Abstract
Purpose of the Review
This review summarizes sex-related changes in the heart and vasculature that occur with aging, both in the presence and absence of cardiovascular disease (CVD).
Recent Findings
In the presence of CVD risk factors and/or overt CVD, sex-specific changes in the number of cardiomyocytes, extent of the myocardial extracellular matrix, and myocellular hypertrophy promote unique patterns of LV remodeling in men and women. In addition, age- and sex-specific vascular stiffening is also well established, driven by changes in endothelial dysfunction, elastin–collagen content, microvascular dysfunction, and neurohormonal signaling. Together, these changes in LV chamber geometry and morphology, coupled with heightened vascular stiffness, appear to drive both age-related increases in systolic function and declines in diastolic function, particularly in postmenopausal women. Accordingly, estrogen has been implicated as a key mediator, given its direct vasodilating properties, association with nitric oxide excretion, and involvement in myocellular Ca2+ handling, mitochondrial energy production, and oxidative stress.
Summary
The culmination of the abovementioned sex-specific cardiac and vascular changes across the lifespan provides important insight into heart failure development, particularly of the preserved ejection fraction variety, while offering promise for future preventive strategies and therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, accounting for 31% of all reported deaths in 2016 [1]. Moreover, nearly 81% of all CVD-attributable deaths were among individuals ≥ 65 years old [2], reinforcing the notion that CVD is predominantly a disease of senescence. While this general pattern is true for both men and women, important sex-specific differences exist. For example, according to the National Health and Nutrition Examination Survey, collected from 2013 to 2016, the prevalence of CVD was lower in premenopausal women compared with age-matched men, yet surpassed that of men after menopause [2]. Despite near universal recognition of this sex-by-age interaction, however, the exact mechanism(s) by which age and sex influence CVD development and progression remains elusive. This review summarizes sex-related changes in the heart and vasculature that occur with aging, both in the presence and absence of cardiovascular disease.
Age–Sex Interaction and the Heart
Left Ventricular Structural Remodeling
Both sex and age are known to impact cardiac morphology (Fig. 1). Data from both the Framingham Heart Study and Multi-Ethnic study of Atherosclerosis (MESA) demonstrate that left ventricular (LV) mass and volume are significantly greater in men than women, even after adjusting for height and body surface area (BSA) [3, 4]. Across the lifespan, absolute LV mass and LVEDV tend to decrease with healthy aging, with LVEDV declining more steeply with age, resulting in a progressive rise in LV concentricity over time [4,5,6,7,8].
Influence of age, sex, and cardiovascular disease on left ventricular structure. Conceptual left ventricular cross-sectional image, at the level of the papillary muscles, showing the most common structural adaptations of the left ventricle (LV) in men and women across the lifespan and in the presence of cardiovascular disease (CVD) or CVD risk factors. Beginning with early adulthood, LV mass and volume are greater in men than women. With healthy aging, mass and volume decline, with LVEDV declining to a greater extent than LV mass, resulting in more concentric hypertrophy. In the presence of CVD risk factors and/or CVD, women tend to develop an eccentric pattern of hypertrophy, while men tend to develop a concentric pattern of hypertrophy
In contrast to healthy aging, however, in the presence of CVD risk factors and/or overt CVD, LV mass increases with age and is associated with sex-specific cardiac remodeling, such that women experience greater concentric hypertrophy, while men tend to develop an eccentric pattern of hypertrophy [9, 10]. Indeed, studies of chronic pressure overload by aortic stenosis have found that women demonstrate more concentric remodeling and less eccentric hypertrophy than men [11,12,13,14,15,16]. Likewise, in a large dataset of 3,745 women and men undergoing both cMRI and invasive coronary angiography, women presented with greater concentric remodeling and less eccentric hypertrophy [17•]. Extrapolating these sex-specific patterns of remodeling may provide insight into disease risk and pathology, where women are two times more likely to develop heart failure with preserved ejection fraction (HFpEF) than men, a condition associated with a clustering of CVD risk factors, and adverse left ventricular remodeling [18, 19]. While LV concentric remodeling is only present in a fraction of the HFpEF population [20], it is overrepresented in women compared with men with HFpEF [21].
At the cellular level, these patterns may be explained by cardiomyocyte loss, an increase in extracellular matrix, and myocellular hypertrophy [22,23,24,25]. Indeed, aging is associated with progressive neurohumoral dysfunction that contributes to cardiomyocyte death [26,27,28,29,30,31] in a sex-specific manner [32]. Among autopsies of 53 men and 53 women, cardiomyocyte death with healthy aging occurs to a greater extent in men than women [33]. This sex difference likely arises from (A) a larger pool of cardiac stem cells in women that allow for greater myocyte turnover compared with men [34] and (B) sex-specific rates of apoptosis. Regarding the latter, men experience greater rates of apoptosis than women when free of any cardiovascular disease [35], after acute myocardial infarction [36], and in heart failure [37].
Collagen content in the human heart nearly doubles over the lifespan (from ~ 3.9 to ~5.9%), independent of pathology [38]. This results in progressive reductions in LV compliance [39, 40] and is regarded as a primary mechanism of age-related diastolic dysfunction [22], discussed in more detail in the following section, “Left Ventricular Diastolic Function.” Whether an age-by-sex interaction exists with collagen content in the heart, however, remains unclear. LV systolic and diastolic stiffness is indeed greater in women than men across the lifespan, with an apparent acceleration of LV systolic and diastolic elastance in women beyond 50 years of age [41,42,43]. In the presence of CVD risk factors, the MESA demonstrated that LV extracellular volume (ECV), measured by gadolinium-enhanced MRI, is greater in women than men until ~ 84 years of age [44]. Likewise, ECV is elevated to a greater extent in women than men with mild aortic stenosis, despite women having fewer comorbidities [45]. To what extent these imaging-based observations reflect expansion of extracellular proteins, however, remains incompletely understood, as biopsy studies have reported opposite results among patients with aortic stenosis [11, 12]. In line with this later observation, a recent imaging study found greater ECV in healthy young women compared with age- and health-matched men, together with greater myocardial blood volume and myocardial resting and peak perfusion, suggesting that women may have greater capillary density, rather than a more developed extracellular matrix per se [46•]. More work is therefore needed to better define the age-by-sex interaction of extracellular proteins like collagen and specific mechanisms driving morphological changes over the lifespan and in the presence of CVD.
Left Ventricular Systolic Function
Clinically, LV ejection fraction (LVEF) is the most widely used measure to assess systolic function, despite its well-recognized shortcomings. Consistent with the age- and sex-related LV structural changes described previously, LVEF tends to be higher in women than men [47, 48], with studies reporting both increases [49,50,51] and decreases [52,53,54,55,56,57] in LVEF with advancing age; the latter of which affecting men more than women. As summarized in Table 1, similar observations have also been made with more advanced measures of LV contractility [41, 60, 61], regional tissue deformation indices [62, 63], and twist mechanics [64,65,66].
Sex hormones seem to be an unlikely source of this age-related rise in systolic function, given that both estrogen and testosterone decline with age. This is not to suggest that estrogen and testosterone are not involved in the mechanical and protein function of ventricular myocytes, which they undoubtedly are [67,68,69,70,71,72,73,74,75,76,77], just that their role in the age-associated rise in systolic function seems improbable. Indeed, ovarian hormone deficiency decreases (not increases) myocellular contractile function, and while this function is often restored with estrogen replacement [68,69,70,71,72,73,74,75,76,77], this fails to explain the rise in systolic function often observed in postmenopausal women. Likewise, while testosterone is strongly implicated in the density of L-type Ca2+ channels, sarcoplasmic reticulum Ca2+ availability, the magnitude of the Ca2+ transient, and the maximal myofilament responsiveness [67], it seems unlikely to explain the heightened systolic function observed with age, especially considering that testosterone levels decline with age, in both men and women [78,79,80]. In fact, given that the age-associated rise in systolic function is attenuated in men compared with women, for whom testosterone plays a much more dominant role across the lifespan, argues against testosterone being a contributory mechanism.
In contrast to the sex hormone hypothesis, many believe that this “heightened” contractility is reflective of a necessary adaptation to maintain optimal output in the face of higher resistance. Indeed, it is now well established that large-artery stiffness increases with age [41, 81,82,83] and is higher in women [41, 81, 84,85,86], independent of vascular disease or risk factors [41, 81, 82, 87]. To maintain optimal output, the left ventricle must therefore develop greater systolic stiffness [88,89,90,91,92]. That end-systolic elastance is elevated in women, particularly in older women, supports this interpretation [41, 59]. The exact mechanism for this augmented systolic performance, however, remains incompletely understood. To date, there is no clear evidence for heightened inotropy (e.g. circulating catecholamines, calcium affinity, etc.). Instead, alterations in chamber geometry with age and sex is likely to play the most dominant role. In accordance with the left ventricle’s unique helical muscle fiber orientation, contraction of the endocardial fibers contributes to longitudinal shortening, while contraction of the epicardial fibers contributes to circumferential shortening and left ventricular twist [93]. Age, along with presence of cardiovascular risk factors with/without overt structural remodeling, is associated with impaired subendocardial function [94], giving rise to reduced global longitudinal shortening [95, 96], for which arterial stiffness is a likely contributor [97], particularly in women [98]. At the same time, subepicardial function remains relatively unaffected, allowing for the longer lever arm of the epicardial fibers to dominate, resulting in increased circumferential shortening and increased left ventricular twist, together of which help to maintain (and even augment) left ventricular ejection fraction [99, 100] (Fig. 2). Accordingly, given the structural changes that occur with age (i.e. concentric remodeling, subendocardial dysfunction, sphericity), especially in women, and in the presence of cardiovascular disease/risk factors [4, 41, 64, 94, 101, 102], mechanical factors seem to play the most influential role.
Twist mechanics and altered strain contributions to left ventricular ejection fraction with age, sex, and cardiovascular disease. a Left ventricular myofiber architecture, changing from a left-handed helix in the subepicardium to a right-handed helix in the subendocardium. Contraction of these two opposing myofiber layers gives rise to circumferential and longitudinal shortening about the long axis of the cylinder. Note the longer lever arm of the subepicardial fibers compared with the subendocardial fibers. When both layers contract simultaneously, the epicardial fibers have a mechanical advantage, dominating the overall direction and magnitude of rotation. This mechanical advantage is augmented in conditions with impaired subendocardial function and/or a greater subepicardial radius (i.e. concentric hypertrophy). b Conceptual model illustrating patterns of change in left ventricular tissue deformation, twist mechanics, and ejection fraction through the onset of early mechanical dysfunction (associated with age, sex, and cardiovascular comorbidities), heart failure with preserved ejection fraction (HFpEF), and heart failure with reduced ejection fraction (HFrEF)
Left Ventricular Diastolic Function
Both age and female sex are associated with increased LV stiffness, related to concentric remodeling, increased collagen deposition, and loss of estrogen. As a result, the LV end-diastolic pressure–volume relationship is shifted leftward with healthy aging [39, 40], a result which is augmented in elderly females [103]. Moreover, age and female sex appear to affect other components of diastole, including early and late diastolic filling patterns [58, 59, 104, 105], as summarized in Table 2.
The majority of results to date suggest that postmenopausal status is strongly related to impaired LV relaxation, with most population-based studies showing accelerated age-related impairments in LV relaxation in women after 50 years of age (the average onset of natural menopause). While the exact mechanism for this age-by-sex interaction remains incompletely understood, estrogen is likely a key mediator for age-related diastolic dysfunction in women. Indeed, estrogen is a direct vasodilator [106, 107]; it promotes nitric oxide excretion [108, 109] and directly impacts myocellular calcium (Ca2+) handling; all of which could impact diastolic performance.
Myocardial relaxation is inherently dependent on the removal of Ca2+ from the cytosol, primarily through sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) uptake into the sarcoplasmic reticulum and sodium/calcium exchanger (NCX) extrusion from the cell [110, 111]. Although the effect of sex hormones on NCX remains inconclusive, SERCA2a’s response to hormonal changes has been well documented, at least in preclinical models of human aging. For example, ovariectomy (OVX) in middle and old age normotensive rats reduces phosphorylated phospholamban (PLB), responsible for facilitating SERCA2a activity, resulting in decreased lusitropy and increased cardiac filling pressures, with the older OVX rats experiencing the worst diastolic dysfunction. That G protein–coupled estrogen receptor (GPER) activation significantly improves LV lusitropy in this model—resulting in greater SERCA2a expression and reduced interstitial collagen content—strongly supports estrogen as a principle determinant of age-by-sex-related diastolic dysfunction [112]. Similar results have also been reported across different strains of OVX rats and experimental models, including direct GPER knockout in transgenic mice [70, 71, 113]. Furthermore, estrogen treatment in a well-established translational nonhuman primate model of menopause preserved diastolic function, in part, by modulating calcium homeostasis [114]. Although not always consistent, the beneficial effect of estrogen on diastolic function has also been demonstrated by hormone replacement therapy trials in women, as reviewed by Maslov and colleagues [115]. Less work has been performed evaluating the influence of male gonads and associated sex hormones, on diastolic (dys)function. In male mice, following bilateral removal of the testes (GDX), evidence of diastolic dysfunction has indeed been observed in both isolated myocyte preparations and in vivo [116]. It remains unclear, however, whether this effect is directly related to testosterone itself or the reduction in estradiol via aromatization of testosterone. While direct cardiomyocyte treatment with testosterone influences Ca2+-related gene expression [117], more work in this area is needed.
Diastole is a highly energy-dependent process [118]. Under normal conditions, the majority of ATP is produced from oxidative phosphorylation in the mitochondria. Impairments in ATP generation, whether from impaired oxygen delivery or oxidative phosphorylation, could therefore have direct effects on diastolic function. We and others have described clear sex differences in the presentation of myocardial ischemia, which often develops in the presence of age and/or cardiovascular risk factors. For a more detailed review of sex-specific patterns of myocardial ischemia, the reader is directed to the following comprehensive reviews: [119, 120]. While we have observed some evidence to support a role for myocardial ischemia in the development of diastolic dysfunction in women with signs and symptoms of ischemia with no obstructive coronary artery disease [121,122,123], investigations are currently underway to evaluate both the direct and indirect effect of myocardial ischemia on diastolic function in this bourgeoning clinical population.
Abnormalities in mitochondrial energy production can also contribute to impaired diastolic function via oxidative stress, as is increasingly recognized in the pathogenesis of heart failure [124, 125]. Upon ischemia/reperfusion, female Sprague Dawley rat hearts express lower rates of ROS production compared with age-matched male hearts via posttranslational modification of mitochondrial proteins [126], with estrogen being strongly implicated as the principle cardioprotective agent. Indeed, preclinical ischemia/reperfusion studies incorporating OVX with and without exogenous estradiol treatment suggest that estrogen promotes electron transport chain activity and ATP production [127], upregulates mitochondrial antioxidants [128], and downregulates mitochondrial apoptotic pathways [129]. With regard to cardiac pathology, estradiol treatment in an OVX mouse model of hypertrophic cardiomyopathy prevents energy dysregulation, reduces ROS formation, and improves diastolic function [130]. ROS production also serves as a scavenger to nitric oxide, a key regulator of normal diastolic function [131,132,133,134,135,136]. Indeed, cardiomyocytes possess both the “endothelial” and “neuronal” isoforms of nitric oxide synthase (NOS), with neuronal NOS most strongly implicated in cardiac relaxation, via effects on phospholamban phosphorylation [132,133,134,135,136]. Uncoupling of NOS often occurs during oxidative depletion of its co-factor tetrahydrobiopterin (BH4), leading to production of superoxide instead of NO. Estrogen is known to modulate BH4, and therefore may represent a key source of diastolic dysfunction in aged postmenopausal women. For example, OVX rats demonstrate reduced cardiac BH4 concentration, and BH4 treatment after OVX improves lusitropy and reduces cardiac filling pressures, collagen content, and ROS production [137].
As mentioned, estrogen is also a direct vasodilator of the arterial system [106, 107]. While this may explain at least part of the female-dominant pattern of nonobstructive coronary artery disease we and others have observed in middle-aged women [138,139,140], it may also provide insight into the accelerated impairment in early diastolic function seen in older women. For example, ventricular-arterial coupling is an important contributor to cardiac mechanics and hemodynamic control. Alterations in the stiffness of the central vascular system elevate cardiac afterload and compromise cardiac efficiency [141,142,143], with the added potential of decreasing coronary perfusion [144, 145]. While this mechanism of diastolic dysfunction has been implicated in hypertension, diabetes, and heart failure [146,147,148], the age-by-sex interaction of this proposed mechanism has not been well described, warranting further investigation.
Age–Sex Interaction and the Vasculature
The vascular system is commonly divided into two levels: the macrovasculature and microvasculature. The macrovasculature is composed of large elastic arteries that buffer intermittent increases in pulsatility following left ventricular ejection and muscular arteries that serve as conduit vessels to supply blood to the microvasculature (< 300 μm in diameter), for subsequent tissue perfusion and oxygenation. The microvasculature is therefore composed of arterioles, capillaries, and venules. As mentioned, vascular stiffness increases with age [81,82,83], independent of vascular disease or risk factors [81, 82, 87], and is higher in women [81, 84,85,86]. Multiple mechanisms have been proposed to explain age- and sex-dependent vascular stiffening, including endothelial dysfunction, changes in vascular protein composition (i.e. elastin–collagen content), microvascular dysfunction, and neurohormonal signaling, each of which is discussed in more detail herein.
Endothelial (Dys)function
The vascular endothelium, a single-cell layer lining the inner lumen of all blood vessels, plays a pivotal role in blood flow regulation by synthesizing and secreting vasoactive molecules, principally nitric oxide (vasodilator) and endothelin-1 (vasoconstrictor).
Endothelium-dependent vasodilation may be invoked by either chemical (acetylcholine) or mechanical (increase in blood flow and shear stress) stimuli, the latter of which is the principle of flow-mediated dilation (FMD), an index of coronary endothelial health/function [149] along with overall endothelial function. Both acetylcholine-mediated vasodilation and flow-mediated dilation (FMD) decrease with age in men, but remain preserved in women typically until the onset of menopause, after which endothelial dependent vasodilation markedly declines [150, 151]. In accordance with the biological timeline of these results, the majority of work strongly implicates estrogen and testosterone as primary mediators of endothelial-dependent vasodilation. Indeed, both estrogen and testosterone increase NO production via receptor-mediated activation of endothelial NO synthase. Accordingly, endothelial-dependent vasodilation declines with age in both men and women [150,151,152,153] and is attenuated in premenopausal women treated with a gonadotropin-releasing hormone antagonist (GnRH-ant) [154] and young men treated with an aromatase inhibitor, which blocks endogenous production of estrogen [155], and restored by estradiol treatment [154, 156,157,158,159]. Less clear is the role of testosterone in the regulation of endothelial function, as results from several cross-sectional studies evaluating FMD in men with low serum testosterone remain equivocal [160,161,162,163,164]. Both testosterone and estrogen possess antioxidant and anti-inflammatory properties that are lost in hormone-deficient states, regarded as the principle mechanism linking sex hormones with endothelial-dependent vasodilation [165,166,167,168].
Less established, but increasingly recognized, is the role of endothelin-1 on endothelial function both with aging and between sexes. Endothelin-1 is a potent vasoconstrictor produced and released by endothelial cells that acts on two receptor subtypes, ETA and ETB, located on the vascular smooth muscle [169]. In addition, ETB receptors are also located on the endothelium and mediate vasodilation [169, 170]. Emerging evidence suggests that endothelin-1 receptors may be sexually dimorphic [171•] [172], with endothelin-1 preferentially binding to ETB receptors in women [173]. Moreover, endothelin-1-mediated vasoconstriction appears to be augmented with age [174, 175], with ETB-mediated vasodilation potentially lost in postmenopausal women [176]. Whether targeting endothelin receptors can improve cardiovascular disease outcomes, quality of life, and overall survival remains largely unknown, but with the advent of endothelin receptor antagonists, has great potential of being addressed within the next decade.
Elastin–Collagen Content
Large blood vessels like the aorta are inherently “elastic,” facilitating blood vessel distension with each heartbeat (i.e. stroke volume), dampening velocity and pressure fluctuations, and maintaining consistent unidirectional blood flow. Vascular elasticity is predominantly mediated by the balance between collagen—a stiff scaffolding protein—and elastin—an elastic protein designed to facilitate the repetitive distention of the vessel. In male rodents, aging is associated with a progressive shift in the collagen–elastin ratio, whereby elastin is degraded with age and collagen expression is increased [177,178,179]. To our knowledge, however, this work has not been replicated in female rodents, rendering our understanding of sex-by-age-related differences in vascular protein composition incomplete. In nonhuman primates, aortic stiffness increases with age to a greater extent in male versus female monkeys, attributable to preserved collagen but decreased elastin among old male monkeys that was larger in magnitude than that observed in old female monkeys [180]. Notably, several cross-species differences in specific collagen isoform changes with aging appear to exist, at least between mice and monkeys, highlighting the need to extend these observations to humans (of both sexes). Unfortunately, aside from a limited number of autopsy studies completed more than three decades ago [181,182,183,184,185], our clinical understanding remains limited. Nevertheless, despite several challenges with interpreting these early data, including issues surrounding differences in both the location of dissection (abdominal aorta vs thoracic) and prior health status of the individuals included, it does appear that human aging is indeed associated with a similar shift in collagen–elastin ratio. While the exact mechanism driving age (and potentially sex) related changes in the balance between collagen and elastin remains incompletely understood, reactive oxygen species and inflammation—which could degrade elastin and increase the deposition of collagen—are thought to play a major role [168]. More work is needed, however, to truly address this question.
Microvascular (Dys)function
We and others have shown that coronary microvascular dysfunction (CMD) is more prevalent in women than men [186,187,188], and several reports of “microvascular dysfunction” in HFpEF have also recently emerged, touting microvascular dysfunction as a promising therapeutic target in this burgeoning condition that predominantly impacts older women [189, 190]. This has led to the hypothesis that risk factor conditions (age, obesity, dysglycemia, hyperlipidemia), including loss of estrogen, promote a pro-inflammatory, pro-oxidative state, rending the microvasculature vulnerable [191, 192]. Thus, while “microvascular dysfunction” may present itself in specific end-organs like the myocardium (i.e. coronary microvascular dysfunction, and associated ischemia, structural remodeling, systolic/diastolic dysfunction), this conceptual framework suggests that microvascular dysfunction is likely systemic in nature.
The assessment of “microvascular function” has therefore taken a broad approach in recent years, ranging from circulating biomarkers (sICAM-1 [soluble intercellular adhesion molecule-1], sVCAM-1 [soluble vascular adhesion molecule-1], sE-selectin [soluble E-selectin], and vWF [von Willebrand factor]), structural imaging approaches like optical coherence tomography [193], and darkfield microscopy [194,195,196] to limb reperfusion measurements following a brief period of tissue ischemia (i.e. reactive hyperemia) [197, 198]. While each of these endpoints have been studied in the context of specific cardiovascular and/or metabolic diseases, unlike studies evaluating macrovascular endothelial function, population studies exploring sex and the influence of healthy aging on microvascular endpoints remain limited. More work is therefore needed to fully elucidate the impact of age and sex on microvascular dysfunction, along with specific mechanisms contributing to its prevalence.
Neurovascular Control
Accumulating evidence suggests that both sex and age influence autonomic neural control of vascular tone. For example, the incidence of orthostatic intolerance is much higher in young women than young men, related to an apparent attenuation of peripheral vasoconstrictor responsiveness to sympathetic activity [199,200,201]. Where a significant relationship exists between sympathetic nerve activity and total peripheral resistance in young men, this relationship is absent in young women [202], attributable to greater β-adrenergic-mediated vasodilation in young women [203, 204]. Indeed, the relationship between sympathetic nerve activity and total peripheral resistance is restored in young women via systemic β-adrenoreceptor blockade [204]. After ~ 40 years of age, however, the autonomic nervous system plays a much more dominant role in the control of blood pressure [205], a response largely attributable to a reduction in β2-adrenergic receptor (β2AR)–mediated vasodilation [203, 206, 207]. While the exact mechanism for this finding remains incompletely understood, reduced NO bioavailability has been implicated [207]. Moreover, aged postmenopausal women have greater vasoconstrictor responses to norepinephrine [203], which may be related to greater sympathetic transduction of sympathetic nerve activity [208]. It is interesting to consider these findings in the context of popular cardiovascular therapeutics, particularly beta- and alpha-blockers. Caution may therefore be warranted as we promote certain classes of drugs that have worked well in male-dominated conditions like heart failure with reduced ejection fraction (HFrEF) to more female-dominant conditions like HFpEF.
Conclusions
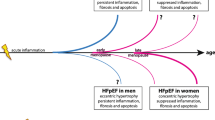
Taken together, there is clear evidence that both age and sex influence the cardiovascular system. Sex-specific cardiomyocyte loss, an increase in extracellular matrix, and myocellular hypertrophy work in tandem in the presence of CVD risk factors and/or overt CVD to promote unique patterns of LV remodeling in women and men. In addition, age- and sex-specific vascular stiffening is also well established, driven by changes in endothelial dysfunction, elastin–collagen content, microvascular function, and neurohormonal signaling. Together, these changes in LV chamber geometry and morphology, coupled with heightened vascular stiffness, appear to drive both age-related increases in systolic function and declines in diastolic function, particularly in postmenopausal women. Estrogen is indeed implicated as an important mediator of the aforementioned changes, given that it is a direct vasodilator, promotes nitric oxide excretion, and impacts myocellular Ca2+ handling, mitochondrial energy production, and oxidative stress. The culmination of these sex-specific cardiac and vascular changes across the lifespan may provide key insight into heart failure development, particularly of the preserved ejection fraction variety. While knowledge gaps remain, as outlined herein, the collective insight currently available offers great promise for future preventive strategies and therapeutic approaches.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Organization WH. Cardiovascular diseases (CVDs) 2017 [Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39(6):1055–60.
Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2(3):191–8.
Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–65.
Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90(11):1231–6.
Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): a quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63(2):137–46.
Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study). Am J Cardiol. 1989;64(16):1066–8.
Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, et al. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122(6):570–8.
Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119(24):3085–92.
Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mahmoodzadeh S, et al. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging. 2014;7(11):1073–80.
Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16(11):1160–7.
Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation. 2010;122(11 Suppl):S23–8.
Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71(8):860–71.
Dobson LE, Fairbairn TA, Musa TA, Uddin A, Mundie CA, Swoboda PP, et al. Sex-related differences in left ventricular remodeling in severe aortic stenosis and reverse remodeling after aortic valve replacement: a cardiovascular magnetic resonance study. Am Heart J. 2016;175:101–11.
Singh A, Chan DCS, Greenwood JP, Dawson DK, Sonecki P, Hogrefe K, et al. Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. JACC Cardiovasc Imaging. 2019;12(1):96–105.
• Miller RJH, Mikami Y, Heydari B, Wilton SB, James MT, Howarth AG, et al. Sex-specific relationships between patterns of ventricular remodelling and clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020. This study confirms that sex differences in LV remodeling have serious adverse implications.
Ho JE, Gona P, Pencina MJ, Tu JV, Austin PC, Vasan RS, et al. Discriminating clinical features of heart failure with preserved vs. reduced ejection fraction in the community. Eur Heart J. 2012;33(14):1734–41.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9.
Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, et al. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7(1):104–15.
Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):535–42.
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–54.
Donekal S, Venkatesh BA, Liu YC, Liu CY, Yoneyama K, Wu CO, et al. Interstitial fibrosis, left ventricular remodeling, and myocardial mechanical behavior in a population-based multiethnic cohort: the Multi-Ethnic Study of Atherosclerosis (MESA) study. Circ Cardiovasc Imaging. 2014;7(2):292–302.
Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46(7):549–59.
Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol. 1987;10(2 Suppl A):42A–7A.
Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic peptide. Hypertension. 2011;57(2):201–7.
Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293(3):H1351–8.
Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524–30.
Vasan RS, Sullivan LM, D'Agostino RB, Roubenoff R, Harris T, Sawyer DB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139(8):642–8.
Luong N, Davies CR, Wessells RJ, Graham SM, King MT, Veech R, et al. Activated FOXO-mediated insulin resistance is blocked by reduction of TOR activity. Cell Metab. 2006;4(2):133–42.
Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11(4):725–33.
Kessler EL, Rivaud MR, Vos MA, van Veen TAB. Sex-specific influence on cardiac structural remodeling and therapy in cardiovascular disease. Biol Sex Differ. 2019;10(1):7.
Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–79.
Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107(11):1374–86.
Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, et al. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci. 2001;56(11):M719–23.
Biondi-Zoccai GG, Abate A, Bussani R, Camilot D, Giorgio FD, Marino MP, et al. Reduced post-infarction myocardial apoptosis in women: a clue to their different clinical course? Heart. 2005;91(1):99–101.
Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85(9):856–66.
Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122(10):1049–58.
Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, et al. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol. 2012;590(8):1871–80.
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110(13):1799–805.
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254–62.
Claessens TE, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Gillebert TC, et al. Noninvasive assessment of left ventricular and myocardial contractility in middle-aged men and women: disparate evolution above the age of 50? Am J Physiol Heart Circ Physiol. 2007;292(2):H856–65.
Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6(5):944–52.
Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62(14):1280–7.
Tastet L, Kwiecinski J, Pibarot P, Capoulade R, Everett RJ, Newby DE, et al. Sex-related differences in the extent of myocardial fibrosis in patients with aortic valve stenosis. JACC Cardiovasc Imaging. 2020;13(3):699–711.
• Nickander J, Themudo R, Sigfridsson A, Xue H, Kellman P, Ugander M. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males - an adenosine stress cardiovascular magnetic resonance study. Sci Rep. 2020;10(1):10380 This novel imaging study raises important questions about sex-specific differences in myocardial perfusion and extracellular volume fraction that needs further investigation.
Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113(12):1597–604.
Maredziak M, Bengs S, Portmann A, Haider A, Wijnen WJ, Warnock GI, et al. Microvascular dysfunction and sympathetic hyperactivity in women with supra-normal left ventricular ejection fraction (snLVEF). Eur J Nucl Med Mol Imaging. 2020.
Gebhard C, Stahli BE, Gebhard CE, Tasnady H, Zihler D, Wischnewsky MB, et al. Age- and gender-dependent left ventricular remodeling. Echocardiography. 2013;30(10):1143–50.
Ruan Q, Rao L, Middleton KJ, Khoury DS, Nagueh SF. Assessment of left ventricular diastolic function by early diastolic mitral annulus peak acceleration rate: experimental studies and clinical application. J Appl Physiol (1985). 2006;100(2):679–84.
Kaku K, Takeuchi M, Otani K, Sugeng L, Nakai H, Haruki N, et al. Age- and gender-dependency of left ventricular geometry assessed with real-time three-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24(5):541–7.
Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–82.
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202.
Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, et al. Prevalence of heart failure and left ventricular dysfunction in the general population. The Rotterdam Study Eur Heart J. 1999;20(6):447–55.
McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350(9081):829–33.
Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358(9280):439–44.
Devereux RB, Roman MJ, Paranicas M, Lee ET, Welty TK, Fabsitz RR, et al. A population-based assessment of left ventricular systolic dysfunction in middle-aged and older adults: the Strong Heart Study. Am Heart J. 2001;141(3):439–46.
Hayashi S, Yamada H, Nishio S, Hotchi J, Bando M, Takagawa Y, et al. Age- and gender-specific changes of tricuspid annular motion velocities in normal hearts. J Cardiol. 2015;65(5):397–402.
Hoshida S, Shinoda Y, Ikeoka K, Fukuoka H, Inui H, Watanabe T. Age- and sex-related differences in diastolic function and cardiac dimensions in a hypertensive population. ESC Heart Fail. 2016;3(4):270–7.
Hayward CS, Kalnins WV, Kelly RP. Gender-related differences in left ventricular chamber function. Cardiovasc Res. 2001;49(2):340–50.
Celentano A, Palmieri V, Arezzi E, Mureddu GF, Sabatella M, Di Minno G, et al. Gender differences in left ventricular chamber and midwall systolic function in normotensive and hypertensive adults. J Hypertens. 2003;21(7):1415–23.
Gruner Svealv B, Fritzon G, Andersson B. Gender and age related differences in left ventricular function and geometry with focus on the long axis. Eur J Echocardiogr. 2006;7(4):298–307.
Foll D, Jung B, Schilli E, Staehle F, Geibel A, Hennig J, et al. Magnetic resonance tissue phase mapping of myocardial motion: new insight in age and gender. Circ Cardiovasc Imaging. 2010;3(1):54–64.
Yoneyama K, Gjesdal O, Choi EY, Wu CO, Hundley WG, Gomes AS, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126(21):2481–90.
Hung CL, Goncalves A, Shah AM, Cheng S, Kitzman D, Solomon SD. Age- and sex-related influences on left ventricular mechanics in elderly individuals free of prevalent heart failure: the ARIC study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2017;10(1):e004510.
Nio AQX, Stohr EJ, Shave RE. Age-related differences in left ventricular structure and function between healthy men and women. Climacteric. 2017;20(5):476–83.
Ayaz O, Howlett SE. Testosterone modulates cardiac contraction and calcium homeostasis: cellular and molecular mechanisms. Biol Sex Differ. 2015;6:9.
Machuki JO, Zhang HY, Geng J, Fu L, Adzika GK, Wu L, et al. Estrogen regulation of cardiac cAMP-L-type Ca(2+) channel pathway modulates sex differences in basal contraction and responses to beta2AR-mediated stress in left ventricular apical myocytes. Cell Commun Signal. 2019;17(1):34.
Parks RJ, Bogachev O, Mackasey M, Ray G, Rose RA, Howlett SE. The impact of ovariectomy on cardiac excitation-contraction coupling is mediated through cAMP/PKA-dependent mechanisms. J Mol Cell Cardiol. 2017;111:51–60.
Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, et al. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol. 2003;284(5):H1800–7.
Turdi S, Huff AF, Pang J, He EY, Chen X, Wang S, et al. 17-beta estradiol attenuates ovariectomy-induced changes in cardiomyocyte contractile function via activation of AMP-activated protein kinase. Toxicol Lett. 2015;232(1):253–62.
Ribeiro RF Jr, Pavan BM, Potratz FF, Fiorim J, Simoes MR, Dias FM, et al. Myocardial contractile dysfunction induced by ovariectomy requires AT1 receptor activation in female rats. Cell Physiol Biochem. 2012;30(1):1–12.
Paigel AS, Ribeiro RF Jr, Fernandes AA, Targueta GP, Vassallo DV, Stefanon I. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod Biol Endocrinol. 2011;9:54.
Bupha-Intr T, Wattanapermpool J, Pena JR, Wolska BM, Solaro RJ. Myofilament response to Ca2+ and Na+/H+ exchanger activity in sex hormone-related protection of cardiac myocytes from deactivation in hypercapnic acidosis. Am J Phys Regul Integr Comp Phys. 2007;292(2):R837–43.
Wu Q, Zhao Z, Sun H, Hao YL, Yan CD, Gu SL. Oestrogen changed cardiomyocyte contraction and beta-adrenoceptor expression in rat hearts subjected to ischaemia-reperfusion. Exp Physiol. 2008;93(9):1034–43.
Curl CL, Wendt IR, Canny BJ, Kotsanas G. Effects of ovariectomy and 17 beta-oestradiol replacement on [Ca2+]i in female rat cardiac myocytes. Clin Exp Pharmacol Physiol. 2003;30(7):489–94.
Kam KW, Kravtsov GM, Liu J, Wong TM. Increased PKA activity and its influence on isoprenaline-stimulated L-type Ca2+ channels in the heart from ovariectomized rats. Br J Pharmacol. 2005;144(7):972–81.
Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89(12):5920–6.
Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2(6):e000272.
Pluchino N, Carmignani A, Cubeddu A, Santoro A, Cela V, Errasti T. Androgen therapy in women: for whom and when. Arch Gynecol Obstet. 2013;288(4):731–7.
Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45.
Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Pt 1):1456–62.
Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–9.
Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37(5):1374–80.
Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, et al. Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens. 2001;19(12):2197–203.
Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30(7):1863–71.
Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–10.
Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32(5):1221–7.
Little WC, Cheng CP. Left ventricular-arterial coupling in conscious dogs. Am J Phys. 1991;261(1 Pt 2):H70–6.
Burkhoff D, de Tombe PP, Hunter WC, Kass DA. Contractile strength and mechanical efficiency of left ventricle are enhanced by physiological afterload. Am J Phys. 1991;260(2 Pt 2):H569–78.
van der Velde ET, Burkhoff D, Steendijk P, Karsdon J, Sagawa K, Baan J. Nonlinearity and load sensitivity of end-systolic pressure-volume relation of canine left ventricle in vivo. Circulation. 1991;83(1):315–27.
Saba PS, Ganau A, Devereux RB, Pini R, Pickering TG, Roman MJ. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. 1999;17(7):1007–15.
Sengupta PP, Khandheria BK, Narula J. Twist and untwist mechanics of the left ventricle. Heart Fail Clin. 2008;4(3):315–24.
Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291(4):H1573–9.
Bianco CM, Farjo PD, Ghaffar YA, Sengupta PP. Myocardial mechanics in patients with normal LVEF and diastolic dysfunction. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):258–71.
Szelenyi Z, Fazakas A, Szenasi G, Tegze N, Fekete B, Molvarec A, et al. The mechanism of reduced longitudinal left ventricular systolic function in hypertensive patients with normal ejection fraction. J Hypertens. 2015;33(9):1962–9 discussion 9.
Bell V, McCabe EL, Larson MG, Rong J, Merz AA, Osypiuk E, et al. Relations between aortic stiffness and left ventricular mechanical function in the community. J Am Heart Assoc. 2017;6(1):e004903.
Yoshida Y, Nakanishi K, Daimon M, Ishiwata J, Sawada N, Hirokawa M, et al. Sex-specific difference in the association between arterial stiffness and subclinical left ventricular dysfunction. Eur Heart J Cardiovasc Imaging. 2020.
Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19(9):1077–84.
Buchalter MB, Rademakers FE, Weiss JL, Rogers WJ, Weisfeldt ML, Shapiro EP. Rotational deformation of the canine left ventricle measured by magnetic resonance tagging: effects of catecholamines, ischaemia, and pacing. Cardiovasc Res. 1994;28(5):629–35.
Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112(7):984–91.
Nikitin NP, Witte KK, Thackray SD, de Silva R, Clark AL, Cleland JG. Longitudinal ventricular function: normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color Doppler tissue imaging. J Am Soc Echocardiogr. 2003;16(9):906–21.
Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127(1):55–62.
Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, et al. Age- and gender-specific changes in the left ventricular relaxation: a Doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging. 2009;2(1):41–6.
Daimon M, Watanabe H, Abe Y, Hirata K, Hozumi T, Ishii K, et al. Gender differences in age-related changes in left and right ventricular geometries and functions. Echocardiography of a healthy subject group. Circ J. 2011;75(12):2840–6.
Sudhir K, Chou TM, Mullen WL, Hausmann D, Collins P, Yock PG, et al. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26(3):807–14.
Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, Gerstenblith G, et al. Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in postmenopausal women. Circulation. 1994;89(1):52–60.
Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81(5):885–92.
Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Phys. 1997;273(1 Pt 1):L119–26.
Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476(2):279–93.
Bers D Excitation-contraction coupling and cardiac contractile force: Springer Science & Business Media; 2001.
Alencar AK, da Silva JS, Lin M, Silva AM, Sun X, Ferrario CM, et al. Effect of age, estrogen status, and late-life GPER activation on cardiac structure and function in the Fischer344xBrown Norway Female Rat. J Gerontol A Biol Sci Med Sci. 2017;72(2):152–62.
Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc Res. 2012;94(1):96–104.
Michalson KT, Groban L, Howard TD, Shively CA, Sophonsritsuk A, Appt SE, et al. Estradiol treatment initiated early after ovariectomy regulates myocardial gene expression and inhibits diastolic dysfunction in female cynomolgus monkeys: potential roles for calcium homeostasis and extracellular matrix remodeling. J Am Heart Assoc. 2018;7(21):e009769.
Maslov PZ, Kim JK, Argulian E, Ahmadi A, Narula N, Singh M, et al. Is cardiac diastolic dysfunction a part of post-menopausal syndrome? JACC Heart Fail. 2019;7(3):192–203.
Ayaz O, Banga S, Heinze-Milne S, Rose RA, Pyle WG, Howlett SE. Long-term testosterone deficiency modifies myofilament and calcium-handling proteins and promotes diastolic dysfunction in the aging mouse heart. Am J Physiol Heart Circ Physiol. 2019;316(4):H768–H80.
Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36(4):197–202.
Pouleur H. Diastolic dysfunction and myocardial energetics. Eur Heart J. 1990;11(Suppl C):30–4.
Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004437.
Sobhani K, Nieves Castro DK, Fu Q, Gottlieb RA, Van Eyk JE, Noel Bairey Merz C. Sex differences in ischemic heart disease and heart failure biomarkers. Biol Sex Differ. 2018;9(1):43.
Nelson MD, Szczepaniak LS, Wei J, Haftabaradaren A, Bharadwaj M, Sharif B, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging. 2014;7(3):510–6.
Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C, et al. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol. 2017;40(5):300–6.
Zamani SK, Samuel TJ, Wei J, Thomson LEJ, Tamarappoo B, Sharif B, et al. Left atrial stiffness in women with ischemia and no obstructive coronary artery disease: novel insight from left atrial feature tracking. Clin Cardiol. 2020.
Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, et al. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14(4):238–50.
Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139(11):1435–50.
Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106(11):1681–91.
Lancaster TS, Jefferson SJ, Hunter JC, Lopez V, Van Eyk JE, Lakatta EG, et al. Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiol Genomics. 2012;44(20):957–69.
Liu H, Yanamandala M, Lee TC, Kim JK. Mitochondrial p38beta and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes. PLoS One. 2014;9(1):e85272.
Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Phys Regul Integr Comp Phys. 2010;298(6):R1597–606.
Chen Y, Zhang Z, Hu F, Yang W, Yuan J, Cui J, et al. 17beta-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. J Steroid Biochem Mol Biol. 2015;147:92–102.
Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121(4):519–28.
Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Phys Cell Phys. 2008;294(6):C1566–75.
Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol. 2008;45(5):625–32.
Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, et al. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102(2):242–9.
Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117(20):2626–36.
Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105(12):1503–8.
Jessup JA, Zhang L, Presley TD, Kim-Shapiro DB, Wang H, Chen AF, et al. Tetrahydrobiopterin restores diastolic function and attenuates superoxide production in ovariectomized mRen2.Lewis rats. Endocrinology. 2011;152(6):2428–36.
Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–44.
Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9.
Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl):S4–S20.
Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985). 2008;105(4):1342–51.
Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50(16):1570–7.
Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21(2):183–94.
Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21(4):402–24.
Tritakis V, Tzortzis S, Ikonomidis I, Dima K, Pavlidis G, Trivilou P, et al. Association of arterial stiffness with coronary flow reserve in revascularized coronary artery disease patients. World J Cardiol. 2016;8(2):231–9.
Ikonomidis I, Lekakis J, Papadopoulos C, Triantafyllidi H, Paraskevaidis I, Georgoula G, et al. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am J Hypertens. 2008;21(7):806–13.
van Schinkel LD, Auger D, van Elderen SG, Ajmone Marsan N, Delgado V, Lamb HJ, et al. Aortic stiffness is related to left ventricular diastolic function in patients with diabetes mellitus type 1: assessment with MRI and speckle tracking strain analysis. Int J Card Imaging. 2013;29(3):633–41.
Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, et al. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC Study (Health, Aging, and Body Composition). Hypertension. 2017;69(2):267–74.
Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–41.
Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–6.
Skaug EA, Aspenes ST, Oldervoll L, Morkedal B, Vatten L, Wisloff U, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol. 2013;20(4):531–40.
Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–700.
Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab. 2013;98(11):4507–15.
Yim SF, Lau TK, Sahota DS, Chung TK, Chang AM, Haines CJ. Prospective randomized study of the effect of "add-back" hormone replacement on vascular function during treatment with gonadotropin-releasing hormone agonists. Circulation. 1998;98(16):1631–5.
Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93(11):1127–33.
Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, et al. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med. 1994;121(12):936–41.
Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21(9):1512–9.
Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol. 2008;294(4):H1630–7.
Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, et al. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol. 2011;301(4):H1716–22.
Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30(11):1029–34.
Empen K, Lorbeer R, Dorr M, Haring R, Nauck M, Glaser S, et al. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol. 2012;32(2):481–6.
Makinen JI, Perheentupa A, Irjala K, Pollanen P, Makinen J, Huhtaniemi I, et al. Endogenous testosterone and brachial artery endothelial function in middle-aged men with symptoms of late-onset hypogonadism. Aging Male. 2011;14(4):237–42.
Corrigan FE 3rd, Al Mheid I, Eapen DJ, Hayek SS, Sher S, Martin GS, et al. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol. 2015;194:94–9.
Sansone A, Rastrelli G, Cignarelli A, de Rocco PM, Condorelli RA, Giannetta E, et al. Effect of treatment with testosterone on endothelial function in hypogonadal men: a systematic review and meta-analysis. Int J Impot Res. 2019;32(4):379–86.
Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol. 2019;317(2):H395–404.
Moreau KL. Intersection between gonadal function and vascular aging in women. J Appl Physiol (1985). 2018;125(6):1881–7.
Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol Heart Circ Physiol. 2019;316(3):H522–H6.
Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11(1):18.
Haynes WG. Endothelins as regulators of vascular tone in man. Clin Sci (Lond). 1995;88(5):509–17.
Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, et al. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci U S A. 1994;91(11):4892–6.
• Kuczmarski AV, Shoemaker LN, Hobson JC, Edwards DG, Wenner MM. Altered endothelial ETB receptor expression in postmenopausal women. Am J Physiol Heart Circ Physiol. 2020;319(1):H242–H7 This study provides novel data demonstrating attenuated endothelial ETB receptor expression in postmenopausal women, providing mechanistic insight into vascular endothelial dysfunction in the population, with important potential therapeutic implications.
Kitada K, Ohkita M, Matsumura Y. Pathological importance of the endothelin-1/ET(B) receptor system on vascular diseases. Cardiol Res Pract. 2012;2012:731970.
Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285(2):511–7.
Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–32.
Westby CM, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin Sci (Lond). 2011;120(11):485–91.
Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT, Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Phys Regul Integr Comp Phys. 2017;313(1):R51–R7.
Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33(1):116–23.
O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50(1):1–13.
Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother. 2003;57(5-6):195–202.
Qiu H, Depre C, Ghosh K, Resuello RG, Natividad FF, Rossi F, et al. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116(6):669–76.
Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245(1):73–84.
Spina M, Garbisa S, Hinnie J, Hunter JC, Serafini-Fracassini A. Age-related changes in composition and mechanical properties of the tunica media of the upper thoracic human aorta. Arteriosclerosis. 1983;3(1):64–76.
Andreotti L, Bussotti A, Cammelli D, di Giovine F, Sampognaro S, Sterrantino G, et al. Aortic connective tissue in ageing--a biochemical study. Angiology. 1985;36(12):872–9.
Maurel E, Shuttleworth CA, Bouissou H. Interstitial collagens and ageing in human aorta. Virchows Arch A Pathol Anat Histopathol. 1987;410(5):383–90.
Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35(10):615–21.
Bairey Merz CN. Women and ischemic heart disease: paradox and pathophysiology. JACC Cardiovasc Imaging. 2011;4(1):74–7.
Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–92.
Nelson MD. Left ventricular diastolic dysfunction in women with nonobstructive ischemic heart disease: insights from magnetic resonance imaging and spectroscopy. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R322–r9.
Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39(37):3439–50.
Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845–54.
Wei J, Nelson MD, Sharif B, Shufelt C, Bairey Merz CN. Why do we care about coronary microvascular dysfunction and heart failure with preserved ejection fraction: addressing knowledge gaps for evidence-based guidelines. Eur Heart J. 2018;39(37):3451–3.
Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J. 2018;39(10):850–2.
Lipecz A, Miller L, Kovacs I, Czakó C, Csipo T, Baffi J, et al. Microvascular contributions to age-related macular degeneration (AMD): from mechanisms of choriocapillaris aging to novel interventions. Geroscience. 2019;41(6):813–45.
Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc. 2020;9(9):e015716.
Pranskunas A, Tamosuitis T, Balciuniene N, Damanskyte D, Sneider E, Vitkauskiene A, et al. Alterations of conjunctival glycocalyx and microcirculation in non-septic critically ill patients. Microvasc Res. 2018;118:44–8.
Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, et al. Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2018;315(3):H531–H9.
• Rosenberry R, Trojacek D, Chung S, Cipher DJ, Nelson MD. Interindividual differences in the ischemic stimulus and other technical considerations when assessing reactive hyperemia. Am J Phys Regul Integr Comp Phys. 2019;317(4):R530–R8 This study raises important methodological considerations regarding reactive hyperemia, a common measures of peripheral microvascular function. The considerations raised have direct implications for sex and age related differences previously reported.
Rosenberry R, Nelson MD. Reactive hyperemia: a review of methods, mechanisms, and considerations. Am J Phys Regul Integr Comp Phys. 2020;318(3):R605–R18.
Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol. 2003;91(8):1006–8 A8.
Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286(1):H449–57.
Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111(4):494–8.
Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53(3):571–6.
Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol. 2011;589(Pt 21):5285–97.
Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36(4):1233–8.
Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45(4):522–5.
Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, et al. Forearm vasodilator responses to a beta-adrenergic receptor agonist in premenopausal and postmenopausal women. Phys Rep. 2014;2(6):e12032.
Harvey RE, Ranadive SM, Limberg JK, Baker SE, Nicholson WT, Curry TB, et al. Forearm vasodilatation to a beta2 -adrenergic receptor agonist in premenopausal and postmenopausal women. Exp Physiol. 2020;105(5):886–92.
Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, et al. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol. 2016;594(17):4753–68.
Funding
This work was supported by National Institutes of Health grant nos. R01HL136601, HL090957, R01HL146158, N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, U01 64829, U01 HL649141, U01 HL649241, R03 AG032631, and R01HL146158.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
C.N.B.M receives funding from Abbott Diagnostics, Sanofi (paid through CSMC) and serves as Board of Directors for iRhythm. Andrew Oneglia and Dr. Michael Nelson declare that they have no conflict of interest. Dr. Bairey Merz reports personal fees from iRhythm, personal fees from Med Intelligence, personal fees from Bayer Advisory Board, grants from California Institute for Precision Medicine, grants from CDMRP Department of Defense, grants from NHLBI subcontract to Research Triangle Institute (RTI) International, grants from the NIH, and grants from Sanofi, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Heart Failure
Rights and permissions
About this article
Cite this article
Oneglia, A., Nelson, M.D. & Merz, C.N.B. Sex Differences in Cardiovascular Aging and Heart Failure. Curr Heart Fail Rep 17, 409–423 (2020). https://doi.org/10.1007/s11897-020-00487-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-020-00487-7