Abstract
Purpose of Review
The diagnosis of gastric neuroendocrine tumors (NETs) is being made with increased frequency likely as a result of more upper endoscopies being done for unrelated reasons. It is therefore vital that gastroenterologists become familiar with the basic work-up and management of patients found to have these tumors. This review describes the classification, pathophysiology, clinical characteristics, and treatment options of the different gastric NETs.
Recent Findings
In addition to the three traditional subtypes of gastric NETs, additional cases associated with achlorhydria and appropriate hypergastrinemia may exist. The management of gastric NETs between 1 and 2 cm in size remains controversial and needs to be individualized.
Summary
Gastric NETs are uncommon but are now diagnosed more frequently. This review highlights the role of hypergastrinemia in their development and the controversies around their management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroenteropancreatic neuroendocrine tumors (GEPNETs) are commonly divided into pancreatic neuroendocrine tumors (pNETs) and (luminal) carcinoid tumors with the latter being further subdivided into foregut, midgut, and hindgut subtypes [1]. However, in this context, the term “carcinoid” (i.e., carcinoma-like) is better replaced by luminal neuroendocrine tumors (NETs), which better describes their mixed neuro- and endocrinological origins. Luminal NETs originate from enterochromaffin (EC) and enterochromaffin-like (ECL) enteroendocrine cells lining the gastrointestinal tract, which control gastrointestinal motility and secretion.
Gastric NETs are generally slow growing and often-indolent tumors but can also be very aggressive and metastasize widely. They are frequently multi-focal and are being diagnosed with increased frequency, often incidentally, likely on the basis of increased endoscopies being done for other reasons [2]. Within the foregut group of luminal NETs, the incidence of gastric NETs has increased 15-fold from 1973 to 2012 based on the Surveillance, Epidemiology, and End Results (SEER) database, with the most recent annual incidence estimated at 0.5 per 100,000 persons [3]. Gastric NETs are relatively rare lesions representing about 7% of all carcinoid tumors and less than 1% of all stomach neoplasms [4]. In addition, gastric NETs have a higher incidence among obese patients, and routine pre-bariatric surgery upper endoscopy may provide an opportunity for their diagnosis [5].
Classification
In 2010, the World Health Organization (Table 1, WHO histological grading of gastric NETs) classified GEPNETs into well-differentiated low- and intermediate-grade (G1, G2) NETs and poorly differentiated high-grade (G3) neuroendocrine carcinomas (NECs) based on elevated mitotic rate and Ki-67 index [6••]. Higher grade tumors are more likely to be associated with more angioinvasion, metastases, and muscularis mucosa invasion. Gastric NETs are broadly divided into three subtypes with different clinical characteristics, pathophysiology, aggressiveness, and prognosis (Table 2 characteristics of gastric NETs). Type I gastric NETs are associated with chronic atrophic gastritis that may or may not occur in the setting of pernicious anemia. Type II gastric NETs are usually found in patients with multiple endocrine neoplasia (MEN)-1-associated gastrinomas, which cause Zollinger-Ellison syndrome (ZES). Type III gastric NETs are sporadic, are not associated with any predisposing condition, and have a high rate of metastasis at diagnosis and often a poor prognosis. There has been described an even rarer subtype of gastric NET, the so-called type IV with similar features to type II except for achlorhydria [8]. However, the existence of this type as a separate entity is debated. Although proton pump inhibitor (PPI)–associated ECL cell hyperplasia is well recognized in humans, macroscopic carcinoids secondary to PPI use is felt to be very rare [9, 10].
Pathophysiology
Type I and type II gastric NETs are both associated with elevated fasting serum gastrin levels but the mechanism for this hypergastrinemia differs in the two types. Normally, in response to food consumed, G cells in the gastric antrum release gastrin, which stimulates ECL cells to produce histamine. This histamine then acts on parietal cells in the fundus to stimulate acid production and secretion. Acidification of gastric luminal contents initiates a feedback loop to downregulate gastrin release mediated primarily by somatostatin-producing D cells (in the gastric antrum), which directly inhibits further release of gastrin from G cells (Fig. 1a, physiological acid secretion from parietal cells).
a Physiological acid secretion from parietal cells: G cells release gastrin, which stimulates ECL cells to produce histamine. Histamine acts on parietal cells to stimulate acid production and ↓pH in the gastric lumen. This initiates an inhibitory feedback loop to downregulate gastrin release mediated by somatostatin-producing D cells which directly inhibits further release of gastrin from G cells. b Pathophysiology of type I gastric NETs: chronic atrophic gastritis leads to loss of parietal cells. This culminates in achlorhydria and ↑pH in the gastric lumen, which leads to D cell suppression and G cell hyperplasia-induced hypergastrinemia. This causes ECL cell hyperplasia and “ECLoma” formation. c Pathophysiology of type II gastric NETs: an ectopic gastrin-producing G cell neoplasia (gastrinoma) usually located in the duodenum or pancreas, leading to hypergastrinemia and resultant excess acid production that is independent of the inhibitory feedback loop described above
In type 1 gastric NETs, chronic atrophic gastritis (which, if associated with parietal cell antibodies and/ or intrinsic factor antibodies, is called pernicious anemia) leads to progressive loss of parietal cells. This culminates in achlorhydria, which in turn leads to D cell suppression and G cell hyperplasia–induced hypergastrinemia (termed appropriate hypergastrinemia, because it is an appropriate physiologic response to achlorhydria) (Fig. 1b, pathophysiology of type I gastric NETs). Gastrin has a trophic effect on ECL cells, with hypergastrinemia causing ECL cell hypertrophy and hyperplasia [11••]. This ultimately leads to ECL cell NETs (the so-called ECLomas) [12].
Conversely, in type II gastric NETs, the hypergastrinemia and resultant excess acid production is due to an ectopic gastrin-producing G cell neoplasia (gastrinoma) usually located in the duodenum or pancreas (termed inappropriate hypergastrinemia because it occurs in the presence of gastric acid hypersecretion and is independent of the inhibitory feedback loop described above) as a result of ZES (exclusively when associated with the MEN-1 syndrome) [13••] (Fig. 1c, pathophysiology of type II gastric NETs). It is unclear why type II gastric carcinoids are not associated with sporadic gastrinomas.
In type III gastric NETs (not associated with any other known clinical condition), both the gastrin and acid production are normal. In these circumstances, the cell of origin (an ECL, EC, or X cell) does not participate in the acid secretory feedback loop described above [14]. Type IV carcinoids are felt to occur as a result of appropriate hypergastrinemia due to a block of acid secretory capability of uncertain cause although it is unclear whether this is a distinct entity or not [8, 15]. Certain investigators also believe that drug-induced achlorhydria after prolonged PPI exposure can rarely lead to gastric carcinoids in a similar manner and a few case reports documenting such a condition have been published [9]. However, this too is debated with most authorities feeling that macroscopic carcinoids do not occur by this mechanism in humans (which is nevertheless well recognized in animal models) [10].
Clinical Presentation
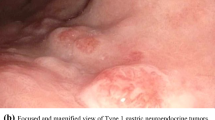
Unlike other gastrointestinal NETs, which may cause carcinoid syndrome, gastric NETs are typically nonfunctional [11••]. Gastric NETs may be diagnosed incidentally, or as part of the work-up for non-specific abdominal pain, anemia, or upper gastrointestinal bleeding. At endoscopy, these lesions are submucosal in appearance. The incidental finding of these tumors in otherwise asymptomatic patients warrants increased awareness among endoscopists. Type I and II gastric NETs are generally diagnosed endoscopically as multiple, small nodules in clusters, less than 1–2 cm in size and are mostly located in the gastric fundus and body. Patients with type II gastric NETs may present with ZES symptoms including abdominal pain due to multiple peptic ulcers and secretory diarrhea due to excessive gastric acid production by gastrinomas. In MEN-1 syndrome, hyperparathyroidism usually precedes the gastrinoma diagnosis by a decade or more [16]. Type III gastric NETs usually appear endoscopically as solitary lesions, often larger than 2 cm in size and are mostly located in the body or fundus. These tumors commonly present with lymph node involvement at diagnosis and may have already metastasized widely. Occasionally, they produce histamine and/or 5-hydroxytryptophan leading to an atypical carcinoid syndrome characterized by a more intense pruritic rash and flushing than typical carcinoid syndrome patients [17].
Diagnostic Work-up
In addition to taking biopsies of the actual carcinoids themselves to establish a histological diagnosis, endoscopists should also obtain biopsies of the normal surrounding mucosa in the body and fundus to assess for underlying atrophic gastritis, intestinal metaplasia, and ECL cell hyperplasia as well as biopsies of the antrum to assess for G cell hyperplasia and H. pylori status. While the latter condition is associated with both appropriate (pangastritis) and inappropriate (antral predominant disease) hypergastrinemia, gastric carcinoids do not typically occur. At the time of endoscopy, measurement of gastric pH and fasting gastrin levels (off PPIs ideally) should be taken in all patients diagnosed with gastric NETs to permit accurate subtyping because therapy differs considerably between the subtypes. In patients likely to have type II gastric NETs, all of whom also have ZES, it should be stressed that withdrawal of antisecretory therapy is not without risk and care should be taken to prevent rebound acid secretion, which can lead to complications such as peptic ulceration and GI bleeding [18•].
Type I gastric NETs present with a high gastric pH > 4–7, whereas type II NETs are associated with a low gastric pH < 2. Both type I and type II NETs have an elevated fasting serum gastrin (easily drawn immediately before or just after endoscopy). However, type III gastric NETs are associated with a normal fasting gastric pH (< 4) and a normal fasting serum gastrin. If not already known beforehand, identification of a type II carcinoid de novo requires additional work-up for MEN-1 syndrome. Testing for anti-parietal cell and anti-intrinsic factor antibodies can be considered in patients with type I NETs but more important clinically is to check vitamin B12 levels, the deficiency of which may not be readily apparent, but which has significant health consequences and is easily treatable. In addition, Addison’s disease and hypothyroidism should be tested for, if symptoms are compatible, and treated if present. Serum chromogranin A (CgA) and serotonin levels as well as serum or urine 5-HIAA should also be considered as part of the work-up for patients with type III NETs if there are associated symptoms suggestive of the carcinoid syndrome. Endoscopic ultrasound (EUS) should be performed to assess depth of invasion and lymph node involvement especially for larger lesions (> 1–2 cm). CT or MRI imaging can be done if metastatic disease is suspected but functional imaging using 68-gallium DOTATATE PET/CT is most accurate for staging.
Treatment of Localized Disease (Endoscopic, Surgical, or Medical)
The management of gastric NETs depends on the tumor subtype, degree of differentiation, extent of invasion, and presence or absence of poor prognostic features. Type I and type II gastric NETs especially those < 1 cm in size may be treated with endoscopic removal, or if not removed, then monitored by close endoscopic surveillance. Types I and II tumors 1–2 cm in size and those with submucosal invasion seen on EUS can often be removed by snare polypectomy or endoscopic mucosal resection (EMR). Those lesions not removed by EMR should be closely surveyed every 1–3 years although there is much controversy regarding the appropriate frequency of monitoring depending on tumor type, number, and size. Patients with 6 or less type I or II tumors > 2 cm in size should be individualized and could undergo endoscopic resection (if possible) or considered for surgical resection [19••]. It should be noted that after diagnosing a type II gastric NET, further treatment also involves localizing and treating the gastrinoma itself and, if necessary, removing it surgically [20]. In addition, screening for other associated tumors in the pituitary and parathyroid is required. These patients should remain on high-dose PPIs since MEN-1-associated ZES generally occurs in a setting of multiple primary gastrinomas that are not generally resectable. Type III gastric NETs should be managed aggressively (like gastric adenocarcinomas) with partial gastrectomy and lymph node dissection although selected lesions < 1–2 cm in size can be treated endoscopically with EMR or endoscopic submucosal dissection (ESD) and followed up very closely.
For recurrent or multi-focal type I gastric NETs, antrectomy although more invasive than endoscopic resection is a potential treatment option. Antrectomy removes the source of the hypergastrinemia and prevents ongoing ECL cell hypertrophy and hyperplasia. Patients treated with antrectomy have a lower risk of recurrence and need fewer follow-up endoscopies than patients who receive endoscopic resection or endoscopic surveillance alone [21]. However, given the improvement in endoscopic therapy and the nutritional side effects of gastric resection, this option should only be considered in patients with extensive recurrent disease. It should be noted that this approach will not work for type II carcinoids as the gastrin source is ectopic. In theory, proximal gastrectomy (to include the cardia, fundus, and body) is a potential approach to both type I and II gastric carcinoids but the nutritional side effects of this approach limits its utility as well.
As an alternative, medical management of gastric NETs with somatostatin analogues (SSA) has also been studied. A review of Canadian patients with GEPNETs who received monthly low-dose octreotide (< 30 mg) compared to monthly higher doses (≥ 30 mg) showed the median overall survival was better in the higher dose group, 66 months compared to 22 months (multivariate HR 0.5, p < 0.01) [22]. In another study, intermittent treatment of recurrent type 1 gastric NETs with SSAs was also effective in patients that could not be managed endoscopically [23]. The mechanism by which SSAs exhibit a direct anti-proliferative effect on the ECL cells is by diminishing tumor load and reducing ECL cell density [24]. In addition, SSAs used in 3 patients with type II gastric NETs resulted in reduction in size and number of tumors and decrease in serum gastrin levels in all [25].
A novel agent that is a gastrin/cholecystokinin receptor antagonist has been studied for the use in patients with type I gastric NETs. In an open-label study, netazepide was given to 8 patients with multiple type 1 gastric NETs once daily for 12 weeks. All patients had a reduction in the number and size of their tumors and a reduced serum CgA, although their serum gastrin remained unchanged throughout treatment [26]. In another study, netazepide given to 16 type I gastric NET patients for 52 weeks cleared all tumors in 5 patients, cleared all but one tumor in one patient, reduced the number of tumors and size of the largest one in the other patients, and normalized CgA levels in all treated patients [27•]. It should be noted that tumors will regrow if this treatment is stopped, so it should be given continuously. These studies found this agent to be safe and well tolerated. Trials are ongoing with this investigational agent.
Treatment of Metastatic Disease
Metastatic liver disease from gastric NETs is managed using multiple treatment modalities and should be individualized and undertaken as part of a multi-disciplinary team approach. As indicated above, SSAs may be used for their anti-proliferative effect on ECL cells and a decrease in tumor burden. Other therapies include liver-directed approaches (embolic or radiotherapeutic), surgery, chemotherapy, small molecule therapies, and peptide receptor radiotherapy in various sequences or concurrently. An in-depth discussion on this topic is beyond the scope of this manuscript.
Controversies in Management
The optimal management of type I gastric NETs < 1 cm in size remains controversial in terms of deciding between endoscopic removal versus endoscopic surveillance and both modalities appear appropriate. The treatment of type I gastric NETs between 1 and 2 cm in size is also unclear. The European Neuroendocrine Tumor Society (ENETS) guidelines have suggested endoscopic removal of type I tumors larger than 1 cm in size without invasion of the muscularis propria and lymph nodes and surgical resection of tumors with involvement beyond the submucosa or lymph nodes or with distant disease [28]. However, the National Comprehensive Cancer Network (NCCN) guidelines have recommended either endoscopic resection or observation of local type I tumors 1–2 cm in diameter [29•]. It should be noted that patients with type I gastric carcinoids are also predisposed to developing gastric adenocarcinoma along the well-established Correa cascade and this is another important reason for interval surveillance of patients with atrophic gastritis. Also, the optimal interval of endoscopic surveillance for type I lesions following endoscopic resection is unclear but suggested at 1–3-yearly intervals. Lastly, the NCCN guidelines suggest that endoscopic or wedge resection can be considered for type III lesions < 2 cm [30].
Prognosis
The clinical outcome of patients with gastric NETs is dependent on the tumor subtype, stage, and grade. Even untreated patients with type I gastric NETs generally have an excellent prognosis with a disease-specific survival approaching 100% [31]. Type II gastric NETs, like type I, are usually detected at an early stage and thus have an excellent long-term prognosis [32]. However, patients from one large series with MEN-1 and ZES who had metastatic disease often died from other NETs especially pancreatic or thymic lesions [33]. Therefore, the limiting prognostic issues here are whether they develop metastatic pancreatic or thymic primary NETs rather than gastric NETs. However, it may not be possible to determine the actual source of liver metastases in MEN-1 patients with multiple potential metastatic primaries. Type III tumors have a prognosis like that of gastric adenocarcinomas underscoring the importance of measuring gastrin levels in all gastric carcinoid patients in order to permit prognostic differentiation by type.
Conclusion
Gastric NETs are being diagnosed at an increased frequency often during upper endoscopies done for unrelated reasons. These often-indolent tumors are subtyped into three categories based on common clinical features, pathophysiology, and prognosis. During upper endoscopy gastric pH, biopsies of normal gastric mucosa and fasting serum gastrin should be collected to assist in subtyping, classification, and ultimately management of these tumors. Given the excellent prognosis of type I gastric NETs, even if untreated, endoscopic surveillance is a reasonable and safe approach for most patients. Type II gastric NETs present as part of the MEN-1 syndrome in the setting of a gastrinoma and the other associated endocrinopathies need to be considered in the management of these patients too. Type III tumors have the worst prognosis with a high risk of metastases. Given the relative rarity of these tumors in clinical practice, their management should be assumed as part of a multi-disciplinary team including gastroenterologists, radiologists, surgeons, oncologists, nuclear medicine physicians, and pathologists with a specific interest and expertise in the diagnosis and management of these tumors. Surgical resection is the preferred approach for type III tumors in the absence of metastases at presentation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Díez M, Teulé A, Salazar R. Gastroenteropancreatic neuroendocrine tumors: diagnosis and treatment. Ann Gastroenterol. 2013;26(1):29–36.
Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise. Small tumors, small problems? Endoscopy. 2010;42(8):664–71. https://doi.org/10.1055/s-0030-1255564.
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. https://doi.org/10.1001/jamaoncol.2017.0589.
Nikou GC, Angelopoulos TP. Current concepts on gastric carcinoid tumors. Gastroenterol Res Pract. 2012;2012:287825. https://doi.org/10.1155/2012/287825.
Al-Harbi O, Shakir M, Al-Brahim N. Gastric carcinoid and obesity: association or coincidence? Report of two cases and literature review. Case Rep Gastrointest Med. 2013;2013:848075–4. https://doi.org/10.1155/2013/848075.
•• Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39(6):707–12. https://doi.org/10.1097/MPA.0b013e3181ec124e A landmark review of the pathologic classification of neuroendocrine tumors, highlighting issues regarding stages and grades of NETs and standardizing the way NETs are classified in pathology reports.
Grozinsky-Glasberg S, Alexandraki KI, Angelousie A, Chatzellis E, Sougioultzis S, Kaltsas G. Endocrinol Metab Clin North Am. 2018;47(3):645–660. https://doi.org/10.1016/j.ecl.2018.04.013.
Abraham SC, Aidan Carney J, Ooi A, Choti MA, Argani P. Achlorhydria, parietal cell hyperplasia, and multiple gastric carcinoids: a new disorder. Am J Surg Pathol. 2005;29:969–75.
Waldum HL, Hauso Ø, Brenna E, Qvigstad G, Fossmark R. Does long-term profound inhibition of gastric acid secretion increase the risk of ECL cell-derived tumors in man? Scand J Gastroenterol. 2016;51(7):767–73. https://doi.org/10.3109/00365521.2016.1143527.
Nilsson O, Wängberg B, Johansson L, Modlin IM, Ahlman H. Praomys (Mastomys) natalensis: a model for gastric carcinoid formation. Yale J Biol Med. 1992;65(6):741–51.
•• Corey B, Chen H. Neuroendocrine tumors of the stomach. Surg Clin N Am. 2017;97(2):333–43. https://doi.org/10.1016/j.suc.2016.11.008 A recent review of gastric NETs that provides an algorithm for their management dependent on subtype.
Qvigstad G, Falkmer S, Westre B, Waldum HL. Clinical and histopathological tumour progression in ECL cell carcinoids (“ECLomas”). APMIS. 1999;107(12):1085–92.
•• Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134(7):1842–60. https://doi.org/10.1053/j.gastro.2008.05.021 A review of the physiology and pathophysiology of acid secretion, highlighting diseases of acid hypersecretion and the mechanisms of acid-blocking medications used to treat them.
Wardlaw R, Smith JW. Gastric carcinoid tumors. Ochsner J. 2008;8(4):191–6.
Ooi A, Ota M, Katsuda S, Nakanishi I, Sugawara H, Takahashi I. An unusual case of multiple gastric carcinoids associated with diffuse endocrine cell hyperplasia and parietal cell hypertrophy. Endocr Pathol. 1995;6(3):229–37.
Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83(1):43–83.
Bordi C, D’Adda T, Azzoni C, Canavese G, Brandi ML. Gastrointestinal endocrine tumors: Recent developments. Endocr Pathol. 1998;9:99–115. https://doi.org/10.1007/BF02782603.
• Metz DC. Diagnosis of the Zollinger–Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10(2):126–30. https://doi.org/10.1016/j.cgh.2011.07.012 A practical approach to the diagnosis, work-up, and management of ZES, with particular reference to the important clinical differences between appropriate and inappropriate hypergastrinemia.
•• Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–77. https://doi.org/10.1097/MPA.0b013e31828e34a4 In 2010, the North American Neuroendocrine Tumor Society (NANETS) published a comprehensive and updated guideline addressing the management of NETs. This article addresses controversial topics in the management of NETs.
Dias AR, Azevedo BC, Alban LBV, Yagi OK, Ramos MFKP, Jacob CE, et al. Gastric neuroendocrine tumor: review and update. Arq Bras Cir Dig. 2017;30(2):150–4. https://doi.org/10.1590/0102-6720201700020016.
Jenny HE, Ogando PA, Fujitani K, Warner RR, Divino CM. Laparoscopic antrectomy: a safe and definitive treatment in managing type 1 gastric carcinoids. Am J Surg. 2016;211(4):778–82. https://doi.org/10.1016/j.amjsurg.2015.08.040.
Lau SC, Abdel-Rahman O, Cheung WY. Improved survival with higher doses of octreotide long-acting release in gastroenteropancreatic neuroendocrine tumors. Med Oncol. 2018;35(9):123. https://doi.org/10.1007/s12032-018-1189-1.
Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type 1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47(11):978–83. https://doi.org/10.1016/j.dld.2015.07.155.
Fykse V, Sandvik AK, Qvigstad G, Falkmer SE, Syversen U, Waldum HL. Treatment of ECL cell carcinoids with octreotide LAR. Scand J Gastroenterol. 2004;39(7):621–8.
Tomassetti P, Migliori M, Caletti GC, Fusaroli P, Corinaldesi R, Gullo L. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N Engl J Med. 2000;343(8):551–4.
Fossmark R, Sørdal Ø, Jianu CS, Qvigstad G, Nordrum IS, Boyce M, et al. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin a. Aliment Pharmacol Ther. 2012;36(11–12):1067–75. https://doi.org/10.1111/apt.12090.
• Boyce M, Moore AR, Sagatun L, Parsons BN, Varro A, Campbell F, et al. Netazepide, a gastrin/cholecystokinin-2 receptor antagonist, can eradicate gastric neuroendocrine tumours in patients with autoimmune chronic atrophic gastritis. Br J Clin Pharmacol. 2017;83(3):466–75. https://doi.org/10.1111/bcp.13146 A study of netazepide, an investigational agent that shows promise in treating type I gastric NETs especially in patients who want to avoid regular endoscopies or surgery.
Delle Fave G, O’Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, et al. (Vienna Consensus Conference participants). ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):119–24. https://doi.org/10.1159/000443168.
• Sato Y. Clinical features and management of type I gastric carcinoids. Clin J Gastroenterol. 2014;7:381. https://doi.org/10.1007/s12328-014-0528-9 A recent review of the pathophysiology, diagnosis, management, and prognosis of type I gastric NETs.
Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22(30):6817–28.
Ravizza D, Fiori G, Trovato C, Fazio N, Bonomo G, Luca F, et al. Long-term endoscopic and clinical follow-up of untreated type 1 gastric neuroendocrine tumours. Dig Liver Dis. 2007;39(6):537–43.
Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Management of early gastrointestinal neuroendocrine neoplasms. World J Gastrointest Endosc. 2011;3(7):133–9. https://doi.org/10.4253/wjge.v3.i7.133.
Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore). 2013;92(3):135–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Metz reports personal fees from Ipsen, Novartis, Lexicon, and Takeda and grants from AAA and Lexicon and Emeritus chair of NANETS. Craig Gluckman has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stomach and Duodenum
Rights and permissions
About this article
Cite this article
Gluckman, C.R., Metz, D.C. Gastric Neuroendocrine Tumors (Carcinoids). Curr Gastroenterol Rep 21, 13 (2019). https://doi.org/10.1007/s11894-019-0684-7
Published:
DOI: https://doi.org/10.1007/s11894-019-0684-7