Abstract
Hepatic adenomatosis (HeAs) is a rare clinical entity defined by the presence of 10 or more hepatic adenomas (HA) within the background of an otherwise normal liver parenchyma, in the absence of glycogen storage disease or anabolic steroid use. HA is a benign tumor associated with oral contraceptive use. Recent advances in pathogenesis and classification of HA have questioned the distinction between these two diseases. HA are currently classified into four different subtypes with genotypic and phenotypic correlation: HNF-1a inactivated HA, B-catenin activated HA, inflammatory HA, and undetermined subtype. The clinical presentation of HA depends on the lesion size and the subtype. MRI using hepatospecific contrast agents is helpful in diagnosing the most common subtypes. When diagnosis is uncertain, biopsy with immunohistochemistry is used to diagnose and classify the lesions. Management is governed by the molecular subtype and tumor size. Pregnancy is not routinely discouraged but management is individualized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic adenomatosis (HeAs) is a rare clinical entity defined by the presence of 10 or more hepatic adenomas within the background of an otherwise normal liver parenchyma in the absence of glycogen storage disease or anabolic steroid use [1]. The adenomas in HeAs are histologically similar to benign hepatic adenomas, but HeAs was historically described as a distinct and unique clinical entity. Hepatic adenomas (HA) are benign liver tumors and were long considered to be a homogenous entity until recently when the molecular basis was understood [2, 3]. Armed with molecular classification and understanding of the heterogeneous nature of the disorder, several researchers have been able to convincingly make the argument that HeAs is not a distinct clinical entity per se from HA. It has been proposed that the term HeAs not be used at all in order to avoid further confusion [4].
Hepatic adenomas are the third most frequently encountered benign solid tumor of the liver after hemangioma and focal nodular hyperplasia (FNH). The estimated incidence of these lesions is 1 per million in women not taking oral contraceptives (OCP). With OCP use, the incidence is estimated to be 34 per million [5]. HA are typically found in young women (3rd–5th decade), are usually solitary, and can vary in size from 1 cm to up to 20–30 cm. HA are characterized by monoclonal proliferation of well-differentiated hepatocytes in sheets and cords. They are classically devoid of portal triads and bile ducts. The hepatocytes show excessive glycogen and fat deposition along with sinusoidal dilation secondary to increased arterial pressure, as the portal venous supply is deficient [6••, 7, 8, 9•].
Etiology and Subtypes
Historical risk factors for the development of HA are female gender and OCP use. There was an increasing incidence of HA seen after the introduction of OCPs [10]. Hepatic steatosis has a strong association with the development of HA. Multiple case series have shown not only a correlation between hepatic steatosis and the development of adenomas, but a higher frequency of hepatic steatosis in cases with multiple adenomas than in those with a single adenoma [11, 12•]. Recently, metabolic syndrome and obesity have been identified as an additional risk factors for both development and progression of HA [6••, 13••, 14••]. Adenomas associated with metabolic syndrome are more likely to be multiple and have associated hemangiomas or FNH. Other risk factors include anabolic steroid use and glycogen storage disease Ia and III. HA associated with glycogen storage diseases are more commonly seen in males (2:1), with a sharp uptake after age >25 years and may require management strategies unique to this group of patients [15].
Recently, HA have been classified in to four major molecular subtypes with genotypic phenotypic correlation. These subtypes are (1) hepatocyte nuclear factor-1A mutated adenomas (H-HA), (2) ß-catenin activated adenomas (B-HA), (3) inflammatory adenomas (I-HA), and (4) an undetermined subtype (U-HA). Each of these subtypes has a unique phenotype and is usually associated with a distinct clinical course [2, 3] (see Table 1).
Hepatocyte nuclear factor 1a gene (HNF1A), also known as transcription factor-1 gene, is a tumor suppressor gene located on long arm of chromosome 12. It encodes a transcription factor, which is responsible for hepatocyte differentiation and expression of certain liver specific genes such as albumin and alpha-1 antitrypsin. Non-functioning HNF1A promotes lipogenesis and leads to faulty transport of fatty acids and intracellular deposition of fat. In addition, there is downregulation of liver-type fatty acid binding protein (L-FABP) [16]. Heterozygous germ line mutation causing inactivation of the HNF1A is associated with type 3 maturity-onset diabetes of the young (MODY3). Biallelic inactivation of HNF1A predisposes these patients to develop familial adenomatosis [17]. About 30–35 % of HA are of the H-HA subtype. H-HA are seen in young women with prominent steatosis and have the least likelihood of malignant transformation [2, 6••].
ß-catenin gene plays an important role in hepatocellular development. ß-catenin is transiently activated, undergoes phosphorylation, ubiquitination, and is subsequently degraded in the cytoplasm (facilitated by a set of genes). In mutated hepatocytes, phosphorylation is impaired and ß-catenin is translocated to the nucleus where it acts as a co-transcription factor and promotes tumorigenesis. B-HA are also associated with an overexpression of GLUL, which encodes for glutamine synthase. The exact role of glutamine synthetase in promoting hepatic adenoma formation is not clear, but is helpful in diagnosing B-HA. These tumors show immunohistochemical staining for ß-catenins and glutamine synthetase (diffuse cytoplasmic pattern). Patients with glycogen storage disease and androgen use primarily develop B-HA. Activating ß-catenin mutations are seen in about 10–15 % of hepatic adenomas. B-HA are more likely to be seen in males and have a higher propensity to undergo malignant transformation to hepatocellular carcinoma [2, 6••].
Inflammatory adenomas comprise 40–50 % of all hepatic adenomas and are characterized by activation of the JAK/STAT pathway. Several different mutations can lead to constitutive activation of JAK/STAT pathway in I-HA, with the interlukin-6 signal transducer (IL6ST) gene mutation being most commonly identified. IL6ST encodes for glycoprotein-130 (gp130) which is a component of the interlukin-6 receptor [18]. Activation of IL6ST and other I-HA-associated mutations promote activation of transcription-3 (STAT3) signaling pathway. Mutated hepatocytes show overexpression of inflammatory proteins such as serum amyloid A and C-reactive protein. Up to 25 % of IHCA’s have no identifiable gene defect [6••]. I-HA includes the telangiectatic FNH, which was previously classified as a subtype of FNH [19]. I-HA are associated with steatohepatitis-related risk factors and alcohol use. Histologically, I-HAs show inflammation, sinusoidal dilation, and ductular reaction which can make the distinction from FNH difficult. About 15 % of I-HA also have an activating ß-catenin mutation, with an associated increased risk of malignant transformation. About 10 % of adenomas fall under the U-HA and lack any identified mutation, and cannot be phenotypically classified [6••].
Clinical Features
Most of the patients with hepatic adenomas currently diagnosed are asymptomatic. A minority of patients will have liver enzyme elevations, while they are normal in the vast majority. I-HA are likely to be associated with a systemic inflammatory syndrome and anemia secondary to hepcidin production [20]. Larger adenomas are more likely to present with abdominal discomfort and bleeding. The most commonly reported symptom has been abdominal pain [21, 22]. Hemorrhage has been reported in 21–40 % of cases [7]. Adenomas can bleed intratumorally, within the hepatic parenchyma, or intraperitoneally. Large adenomas (>5 cm) and recent hormone intake is associated with higher risk of bleeding [3, 7, 21, 23]. Patients can present with massive intraperitoneal hemorrhage and shock. Malignant transformation has been reported in about 5–10 % of cases and may be detected by surveillance with tumor markers such as alpha-fetoprotein or increase in adenoma size on imaging [8, 21, 24, 25]. Tumors <5 cm in women rarely undergo malignant transformation [22, 23]. Men and patients who use androgens or anabolic steroids are at a higher risk of malignant transformation [22, 24]. B-HA have a higher risk of malignant transformation. Hepatocellular carcinoma (HCC) in the HA is usually well differentiated and at times associated with a normal AFP level [24, 26]. It is important to mention that HCC development has been reported in HA despite regression in size with OCP discontinuation [27, 28].
The clinical presentation of HeAs as described historically was dependent upon the number of adenomas [1]. We now understand that the risk of complications is not related to the number of adenomas but rather on the size of the largest adenoma and underlying pathologic subtype.
Diagnosis
The diagnosis of HA can be challenging, often requiring a combination of imaging studies and histological confirmation. HA on ultrasound (US) can be hypo, iso, or hyperechoic depending on the fat content and intralesional hemorrhage [29–31]. US has limited utility in diagnosing HA. On computed tomography imaging (CT), HA usually appear round, smooth, with a late enhancing peripheral capsule [32, 33•]. Smaller lesions tend to be homogenously enhancing on arterial imaging while larger lesions may enhance heterogeneously due to areas of necrosis. Hepatic adenomas are notoriously difficult to visualize during delayed venous phase imaging due to the rapid fading of arterial phase contrast enhancement caused by significant internal arteriovenous shunting. Non-contrast CT imaging can detect the presence of hemorrhage, which is highly suggestive of an adenoma, and can also less commonly detect the presence of fat, another distinguishing feature of hepatic adenomas [33•].
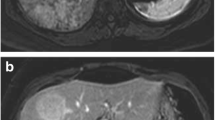
Magnetic resonance imaging (MRI) has proven to be an invaluable imaging modality and can detect the presence of lipid in about 75 % of hepatic adenomas, using in- and out-of-phase imaging and fat-suppression sequences. MRI is also more sensitive in detecting subtle hemorrhage on T1-weighted imaging. Most hepatic adenomas are hyper-intense on T1 imaging and iso or mildly hyper-intense on T2 imaging. In some cases, it is difficult to distinguish hepatic adenomas from FNH, especially in the absence of classical FNH features (presence of a central scar that enhances on delayed images and the absence of fat and/or hemorrhage). In these cases, MRI with the use of hepatocyte-specific contrast agents such as gadoxetate disodium (Eovist) or gadobenate dimeglumine (Multihance) may be of use, as HA will not take up these contrast agents and appear hypo-intense compared to surrounding liver tissue on delayed hepatocyte phase, whereas lesions such as FNH will appear iso-intense or hyper-intense to the liver on hepatocyte phase [33•, 34, 35]. MRI should be used to phenotypically distinguish subsets of hepatic adenomas. H-HA show characteristic MRI findings of diffuse signal drop on opposed phase gradient echo T1w imaging due to extensive intralesional steatosis and arterial phase enhancement which fades in venous and delayed phases. I-HA on the other hand are hyperintense on T2w images due to sinusoidal dilation with arterial enhancement that persists on both venous and delayed phase images [36]. Unfortunately, B-HA do not have any distinguishing MRI features [6••, 24, 37].
Liver biopsy is rarely pursed due to the risk of bleeding and the advances made in MRI imaging in coming to a diagnosis. When diagnosis is uncertain and a biopsy is needed, it is important to remember that a single pass might obscure the diagnosis due to heterogeneous nodule architecture [38]. Nodules will appear well circumscribed with the presence of variable amounts of fat infiltration. “Floating vessels” may also be present and represent focal ectasia of hepatic sinusoids. Classically, there is absence of biliary structures; however, their presence does not necessarily exclude the diagnosis of HA. Some biopsies may be fortuitously obtained between two adenomas, and may reveal presence of portal tracts as a result of entrapment. In hepatic adenomas, as there is no disruption of the reticular meshwork, and reticulin stain will show a normal hepatocyte plate thickness of 1–2 cells, in contrast, a hepatocellular carcinoma can have a thickness greater than three cells [39, 40•].
Immunohistochemical staining should be used during microscopy on all biopsy specimens and helps identify different subtypes of HA, which guides management decisions. While steatosis along with an absence of inflammatory infiltrate is a hallmark feature of H-HA, it cannot be used alone to distinguish this subset of adenomas as a minority of I-HA and B-HA exhibit steatosis as well. The paucity of expression of liver fatty acid-binding protein (L-FABP) has specificity and sensitivity of 100 % for H-HA as it is downregulated in H-HA [9•]. B-HA exhibit positive staining for nucleolar B-catenin as well as cytoplasmic glutamine synthase. The pattern of enhancement with glutamine synthase staining in B-HA should be homogenous, and can be useful in cases where distinction of HA from FNH is difficult. FNH exhibits a speckled or map-like distribution of glutamine synthase staining. Immunohistochemical staining for both B-catenin mutations and glutamine synthetase has a sensitivity of 75–85 % a specificity of 100 % in diagnosing B-HA [2, 24]. I-HA show focal steatosis along with patchy mononuclear inflammation. The neoplastic hepatocytes exhibit staining and over expression of C-reactive protein and serum amyloid A [6••] (Table 1).
Management
Proposed management strategies have centered on accurate imaging as a key component of therapeutic decisions [38, 41••]. The treatment paradigm has shifted from one-size fits all to one based on subtypes with the attendant risk of complications.
Hormone Therapy Cessation
All patients with HA should cease the use of oral contraceptives or hormone therapy. A recent review of HA cases from university hospitals found that after discontinuing oral contraceptives and hormone therapy, 79 % of patients had hepatic adenoma regression [41••]. This approach, however, does not always result in tumor regression [21, 23]. Patients who are unable to stop the OCPs, may be able to take low estrogen formulations, provided the HA is small in size (<5 cm), is of the low-risk steatotic subtype and are closely followed with periodic surveillance. However, the optimal timing and imaging modality for surveillance is not clear [24, 26]. It is also unclear whether the addition of the tumor marker (AFP) aids in the detection of malignant transformation [26].
Resection
Adenomas have long been considered for resection due to the risk of bleeding and malignancy. Laparoscopic resection is preferred when feasible, as a wide margin or regional lymphadenectomy is not needed even in suspected malignancy due to the low risk of vascular invasion or lymph node involvement [22, 24, 42]. The initial approach to non-emergent presentation of HA depends on adenoma size, owing to the correlation between adenoma size and risk of bleeding [43–45]. Resection is recommended for HA 5 cm or larger as smaller tumors rarely bleed or have malignant potential [22, 31, 38]. HA in older women without a history of OCP use should be resected. Other indications for resection include symptomatic adenomas, enlarging size, and when malignancy cannot be ruled out. Adenomas in men regardless of the size and those with positive nuclear immunohistochemical staining for B-catenin on a biopsy should be resected as they carry a higher risk of malignant transformation [3, 6••, 46, 47].
Embolization/Ablation
Transarterial embolization (TAE) and radiofrequency ablation (RFA) have been used in treatment of HA. TAE is often utilized in the initial management of HA after rupture [7]. Selective TAE after initial stabilization is often preferred to emergent surgery, as the surgical mortality rate ranges from 5–10 % and delaying adenoma resection has been correlated with less blood loss, avoidance of postoperative complications, and shorter hospitalizations [22, 23]. TAE has the potential to cause tumor regression, in which case, a less invasive approach would be warranted, whereas persistence or growth of adenomas on surveillance imaging may indicate the need for more invasive modalities of treatment [22, 23, 48, 49].
Limited data mostly in the form of case series is available on the use of RFA for HA. Rhim et al. reported the largest experience of RFA in hepatic adenomas. The largest adenoma in the series was 4.5 cm and all procedures were tolerated well with no complications or recurrence with a mean follow-up of 17.5 months [50]. Fujita et al. reported three patients who underwent lobectomy and RFA. All patients tolerated the procedure well, with no disease recurrence. The longest follow-up was 3 years. Two of the patients went on to have successful pregnancy without any disease recurrence [51]. Traditionally, RFA is considered only in lesions less than 4 cm; however, it may be considered for patients with larger lesions who require resection but are poor surgical candidates [7, 52]. In general, RFA should be used as a treatment option in patients with unresectable disease (i.e., centrally located lesions), patients with underlying hepatic disease precluding resection, or patients showing progression of lesions even after initial surgical therapy [7, 22, 50, 51].
Liver Transplantation
Liver transplant has been used for severe unresectable disease [1, 44]. In a reported series of five patients undergoing hepatectomy followed by orthotropic liver transplantation (OLTx), all patients had greater than 80 % hepatic involvement with transplant being considered in the setting of life-threatening complications and overwhelming burden of disease. Pooled data showed a mean survival of 7.5 years post transplantation. One patient in this series showed metastatic disease 9.5 years after transplantation [53]. There are numerous other case reports in literature where transplantation is shown to be an effective treatment option [54, 55]. Liver transplantation continues to be recommended for patients with unresectable disease, a history of life threatening complications, or malignant degeneration [55]. On the other hand, some have argued for a more restrictive approach given the recent advances in our understanding of this disease and recommend that liver transplantation be offered only in selected cases of multiple, unresectable adenomas in men, or if there is an associated portosystemic venous shunt [6••, 7, 22]. Currently, liver transplantation is not considered as a part of the routine management of HA, except for patients with glycogen storage disease type 1a-associated adenomatosis [56], as most patients with multiple adenomas are usually of the steatotic subtype which have a low propensity of malignant transformation [22, 57].
Pregnancy and HA
Pregnancy is associated with an increasing size and risk of adenomatous rupture [58, 59]. Current approach involves not discouraging pregnancy for adenomas less than 5 cm in size [60•]. Patients with lesions greater than 5 cm or those who have experienced complications in previous pregnancies should have the HA resected prior to planned pregnancy [61••]. If resection is indicated during pregnancy, it should be performed ideally in the second trimester, which has a lower complication rate for both mother and fetus. If hepatic adenomas are diagnosed in the third trimester, a watchful waiting approach should be undertaken due to high risk of complications seen with resection. It is important to mention that patients should be closely monitored throughout the pregnancy, especially in the third trimester due to an increased risk of rupture secondary to high circulating estrogen levels and hyper dynamic circulation [62]. It is our recommendation that patients with adenomas <5 cm get an ultrasound every 3 months and are followed as a high risk pregnancy.
Future Research
Despite the progress in the last decade, many questions still remain unanswered. There is a wide variation in the prevalence of different subtypes HA reported from different parts of the world. Also, genetic mutations in a subset of I-HA and U-HA remain unknown. This does suggest that additional work needs to be done to further elucidate the genetic and environmental factors and their interaction with each other, in understanding the pathogenesis of HA. Further research is also needed in non-invasively diagnosing B-HA which has a higher likelihood of malignant transformation. The identification of signaling pathways in the tumorigenesis of HA has paved the way for the study of targeted inhibition of these pathways in affected patients. A recent publication had made the case for studying currently available JAK1/JAK2 selective tyrosine kinase inhibitor, Ruxolitinib, in I-HA [63].
Conclusion
In conclusion, much progress has been made over the past decade in our understanding of HA. They are currently considered as a heterogeneous disease and are classified in to four major molecular subtypes with genotypic-phenotypic correlation. The use of MRI especially with hepatocyte specific contrast agents (gadobenate dimeglumine or gadoxetate disodium) has been a major advance in non-invasively classifying these lesions. Immunohistochemical staining is part of the diagnostic algorithm and should be routinely done on the rare cases that require a biopsy for diagnosis. HA <5 cm can be managed conservatively with periodic imaging due to the low risk of bleeding or malignant transformation. Pregnancy is not routinely discouraged for HA <5 cm but, rather, an individualized approach is recommended.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, et al. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89(5):1132–8.
Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43(3):515–24.
Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46(3):740–8.
Frulio N, Chiche L, Bioulac-Sage P, Balabaud C. Hepatocellular adenomatosis: what should the term stand for. Clin Res Hepatol Gastroenterol. 2014;38(2).
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, et al. Epidemiology of hepatocellular adenoma: the role of oral contraceptive use. JAMA. 1979;242(7):644–8.
Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors—from molecular classification to personalized clinical care. Gastroenterology. 2013;144(5):888–902. Review article summarizing current understanding of pathological classification of hepatic adenomas using novel immunohistochemical techniques. It also discusses the molecular pathways in adenoma development.
Agrawal S, Agarwal S, Arnason T, Saini S, Belghiti J. Management of hepatocellular adenoma: recent advances. Clin Gastroenterol Hepatol. 2014. doi:10.1016/j.cgh.2014.05.02.
Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford). 2005;7(3):186–96.
Bioulac-Sage P, Cubel G, Balabaud C, Zucman-Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis. 2011;31(1):91–103. Description of immunohistochemistry techniques used to identify different subtypes of hepatocellular adenomas. It also discusses the performance characteristics of these techniques.
Edmondson HA, Reynold TB, Henderson B, Benton B. Regression of liver cell adenomas associated with oral contraceptives. Ann Int Med. 1977;86(2):180–2.
Furlan A, van der Windt DJ, Nalesnik MA, Sholosh B, Ngan KK, Pealer KM, et al. Multiple hepatic adenomas associated with liver steatosis at CT and MRI: a case–control study. AJR Am J Roentgenol. 2008;191(5):1430–5.
Srirattanapong S, Angthong W, Kim BS, Hayashi PH, Gerber DA, Woosley JT, et al. Liver adenomatosis: serial investigation on MRI. Abdom Imaging. 2014;39(2):269–82. This is a retrospective series of 18 patients with liver adenomatosis and summarizes MRI findings and clinical course.
Bunchorntavakul C, Bahirwani R, Drazek D, Soulen MC, Siegelman ES, Furth EE, et al. Clinical history and natural history of hepatocellular adenomas: the impact of obesity. Aliment Pharmacol Ther. 2011;34(6):664–74. The authors evaluate the role of obesity and metabolic syndrome in the development of hepatic adenomas. These disorders play an important role in the inflammatory subtype of hepatic adenomas.
Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma sub-types: the impact of overweight and obesity. Liver Int. 2013;32(8):1217–21. The authors evaluate the role of obesity in hepatic adenoma development.
Parker P, Burr I, Slonim A, Ghishan FK, Greene H. Regression of hepatic adenomas in type 1a glycogen storage disease with dietary therapy. Gastroenterology. 1981;81(3):534–6.
Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Terce F, et al. HNF-1a inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282(19):14437–46.
Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125(5):1470–5.
Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–4.
Paradis V, Benzerkri A, Dargere D, Bieche I, Laurendeau I, Vilgrain V, et al. Telengiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126(5):1323–9.
Sa Cunha A, Blanc JF, Lazaro E, Mellottee L, Le Bail B, Zucman-Rossi J, et al. Inflammatory syndrome with liver adenomatosis: the beneficial effects of surgical management. Gut. 2007;56(2):307–9.
Cho SW, Marsh J, Steel J. Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol. 2008;15:2795–803.
Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137(5):1698–705.
Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640–8.
Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50(2):481–9.
Foster JH, Berman MM. The malignant transformation of liver cell adenomas. Arch Surg. 1994;129(7):712–7.
Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2010;60(1):85–9.
Gordon SC, Reddy KR, Livingstone AS, Jeffers LJ, Schiff ER. Resolution of a contraceptive-steroid-induced hepatic adenoma with subsequent evolution into hepatocellular carcinoma. Ann Int Med. 1986;105(4):547–9.
Tesluk H, Lawrie J. Hepatocellular adenoma: its transformation to carcinoma in a user of oral contraceptives. Arch Pathol Lab Med. 1981;105(6):296–9.
Hussain SM, Semelka RC. Hepatic imaging: comparison of modalities. Radiol Clin N Am. 2005;43(5):929–47. doi:10.1016/j.rcl.2005.05.006. ix.
Hussain SM, Semelka RC. Liver masses. Magn Reson Imaging Clin N Am. 2005;13(2):255–75. doi:10.1016/j.mric.2005.03.007.
Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW, et al. Indications and long-term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001;136(9):1033–8.
Ichikawa T, Federle MP, Grazioli L, Nalesnik M. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000;214(3):861–8. doi:10.1148/radiology.214.3.r00mr28861.
Raman SP, Hruban RH, Fishman EK. Hepatic adenomatosis: spectrum of imaging findings. Abdom Imaging. 2013;38(3):474–81. The authors discuss the imaging characteristics and differential diagnosis of hepatic adenomas.
Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21(4):877–92.
Giovanoli O, Heim M, Terracciano L, Bongartz G, Ledermann HP. MRI of hepatic adenomatosis: initial observations with gadoxetic acid contrast agent in three patients. AJR Am J Roentgenol. 2008;190(5):W290–3.
Shanbhogue A, Shan SN, Zaheer A, Prasad SR, Takahashi N, Vikram R. Hepatocellular adenomas: current update on genetics, taxonomy, and management. J Comput Assist Tomogr. 2011;35(2):159–66.
Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48(3):808–18.
Vetelainen R, Erdogan D, de Graaf W, ten Kate F, Jansen PL, Gouma DJ, et al. Liver adenomatosis: re-evaluation of aetiology and management. Liver Int. 2008;28(4):499–508.
Greaves WO, Bhattacharya B. Hepatic adenomatosis. Arch Pathol Lab Med. 2008;132(12):1951–5.
Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. 2014. The authors review the molecular and genetic biology of hepatic adenomas along with immunohistochemical and histopathological charaterstics.
Van Aalten SM WC, de Man RA, Ijzermans JN, Terkivatan T. Can a decision-making model be justified in the management of hepatocellular adenoma? Liver Int. 2012;32(31):28–37. Summarized current diagnostic and current treatment options for hepatic adenomas. The authors propose a decision model for adenoma management.
Wilson CH, Manas DM, French JJ. Laparoscopic liver resection for hepatic adenoma in pregnancy. J Clin Gastroenterol. 2011;45:828–33.
Yoshidome H, Morita K, Edwards M. Management issues regarding hepatic adenomatosis. Am Surg. 1999;65:1070–6.
Ribeiro A, Burgart LJ, Nagorney DM, Gores GJ. Management of liver adenomatosis: results with a conservative surgical approach. Liver Transplant Surg. 1998;4(5):388–98.
Jenkins RL, Johnson L, Lewis D. Surgical approach to benign liver tumors. Semin Liver Dis. 1994;14:178–89.
Marrero JA, Ahn J, Reddy R. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109(9):1328–47.
Venkatesh SK, Vishal C, Lewis RR. Liver masses: a clinical, radiological, and pathologic perspective. Clin Gastroenterol Hepatol. 2014;12(9):1414–29.
Huurman VA, Schaapherder AF. Management of ruptured hepatocellular adenoma. Dig Surg. 2010;27:56–60.
Kobayashi S, Sakaguchi H, Takatsuka M, Suekane M, Iwai S, Morikawa H, et al. Two cases of hepatocellular adenomatosis treated with transcatheter arterial embolization. Hepatol Int. 2009;3:416–20.
Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular adenoma: initial experience in 10 patients. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e422–7.
Fujita S, Kushihata F, Herrmann GE, Mergo PJ, Liu C, Nelson D, et al. Combined hepatic resection and radiofrequency ablation for multiple hepatic adenomas. J Gastroenterol Hepatol. 2006;21(8):1351–4.
Van Aalten SM, Terkivatan T, van der Linden E, Verheij J, de Man RA, Ijzermans JN. Management of liver adenomatosis by radiofrequency ablation. Dig Surg. 2011;28(3):173–7.
Marino IR, Scantlebury VP, Bronsther O, Iwatsuki S, Starzl TE. Total hepatectomy and liver transplant for hepatocellular adenomatosis and focal nodular hyperplasia. Transpl Int. 1992;5 Suppl 1:S201–5.
Yunta PJ, Moya A, San-Juan F, Lopez-Andujar R, De Juan M, Orbis F, et al. A new case of hepatic adenomatosis treated with orthotopic liver transplantation. Ann Chir. 2001;126(7):672–4.
Wellen JR, Anderson CD, Doyle M, Shenoy S, Nadler M, Turmelle Y, et al. The role of liver transplantation for hepatic adenomatosis in the pediatric population: case report and review of the literature. Pediatr Transplant. 2010;14(3):E16–9.
Lerut JP, Ciccarelli O, Sempoux C, Danse E, DeFlandre J, Horsmans Y, et al. Glycogenosis storage type I diseases and evolution adenomatosis: an indication for liver transplantation. Transpl Int. 2003;16(12):879–84.
Van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, De Man RA, Ijzermans JN. J Hepatol. 2011;55(1):120–5.
Kent DR, Nissen ED, Nissen SE, Ziehm DJ. Effect of pregnancy on liver tumor associated with oral contraceptives. Obstet Gynecol. 1978;51(2):148–51.
Shaked O, Siegelman ES, Olthoff K, Reddy KR. Biologic and clinical features of benign solid and cystic lesions of the liver. Clin Gastroenterol Hepatol. 2011;9(7):547–62.
Broker ME, Ijzermans JN, Van Aalten SM, De Man RA, Terkivatan T. The management of pregnancy in women with hepatocellular adenoma: a plea for an individualized approach. Int J Hepatol. 2012:1–3. The article summarizes the management of hepatic adenomas in pregnant patients and proposes an algorithm for management.
Noels JE, Van Aalten SM, Van der Windt DJ, Kok NF, De Man RA, Terkivatan T, et al. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54(3):553–8. This article describes the outcomes of 17 pregnancies in 12 patients previously diagnosed with hepatic adenomas. The authors make suggestions for management based on their experience.
Cristiano A, Dietrich A, Spina JC, Ariles V, De Santibanes E. Focal nodular hyperplasia and hepatic adenoma: current diagnosis and management. Updat Surg. 2013;66(1):9–21.
Poussin K, Pilati C, Couchy G, Calderado J, Bioulac-Sage P, Bacq Y, et al. Biochemical and functional analyses of gp130 mutants unveil JAK1 as a novel therapeutic target in human inflammatory hepatocellular adenoma. Oncoimmunology. 2013;2(12):e27090.
Acknowledgments
The authors would like to acknowledge the help of Dr. David Reich, Dr. Santiago Munoz, and Dr. Nancy Moshen for their guidance and edits
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Liver
Rights and permissions
About this article
Cite this article
Thapar, M., Grapp, O. & Fisher, C. Management of Hepatic Adenomatosis. Curr Gastroenterol Rep 17, 12 (2015). https://doi.org/10.1007/s11894-015-0434-4
Published:
DOI: https://doi.org/10.1007/s11894-015-0434-4