008) Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28:598–604
Lakhan SE, Avramut M, Tepper SJ (2013) Structural and functional neuroimaging in migraine: insights from 3 decades of research. Headache 53:46–66
Lee HW, Hong SB, Seo DW, Tae WS, Hong SC (2000) Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology 54:849–854
Maizels M, Aurora S, Heinricher M (2012) Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache 52:1553–1565
Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D (2011) Migraine attacks the basal ganglia. Mol Pain 7:71
May A (2008) Chronic pain may change the structure of the brain. Pain 137:7–15
McKendrick AM, Badcock DR (2004) Motion processing deficits in migraine. Cephalalgia 24:363–372
Messina R, Rocca MA, Colombo B, Valsasina P, Horsfield MA, Copetti M, Falini A, Comi G, Filippi M (2013) Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology 268:170–180
Ozge A, Termine C, Antonaci F, Natriashvili S, Guidetti V, Wober-Bingol C (2011) Overview of diagnosis and management of paediatric headache. Part I: diagnosis. J Headache Pain 12:13–23
Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM, Bakkers JT, Hofman PA, van Lew B, Middelkoop HA, van Buchem MA, Ferrari MD, Kruit MC (2012) Structural brain changes in migraine. JAMA 308:1889–1897
Rho YI, Chung HJ, Suh ES, Lee KH, Eun BL, Nam SO, Kim WS, Eun SH, Kim YO (2011) The role of neuroimaging in children and adolescents with recurrent headaches—multicenter study. Headache 51:403–408
Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M (2006) Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37:1765–1770
Rocca MA, Pagani E, Colombo B, Tortorella P, Falini A, Comi G, Filippi M (2008) Selective diffusion changes of the visual pathways in patients with migraine: a 3-T tractography study. Cephalalgia 28:1061–1068
Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A (2009) Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 29:13746–13750
Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125:89–97
Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A (2005) Gray matter decrease in patients with chronic tension type headache. Neurology 65:1483–1486
Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U (2007) Striatal grey matter increase in patients suffering from fibromyalgia—a voxel-based morphometry study. Pain 132(Suppl 1):S109–S116
Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache 48:1044–1055
Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC (2008) Increased gray matter density in young women with chronic vulvar pain. Pain 140:411–419
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17:479–489
Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31:1937–1943
Wiech K, Ploner M, Tracey I (2008) Neurocognitive aspects of pain perception. Trends Cogn Sci 12:306–313
Yu D, Yuan K, Zhao L, Dong M, Liu P, Wang G, Liu J, Sun J, Zhou G, von Deneen KM, Liang F, Qin W, Tian J (2012) Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed 25:806–812
Conflicts of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocca, M.A., Messina, R., Colombo, B. et al. Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol 261, 350–357 (2014). https://doi.org/10.1007/s00415-013-7201-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7201-y