Abstract
Hepatocellular adenomas (HCAs) are a rare benign liver neoplasm mainly observed in young women taking oral contraceptives (OC) (Baum et al., Lancet 2:926–929, 1973; Rooks et al., JAMA 242:644–648, 1979; Carrasco et al., N Engl J Med 310:1120–1121, 1984; Rosenberg, Contraception 43:643–652, 1991; Heinemann et al., Eur J Contracept Reprod Health Care 3:194–200, 1998). Unlike other benign liver diseases such as hemangiomas and focal nodular hyperplasia (FNH) for which the risk of surgery is higher than the risk of spontaneous complications (Schnelldorfer et al., J Am Coll Surg 211(6):724–730, 2010), there is still a surgical indication for HCA because of the risk of bleeding and malignancy (Cho et al., Ann Surg Oncol 15:2795–2803, 2008; Dokmak et al., Gastroenterology 137:1698–1705, 2009; Deneve et al., Ann Surg Oncol 16:640–648, 2009). Major progress has been made in the last decade in the clinical, radiological, and histological understanding of this disease. HCA is no longer considered to be a single entity but is now divided into three radiological (inflammatory, steatotic, and classic) and five histological (inflammatory, HNF1A, b catenin,sonic hedgehog, and unclassified, HNF1A, inflammatory, β-catenin, sonic hedgehog, and unclassified) subtypes with different risks of complications (Nault et al., Gastroenterology 152(4):880–894, 2017). The risk factors for complications are well known and include HCA > 5 cm whatever the subtype (risk of bleeding and malignancy) and male gender whatever the size (risk of malignancy). However, HNF1A (steatotic HCA) has a low risk of complications, and the risk of malignancy is increased in β-catenin HCA with a mutation in exon 3 (Dokmak et al., Gastroenterology 137:1698–1705, 2009; Nault et al., Gastroenterology 152(4):880–894, 2017).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatocellular adenoma
- Liver adenoma
- Genotype classification
- Phenotype classification
- Bleeding
- Malignancy
- Surgery
Risk Factors for HCA
HCA is a benign liver neoplasm that is mainly observed (90%) in young women taking OC [1,2,3,4,5], but it is also rarely observed in men (10%) [6]. In most cases (80–90%), HCA occurs in young women who have been taking OC for many years. Although the exact mechanism of the association between HCA and OC has not been clearly identified, the association is clear because this entity was rarely described before the introduction of OC in the 1970s [1], the incidence of HCA is dose dependent [4, 7] and higher in women taking OC (3–4/100,000) than other women (0.1/100,000) [8, 9], and HCA regresses in some women after OC is withdrawn [10,11,12]. There are probably many other factors related to the development of HCA. Despite the widespread use of low-content estrogen OC, HCA still exists, but it is more frequent in obese patients and in those with the metabolic syndrome and steatohepatitis [6, 13,14,15,16,17,18]. Also, genetic alterations may be responsible such as in HNF1A- and β-catenin-mutated HCA [19, 20]. Androgen also plays a role in the pathogenesis of HCA and has been reported in patients with Fanconi anemia treated with androgens, in athletes who have abused steroids, and in patients with high levels of endogenous androgens [21,22,23,24]. HCA may also occur in association with certain metabolic diseases such as type 1 glycogen storage disease (GSD) [25,26,27] and iron overload related to beta-thalassemia or hemochromatosis [28]. In GSD the development of HCA is related to high triglyceride concentrations [27]. Familial cases (HNF1A) have been reported in patients with maturity-onset diabetes type 3 (MODY 3) [29, 30] and the McCune–Albright syndrome [19]. HCA can also occur in patients with hepatic vascular abnormalities such as portosystemic shunts with portal deprivation [31,32,33], Budd–Chiari syndrome and other vascular diseases [34], and, rarely, cirrhosis [35, 36]. Other rare causes include polycystic ovary syndrome related or not to sodium valproate leading to hyperandrogenemia [37,38,39], patients with Turner’s syndrome receiving growth hormone therapy [40] and Hurler’s syndrome with severe immune deficiencies [41], and adults with history of childhood cancer (leukemia) and treated by hematopoietic stem cell transplants with irradiation or estrogen therapy [42].
HCA is rare in men (10%), and androgen use, metabolic syndrome and steatohepatitis, type 1 GSD, and portosystemic shunts with portal deprivation should be systematically searched for.
Clinical Presentation
The mean age at presentation is 37 (16–62), and the mean size is 8.4 cm (±4.2, range: 1–22) [6]. HCA is usually asymptomatic and discovered incidentally during non-related imaging studies, with abnormal liver function tests, or due to non-specific abdominal pain. Abdominal pain may be present with large or pedunculated HCA. Bleeding usually presents as acute abdominal pain, but hemodynamic instability is rare or is rapidly stabilized with careful treatment. HCA may rarely present with fever, anemia, or pruritus. A single tumor may be present, but multiple tumors (>2 HCA) are more frequently observed [6]. Mild cholestasis or cytolysis is present in two thirds of liver function tests. Alpha-fetoprotein (AFP) should be systematically searched for but is usually normal even when HCA has degenerated. Inflammatory markers (CRP, fibrinogen, and platelets) may be increased with inflammatory HCA [43]. The diagnosis is based on imaging studies including CT scan and MRI [44, 45], and liver biopsy may be necessary in order to confirm the diagnosis or for histological subtyping and management.
Histological Classification
HCA is a soft tumor, and large subcapsular vessels are usually found on macroscopic examination. On cut sections, the tumor is well-delineated, fleshy, and sometimes encapsulated and has a color ranging from white to brown and frequent heterogeneous areas of necrosis and/or hemorrhage (Fig. 12.1). Histologically, HCA consists of a proliferation of benign hepatocytes arranged in a trabecular pattern. However, a normal liver architecture organization is absent. Hepatocytes may have intracellular fat or increased glycogen [46]. However, in the last decade, major progress has been made in the understanding of the histological pathogenesis of the disease, and HCA is no longer considered a single entity. HCA is now classified into at least five histological subtypes with different risks of complication [43, 46, 47].
HNF1A HCA
This subtype is characterized by bi-allellic inactivating mutations of HNF1A (hepatocyte nuclear factor 1 alpha). HNF1A is a key transcription factor that controls several metabolic pathways in the hepatocyte including estrogen metabolism and fatty acid synthesis deregulation with liver fatty acid-binding protein (LFABP), down expression leading to fatty acid accumulation, and steatosis in the tumor hepatocyte. The HNF1A mutation was identified in MODY3 in young patients with a familial context [48] and was later described in some familial cases of adenomatosis and MODY3 [49]. However, adenomatosis is infrequent in MODY3; thus other genetic or environmental factors are probably involved in the development of HCA [47, 50]. The HNF1A subtype is associated with intermediate levels of estrogen exposure [47]. On histology HNF1A is characterized by prominent steatosis associated with an absence of LFABP expression in tumor hepatocytes and high expression in non-tumor hepatocytes [46, 51].
Mutated β-Catenin HCA
This subtype involves mutations of CTNNB1 (protein-coding gene) coding for β-catenin, leading to impaired β-catenin phosphorylation that induces the translocation of β-catenin in the nucleus and expression of Wnt/β-catenin genes such as GLUL (coding for glutamine synthase) and LGR5. These mutations are associated with a higher risk of malignant transformation. These mutations are also observed in colorectal cancer and medulloblastoma. It has recently been shown that mutations on exon 3, but not 7 or 8, are associated with malignancy [47]. These tumors are more related to androgen than estrogen intake, both endogenous and exogenous androgen exposure, and are more frequently observed in men; most HCAs that develop from anabolic steroids are β-catenin mutated [12, 47]. However, women who develop β-catenin HCA have been less exposed to estrogen [47]. Morphologically this subtype is characterized by cellular atypia [52]. Tumor hepatocytes demonstrate strong and homogenous glutamine synthetase positivity (β-catenin target gene) and nuclear expression of β-catenin in some tumor hepatocytes, with high specificity and low sensitivity [52]. These variability and heterogeneity sometimes make the diagnosis difficult by biopsy, and molecular analysis may be needed for an accurate histological diagnosis . For exon 7/exon 8 mutations, glutamine synthetase is less important and heterogeneous, with no β-catenin nuclear staining [53].
Inflammatory HCA
This is the most frequent subtype which is defined by the activation of the IL6/JAK/STAT pathway in tumor hepatocytes with overexpression of acute phase inflammatory proteins such as CRP and SAA. An inflammatory syndrome, anemia and fever, may be observed and is considered to be a paraneoplastic syndrome induced by uncontrolled production of cytokines [43]. Inflammatory HCA can also involve the β-catenin mutation; thus, the Wnt/β-catenin pathway should be searched in the presence of inflammatory HCA. This subgroup is mainly observed in obese patients with extensive exposure to OC [47]. Morphologically these tumors are characterized by the presence of small arteries, inflammatory matrix, and sinusoidal dilatation [54]. Tumor hepatocytes exhibit cytoplasmic expression of SAA and CRP on immunohistochemistry induced by STAT3 activation [46, 51]. They can also contain steatosis and they can be mutated β-catenin [46].
Sonic Hedgehog HCA
The sonic hedgehog mutation (5% of HCA) was recently discovered in the subgroup of unclassified HCA. This mutation results in uncontrolled activation of the sonic hedgehog pathway due to the overexpression of GLI1 [47]. It seems that it is associated with a higher risk of clinical and histological bleeding. It is mainly observed in obese patients with extensive exposure to OC [47].
Unclassified HCA
No genetic alterations can be identified in <10% of HCA.
Radiological Classification
HCAs are usually well-delineated containing fat, vessels, and necrotic or hemorrhagic features. The most marked pathological features are the presence of fat or telangiectatic components; thus, imaging should be fat sensitive (such as MRI) with contrast agents to search for dilated vascular spaces [55]. HCA now includes three subtypes; thus, imaging findings vary depending on the type of HCA.
Steatotic or HNF1A HCA
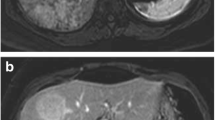
These HCAs are characterized by the presence of a diffuse and homogeneous signal dropout on chemical shift T1 sequences. This corresponds to fat and is the most marked finding with a high (87–91%) sensitivity and (89–100%) specificity on MRI (Fig. 12.2). These tumors are homogenous and moderately hypervascular and often show washout on portal and/or delayed-phase sequences, while they are hypointense on hepatobiliary phase MRI with hepatospecific contrast agents [56, 57].
Typical MR imaging appearance of an HNF-1A-inactivated hepatocellular adenoma located in segment 4 in a young female. The lesion appears hyperintense on T1-weighted images (a) and shows marked and homogeneous signal dropout on chemical shift images due to the presence of fat (b). The fat content is responsible for signal isointensity on T2-weighted images (c), mild contrast enhancement on arterial phase images (d), and pseudo-washout on delayed-phase images (e). The liver parenchyma is normal
Inflammatory HCA
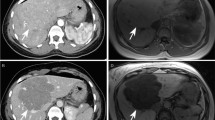
The telangiectatic features of this subtype show a strong, hyperintense signal on T2-weighted sequences, with a diffuse or peripheral (rim-like) image ([58]) and persistent enhancement during the delayed phase that has a high (85–88%) sensitivity and (88–100%) specificity [56, 57]. IHCAs are also markedly hypervascular and heterogeneous (Fig. 12.3). Certain tumors may mimic FNH and be iso- or hyperintense on hepatobiliary phase images with hepatospecific contrast agents [59].
Typical MR imaging appearance of an inflammatory hepatocellular adenoma located in segment 7 in a 37-year-old obese female. The lesion appears isotense on T1-weighted images (arrow in a) and does not contain fat (b). The lesion shows both signal hyperintensity on T2-weighted images (c) and contrast retention on delayed-phase images (d). The liver parenchyma is markedly steatotic (a, b)
The imaging characteristics of the two other subtypes, β-catenin and unclassified HCA (or classic), are less specific, and the features may be similar to other hepatocellular tumors, mainly arterial enhancement and portal or delayed washout (Fig. 12.4). The content is heterogeneous, but with no features to differentiate them from hepatocellular carcinoma or FNH in relation to β-catenin.
Typical CT appearance of a beta-catenin-activated hepatocellular adenoma in a young female. The lesion shows mild contrast enhancement on arterial phase images (a), is heterogeneous on portal venous phase images (b), and shows washout on delayed-phase images (c). The liver parenchyma is normal. Malignant transformation cannot be excluded. The lesion is indistinguishable from a hepatocellular carcinoma
Multiple Adenoma and Adenomatosis
Adenomatosis (>10 HCA) was initially described by Flejou et al. as being more frequent in men with a higher rate of complications [60] and associated with liver steatosis [61]. In fact, multiple HCAs (>2 HCA) are more frequently observed [62] and are not necessarily associated with higher complications. Although we found no clinical difference among the subgroups in a comparison of patients with single or multiple (2–10) [6] HCAs and adenomatosis (>10). Adenomatosis was more frequently associated with microadenomas, obesity, and the steatotic subtype, with a similar risk of complications. The presence of multiple HCAs was not a risk factor for bleeding [6, 63,64,65]. Thus, management should be based on the size and not the number of tumors [6], limiting the indications for liver transplantation.
Risk Factors for Complications
Bleeding
Bleeding is the most frequent complication of HCA, and it may be clinical (acute pain and large zones of bleeding on imaging) or subclinical with small areas of bleeding in HCA discovered on imaging or histology. Although the prevalence of subclinical bleeding is high (30–60%) [6], the clinical impact of this complication is unknown. Clinical bleeding is the most important complication and is observed in 20–25% of cases in surgical series [6, 30, 66]. This may be overestimated because data on prevalence are mainly based on surgical series, which mainly treat complicated HCA. In most cases bleeding HCAs are discovered when the episode of bleeding occurs, and it is less frequent to diagnose bleeding in an observed HCA. The clinical presentation is acute right hypochondrium pain and lower chest pain that can mimic a pulmonary embolism in some patients. Hemodynamic stability must be rapidly obtained following careful reanimation. Bleeding may be intra-tumoral alone with or without parenchymal extension and in 10% of cases associated with intraperitoneal rupture and hemoperitoneum [6]. Biopsy of the viable tissue can be discussed to make certainly the diagnosis of ruptured HCA; however, in patients with complete necrosis at admission, the diagnosis of HCA can be established according to clinical data and prevalence as HCA remains the most frequent cause of liver bleeding in a young female. The main risk factor for bleeding is the presence of inflammatory HCA and tumor size with a 5% risk in HCA < 5 cm and 25% in HCA > 5 cm [6, 63, 66, 67]. Other risk factors are sonic hedgehog HCA [47], exophytic lesions, or lesions located in the left lateral segments and with peripheral arteries visualized on imaging [67], as well as hormone use within the last 6 months [63].
Malignancy
Malignant degeneration is the second complication of HCA. There is no specific clinical or radiological presentation, and in most cases the diagnosis is made following resection. The AFP level is usually normal, and malignant degeneration is suggested in case of rapid growth of an observed or embolized HCA. In clinical practice it may be very difficult to differentiate between malignant HCA and hepatocellular carcinoma (HCC) that develops in a normal liver in young women with or without elevated AFP levels. On histology it can be difficult to differentiate between HCA and well-differentiated HCC, but the presence of both adenomatous tissue and HCC foci is highly suggestive of the diagnosis [68, 69]. The most important risk factors for malignancy are gender and tumor size. The risk of malignant degeneration in men and women is >50% and <5%, respectively [6, 70, 71]. Malignant degeneration is mainly observed in HCA > 5 cm and has been found in large HCA (>8 cm) [63, 72, 73], but it is rare in HCA < 5 cm [6]. Certain retrospective studies have shown an increased risk of malignancy in β-catenin-mutated HCA [6, 65], but further studies are needed to confirm this, especially because recent results show that only mutations on exon 3 but not those on 7 and 8 are at risk of malignant degeneration [53]. Classic HCAs have an increased risk of malignant degeneration [6].
Risk Factors of Complications by Radiological and Histological Subtypes
Certain retrospective studies have correlated the risk of complications with the new phenotype/genotype classification of HCA [6, 65]. The risk of bleeding is increased in inflammatory HCA , and the risk of malignant degeneration is moderate [6]; steatotic HCAs have a very low risk of bleeding (<10%), and malignant degeneration is rare [6]; β-catenin HCAs with an exon 3 mutation have an estimated risk of malignant degeneration of 20% [6, 65], and the risk of bleeding is increased in sonic hedgehog HCA [47], and the risk of malignant degeneration is also increased in classic HCAs [6]. However, there are no prospective studies on the new subtypes and the risk of complications.
Treatment
When a diagnosis is made, underlying risk factors should be managed in all cases. OC should be stopped [74, 75] and weight loss is suggested because obesity is a risk factor. Encouraging results were recently reported with weight loss alone in obese patients [76]. A period of 6 months was usually needed to observe an effect on tumor size, but it seems that a longer period of OC withdrawal is necessary to obtain a significant reduction. If the tumor does not regress to a size without risk (<5 cm), there are several treatment options including surgical resection, embolization, percutaneous ablation, and, more rarely, liver transplantation.
Non-complicated HCA
In men, HCA should be resected whatever the size due to the high risk of malignant degeneration (>50%). However, in GSD the risk of malignant degeneration is low [26] and has only been reported in case reports [77, 78]. Treatment can be less aggressive, and resection can be limited to large HCA in men with GSD and multiple HCAs. In women , because the risk of complications is mainly observed in HCA > 5 cm, only large HCA (>5 cm) should resected while HCA < 5 cm can be observed.
Indications for Resection Based on the New Classification and the Role of Liver Biopsy
Although the new classification has significantly increased the understanding of the disease, its influence on patient management is still limited because of the absence of valid data. HCA subtyping can be obtained from MRI or liver biopsy, and because the accuracy of MRI for the diagnosis and subtyping of HCA is good [57, 79], the usefulness of biopsy is limited for decision-making; thus, the importance of its role is reduced [80]. Although HNFA1 and inflammatory HCA may be diagnosed on MRI, it is less accurate for the diagnosis of the β-catenin subtype. In men, resection is indicated whatever the subtype, and molecular subtyping can play a role in men with multiple HCAs (such as those with GSD) to prevent unnecessary liver transplantation. In women, HCA >5 cm should be resected whatever the subtype and molecular subtyping should be considered in two cases. First is to diagnose β-catenin mutation in HCA <5 cm. There are no valid data to confirm an increased risk of malignant degeneration in β-catenin-mutated HCA <5 cm, and a preoperative diagnosis may be difficult with liver biopsy because this is made indirectly by measuring glutamine synthetase on immunohistochemistry with a heterogeneity of expression in the nucleus and the cytoplasm [81]. Finally, it was recently demonstrated that only the mutation on exon 3 but not 7 and 8 is associated with malignant degeneration [47]. Thus, in our experience management should still be based on gender and size. Second there is a lower risk of complications in steatotic HCA; thus, certain women with large HCA (5–7 cm) may be observed especially if resection is complicated. In these cases, biopsy with molecular subtyping may be needed to confirm the diagnosis of HNF1A [6]. Molecular subtyping may also be systematically performed by biopsy or on the tumor specimen for randomized studies or for the prospective evaluation of liver biopsy.
Bleeding HCA
Stability must be obtained in the presence of hemodynamic instability , and patients should be managed in an intensive care unit [82]. Emergency surgical resection should no longer be performed because this procedure requires a large incision, extended liver resection (resection of HCA and hematoma), and transfusion and is associated with a high morbidity and a long hospital stay [6]. Considerable mortality (12.5%) has been reported following emergency resection [83], and modern management includes stabilization with or without transfusion, arterial embolization, and delayed surgical resection [84,85,86,87]. In case of urgent surgery, packing is preferred to liver resection to decrease morbidity and mortality [84]. Delayed resection is performed 3–4 months following embolization once the parenchymal hematoma has disappeared. This is the best surgical strategy because in some cases resection can be performed by laparoscopic approach including minor liver resection, which is associated with less transfusion, reduced morbidity, and a shorter hospital stay. In certain patients with complete necrosis on imaging, simple observation can be an option and bleeding may result in a spontaneous cure of HCA (Fig. 12.5) . Observation can also be proposed if bleeding HCAs are not completely necrotic but have downsized to <5 cm because recurrent bleeding in the same HCA is rare.
Young female with a long history of oral contraceptive use presented with acute abdominal pain. Bleeding HCA (a) with large subcapsular hematoma was diagnosed. After rapid reanimation and stabilization, the patient was treated by embolization. (b) A few months later, (c) major regression of the hematoma and HCA without any adenomatous tissue. This patient was never operated on and, after a follow-up of 7 years, no recurrence of the disease
Indications for Embolization in Bleeding HCA
The indications for arterial embolization are not well known; however, embolization should be systematically performed in unstable patients, in those with severe deglobulization, and if an arterial blush is seen on imaging. Systematic embolization can also be discussed to stop bleeding, to increase the rate of necrosis (to avoid delayed resection), and to downsizing HCA to <5 cm. Repeat embolization to control recurrent bleeding is rare.
Malignant HCA
If a malignant degeneration is suspected preoperatively (rapid growth, slight elevation of AFP, satellites nodules), anatomical liver resection is recommended similar to HCC, especially in a normal liver, making major liver resection safe. If the diagnosis is made postoperatively, there is no need for additional surgery if the resection is performed with free surgical margins because the risk of satellite nodules or vascular invasion is rare [6]. On the other hand, if resection is not satisfactory or is incomplete, we suggest a second intervention for complete resection.
Surgical Resection
When possible the laparoscopic approach should be the standard procedure because HCA is a benign disease in young women with long-term parietal benefits. The advantages of the laparoscopic approach for morbidity and hospital stay were recently reported compared to open surgery in a large French and European multicentric study on 533 resected HCA [88]. Resection with margins of a few millimeters is sufficient, but care should be taken in some patients because it can be difficult to differentiate between adenomatous tissue and the normal liver parenchyma.
Indications for Other Procedures (Ablation and Embolization)
Although embolization is the first choice for bleeding HCA, its role in non-bleeding HCA is a subject of debate. Many retrospective studies have shown a significant decrease in the size of non-bleeding HCA following classic [89, 90] or bland embolization [91]. For some authors this treatment is mainly effective in patients with multiple and small HCA (<3 cm) [72] and in those with adenomatosis [92]. We feel that this treatment is not effective in non-bleeding HCA, and further studies are needed, especially because embolization can result in severe necrosis on the normal liver. Ablation by radiofrequency and more rarely by microwave [93] has already been described with good results [94,95,96,97,98,99] and a low recurrence rate [96, 97]. However, in most of those studies, ablation was performed on small HCA <5 cm for which general treatment is not needed. However, ablation can be an interesting option for the treatment of limited size HCA (4–5 cm) during pregnancy [100], recurrence after resection [96], difficult intraoperative locations or the need for major liver resection [98], and for small β-catenin-mutated HCA.
Indications for Liver Transplantation
One of the major advantages of the clinical comprehension and genotype phenotype classification (risk factors for complications) of HCA is to limit the indications for liver transplantation, which is therefore rare [62]. In the European Liver Transplant Registry, only 49 liver transplantations were performed between 1986 and 2013 for liver adenomatosis [101]. In women with multiple HCA, only HCA >5 cm should be resected, and remnant HCA <5 cm remains stable in most cases. It should be noted that with the routine and frequent use of modern imaging for abdominal complaints, massive adenomatosis with large HCA involving both liver lobes [102] has become rare. Liver transplantation should only be indicated in symptomatic uncontrolled GSD with multiple HCA [103], men with multiple HCA except GSD (because the risk of malignant degeneration is low), recurrent HCA many years after resection of degenerated HCA, and in patients in which liver resection is a risk due to vascular anomalies [104] (HCA and portacaval shunt) or the presence of underlying liver disease such Budd–Chiari syndrome or other chronic liver diseases.
Pregnancy
Normally pregnancy was contraindicated in patients with HCA due to the risk of disease progression and rupture and reports of maternal and fetal mortality [105]. However, the natural history of HCA from diagnosis to treatment has completely changed in the last 15 years, and HCA is no longer considered to be a contraindication to pregnancy. We followed 15 pregnancies in 11 women including 9 with residual HCA. HCA did not recur in any of the women without residual HCA (six pregnancies), and two of those with residual HCA (n = 9) experienced moderate progression but with no complications. In another study, 17 pregnancies were followed in 12 women with HCA < 5 cm. Progression occurred in four cases, requiring a cesarean in two (>34 weeks) and preventive percutaneous ablation [106]. When the diagnosis of HCA is known before pregnancy (primary diagnosis or residual HCA after resection), it is recommended to treat HCA including those between 3 and 5 cm, and percutaneous ablation could probably play a role in these cases. When the diagnosis is made during pregnancy, the patients with HCA <5 cm can be monitored by ultrasound every 2–3 months and closely observed in HCA >5 cm. In case of disease progression, treatment and indications depend upon the size of HCA and the week of gestation. Surgery should be avoided and replaced by percutaneous ablation or embolization. OC use is not absolutely contraindicated, and low-estrogen content OC or progestative OC can be used once HCA has been managed and the effect of OC withdrawal has been observed on the size of HCA, especially if there are gynecological indications for this treatment.
Follow-Up
After resection of a single HCA , new HCA (<3 cm) may develop in 10–15% of cases, but the patient can be considered cured. After incomplete resection, residual HCA has been shown to progress in 15% and regress in 9% [6, 64, 72, 74]. Follow-up should be mainly radiological, preferable with MRI, but ultrasound can be performed if only the size must be followed. After diagnosis or management, a yearly CT scan or MRI and even ultrasound is sufficient following diagnosis and management. After 5 years and in case of stability, imaging study can be done every 2 years for 5 years, and follow-up may be discontinued after the age of 50 (menopause) because changes are rare after this age. In a recent study, radiological follow-up in 48 women with HCA in the postmenopausal period showed undetectable lesions (44%), stability (33%), or significant regression (19%); thus, follow-up can be discontinued in the postmenopausal period [107]. In all cases , it is very rare to observe complications of residual HCA or newly developed HCA.
References
Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2:926–9.
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW Jr. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–8.
Carrasco D, Barrachina M, Prieto M, Berenguer J. Clomiphene citrate and liver-cell adenoma. N Engl J Med. 1984;310:1120–1.
Rosenberg L. The risk of liver neoplasia in relation to combined oral contraceptive use. Contraception. 1991;43:643–52.
Heinemann LA, Weimann A, Gerken G, Thiel C, Schlaud M, DoMinh T. Modern oral contraceptive use and benign liver tumors: the German Benign Liver Tumor Case-Control Study. Eur J Contracept Reprod Health Care. 1998;3:194–200.
Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698–705.
Gutiérrez Santiago M, García Ibarbia C, Nan Nan DN, Hernández Hernández JL. Hepatic lesions and prolonged use of oral contraceptive. Rev Clin Esp. 2007;207(5):257–8.
Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–2.
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Tyler CW Jr. The association between oral contraception and hepatocellular adenoma—a preliminary report. Int J Gynaecol Obstet. 1977;15:143–4.
Bühler H, Pirovino M, Akobiantz A, Altorfer J, Weitzel M, Maranta E, Schmid M. Regression of liver cell adenoma. A follow-up study of three consecutive patients after discontinuation of oral contraceptive use. Gastroenterology. 1982;82(4):775–82.
Aseni P, Sansalone CV, Sammartino C, Benedetto FD, Carrafiello G, Giacomoni A, Osio C, Vertemati M, Forti D. Rapid disappearance of hepatic adenoma after contraceptive withdrawal. J Clin Gastroenterol. 2001;33(3):234–6.
Svrcek M, Jeannot E, Arrive L, Poupon R, Fromont G, Flejou JF, Zucman-Rossi J, Bouchard P, Wendum D. Regressive liver adenomatosis following androgenic progestin therapy withdrawal: a case report with a 10-year follow-up and a molecular analysis. Eur J Endocrinol. 2007;156:617–21.
Brunt EM, Wolverson MK, Di Bisceglie AM. Benign hepatocellular tumors (adenomatosis) in nonalcoholic steatohepatitis: a case report. Semin Liver Dis. 2005;25(2):230–6.
Smith BM, Hussain A, Jacobs M, Merrick HW III. Ruptured hepatocellular carcinoma in a patient with nonalcoholic steatohepatitis. Surg Obes Relat Dis. 2009;5(4):510–2.
Lefkowitch JH, Antony LV. The evolving role of nonalcoholic fatty liver disease in hepatic neoplasia: inflammatory hepatocellular adenoma in a man with metabolic syndrome. Semin Liver Dis. 2015;35(3):349–54.
Bunchorntavakul C, Bahirwani R, Drazek D, Soulen MC, Siegelman ES, Furth EE, Olthoff K, Shaked A, Reddy KR. Clinical features and natural history of hepatocellular adenomas: the impact of obesity. Aliment Pharmacol Ther. 2011;34(6):664–74.
Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int. 2012;32(8):1217–21.
Chang CY, Hernandez-Prera JC, Roayaie S, Schwartz M, Thung SN. Changing epidemiology of hepatocellular adenoma in the United States: review of the literature. Int J Hepatol. 2013;2013:604860.
Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144(5):888–902.
Jeannot E, Poussin K, Chiche L, Bacq Y, Sturm N, Scoazec JY, Buffet C, Van Nhieu JT, Bellanné-Chantelot C, de Toma C, Laurent-Puig P, Bioulac-Sage P, Zucman-Rossi J. Association of CYP1B1 germ line mutations with hepatocyte nuclear factor 1alpha-mutated hepatocellular adenoma. Cancer Res. 2007;67(6):2611–6.
Bork K, Pitton M, Harten P, Koch P. Hepatocellular adenomas in patients taking danazol for hereditary angio-oedema. Lancet. 1999;353:1066–7.
Nakao A, Sakagami K, Nakata Y, Komazawa K, Amimoto T, Nakashima K, Isozaki H, Takakura N, Tanaka N. Multiple hepatic adenomas caused by long-term administration of androgenic steroids for aplastic anemia in association with familial adenomatous polyposis. J Gastroenterol. 2000;35(7):557–62.
Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77:257–67.
Socas L, Zumbado M, Pérez-Luzardo O, Ramos A, Pérez C, Hernández JR, Boada LD. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39:e27.
Talente GM, Coleman RA, Alter C, Baker L, Brown BI, Cannon RA, Chen YT, Crigler JF Jr, Ferreira P, Haworth JC, Herman GE, Issenman RM, Keating JP, Linde R, Roe TF, Senior B, Wolfsdorf JI. Glycogen storage disease in adults. Ann Intern Med. 1994;120(3):218–26.
Reddy SK, Kishnani PS, Sullivan JA, Koeberl DD, Desai DM, Skinner MA, Rice HE, Clary BM. Resection of hepatocellular adenoma in patients with glycogen storage disease type Ia. J Hepatol. 2007;47:658–63.
Wang DQ, Fiske LM, Carreras CT, Weinstein DA. Natural history of hepatocellular adenoma formation in glycogen storage disease type I. J Pediatr. 2011;159(3):442–6.
Hagiwara S, Takagi H, Kanda D, Sohara N, Kakizaki S, Katakai K, Yoshinaga T, Higuchi T, Nomoto K, Kuwano H, Mori M. Hepatic adenomatosis associated with hormone replacement therapy and hemosiderosis: a case report. World J Gastroenterol. 2006;12(4):652–5.
Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P, Fabre M, Oberti F, Fatome A, Zucman-Rossi J, Bellanne-Chantelot C. Hepatocyte nuclear factor-1 alpha gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab. 2004;89(3):1476–80.
Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford). 2005;7(3):186–96.
Kawakatsu M, Vilgrain V, Belghiti J, Flejou JF, Nahum H. Association of multiple liver cell adenomas with spontaneous intrahepatic portohepatic shunt. Abdom Imaging. 1994;19(5):438–40.
Seyama Y, Sano K, Tang W, Kokudo N, Sakamoto Y, Imamura H, Makuuchi M. Simultaneous resection of liver cell adenomas and an intrahepatic portosystemic venous shunt with elevation of serum PIVKA-II level. J Gastroenterol. 2006;41:909–12.
Pupulim LF, Vullierme MP, Paradis V, Valla D, Terraz S, Vilgrain V. Congenital portosystemic shunts associated with liver tumours. Clin Radiol. 2013;68(7):e362–9.
Sempoux C, Paradis V, Komuta M, Wee A, Calderaro J, Balabaud C, Quaglia A, Bioulac-Sage P. Hepatocellular nodules expressing markers of hepatocellular adenomas in Budd-Chiari syndrome and other rare hepatic vascular disorders. J Hepatol. 2015;63(5):1173–80.
Calderaro J, Nault JC, Balabaud C, Couchy G, Saint-Paul MC, Azoulay D, Mehdaoui D, Luciani A, Zafrani ES, Bioulac-Sage P, Zucman-Rossi J. Inflammatory hepatocellular adenomas developed in the setting of chronic liver disease and cirrhosis. Mod Pathol. 2016;29(1):43–50.
Sasaki M, Nakanuma Y. Overview of hepatocellular adenoma in Japan. Int J Hepatol. 2012;2012:648131.
Toso C, Rubbia-Brandt L, Negro F, Morel P, Mentha G. Hepatocellular adenoma and polycystic ovary syndrome. Liver Int. 2003;23(1):35–7.
Seki A, Inoue T, Maegaki Y, Sugiura C, Toyoshima M, Akaboshi S, Ohno K. Polycystic ovary syndrome and hepatocellular adenoma related to long-term use of sodium valproate in a young woman. No To Hattatsu. 2006;38(3):205–8.
Cazorla A, Félix S, Valmary-Degano S, Sailley N, Thévenot T, Heyd B, Bioulac-Sage P. Polycystic ovary syndrome as a rare association with inflammatory hepatocellular adenoma: a case report. Clin Res Hepatol Gastroenterol. 2014;38(6):e107–10.
Espat J, Chamberlain RS, Sklar C, Blumgart LH. Hepatic adenoma associated with recombinant human growth hormone therapy in a patient with Turner’s syndrome. Dig Surg. 2000;17(6):640–3.
Resnick MB, Kozakewich HP, Perez-Atayde AR. Hepatic adenoma in the pediatric age group. Clinicopathological observations and assessment of cell proliferative activity. Am J Surg Pathol. 1995;19(10):1181–90.
Tonorezos ES, Barnea D, Abou-Alfa GK, Bromberg J, D’Angelica M, Sklar CA, Shia J, Oeffinger KC. Hepatocellular adenoma among adult survivors of childhood and young adult cancer. Pediatr Blood Cancer. 2017;64(4).
Paradis V, Champault A, Ronot M, Deschamps L, Valla DC, Vidaud D, Vilgrain V, Belghiti J, Bedossa P. Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology. 2007;46(1):140–6.
Brancatelli G, Federle MP, Vullierme MP, Lagalla R, Midiri M, Vilgrain V. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30:745–50.
Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, Gambarini S, Donato F, Colagrande S. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520–9.
Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, Balabaud C, Zucman-Rossi J. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–8.
Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S, GENTHEP Investigators, Bioulac-Sage P, Letouzé E, Zucman-Rossi J. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152(4):880–94.
Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI, et al. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384:455–8.
Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, Laurent C, Bourlier P, Pariente D, de Muret A, Fabre M, Bioulac-Sage P, Zucman-Rossi J. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125:1470–5.
Iwen KA, Klein J, Hubold C, Lehnert H, Weitzel JM. Maturity-onset diabetes of the young and hepatic adenomatosis—characterisation of a new mutation. Exp Clin Endocrinol Diabetes. 2013;121(6):368–71.
Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Tercé F, Auffray C, Bioulac-Sage P, Zucman-Rossi J. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282:14437–46.
Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–24.
Rebouissou S, Franconi A, Calderaro J, Letouze E, Imbeaud S, Pilati C, Nault JC, Couchy G, Laurent A, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Genotype-phenotype correlation of CTNNB1 mutations reveals different β-catenin activity associated with liver tumor progression. Hepatology. 2016;64(6):2047–61.
Bioulac-Sage P, Rebouissou S, Sa Cunha A, Jeannot E, Lepreux S, Blanc JF, Blanc JF, Blanché H, Le Bail B, Saric J, Laurent-Puig P, Balabaud C, Zucman-Rossi J. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211–8.
Belghiti J, Dokmak S, Vilgrain V, Paradis V. Benign liver lesions, hepatocellular adenoma. Blumgart’s surgery of the liver, biliary tract and pancreas, vol. 2. 5th ed. Philadelphia, PA: Elsevier Saunders; 2012.
Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808–18.
Ronot M, Bahrami S, Calderaro J, Valla DC, Bedossa P, Belghiti J, Vilgrain V, Paradis V. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53:1182–91.
van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, Ijzermans JN. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011;261(1):172–81.
Bieze M, van den Esschert JW, Nio CY, Verheij J, Reitsma JB, Terpstra V, van Gulik TM, Phoa SS. Diagnostic accuracy of MRI in differentiating hepatocellular adenoma from focal nodular hyperplasia: prospective study of the additional value of gadoxetate disodium. AJR Am J Roentgenol. 2012;199:26–34.
Fléjou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, Benhamou JP. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89:1132–8.
Veteläinen R, Erdogan D, de Graaf W, ten Kate F, Jansen PL, Gouma DJ, van Gulik TM. Liver adenomatosis: re-evaluation of aetiology and management. Liver Int. 2008;28:499–508.
Dokmak S, Cauchy F, Belghiti J. Resection, transplantation and local regional therapies for liver adenomas. Expert Rev Gastroenterol Hepatol. 2014;8(7):803–10.
Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, Martin RC, D’Angelica M, Staley CA, Choti MA, Jarnagin WR, Schulick RD, Kooby DA. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16:640–8.
Toso C, Majno P, Andres A, Rubbia-Brandt L, Berney T, Buhler L, Morel P, Mentha G. Management of hepatocellular adenoma: solitary-uncomplicated, multiple and ruptured tumors. World J Gastroenterol. 2005;11(36):5691–5.
Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H, Zucman-Rossi J, Balabaud C, Saric J. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481–9.
van Aalten SM, de Man RA, IJzermans JN, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911–6.
Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg. 2014;101(7):847–55.
Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85–9.
Singhi AD, Jain D, Kakar S, Wu TT, Yeh MM, Torbenson M. Reticulin loss in benign fatty liver: an important diagnostic pitfall when considering a diagnosis of hepatocellular carcinoma. Am J Surg Pathol. 2012;36:710–5.
Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig Surg. 2010;27:32–8.
Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509–22.
Karkar AM, Tang LH, Kashikar ND, Gonen M, Solomon SB, Dematteo RP, D’Angelica MI, Correa-Gallego C, Jarnagin WR, Fong Y, Getrajdman GI, Allen P, Kingham TP. Management of hepatocellular adenoma: comparison of resection, embolization and observation. HPB (Oxford). 2013;15(3):235–43.
Bossen L, Grønbaek H, Lykke Eriksen P, Jepsen P. Men with biopsy-confirmed hepatocellular adenoma have a high risk of progression to hepatocellular carcinoma: a nationwide population-based study. Liver Int. 2017;37(7):1042–6.
van der Windt DJ, Kok NF, Hussain SM, Zondervan PE, Alwayn IP, de Man RA, IJzermans JN. Case-orientated approach to the management of hepatocellular adenoma. Br J Surg. 2006;93:1495–502.
Sinclair M, Schelleman A, Sandhu D, Angus PW. Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy. Hepatology. 2017;66(3):989–91.
Dokmak S, Belghiti J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. 2015;35:2228–32.
Cassiman D, Libbrecht L, Verslype C, Meersseman W, Troisi R, Zucman-Rossi J, Van Vlierberghe H. An adult male patient with multiple adenomas and a hepatocellular carcinoma: mild glycogen storage disease type Ia. J Hepatol. 2010;53(1):213–7.
Iguchi T, Yamagata M, Sonoda T, Yanagita K, Fukahori T, Tsujita E, Aishima S, Oda Y, Maehara Y. Malignant transformation of hepatocellular adenoma with bone marrow metaplasia arising in glycogen storage disease type I: a case report. Mol Clin Oncol. 2016;5(5):599–603.
Mounajjed T, Wu TT. Telangiectatic variant of hepatic adenoma: clinicopathologic features and correlation between liver needle biopsy and resection. Am J Surg Pathol. 2011;35:1356–63.
Terkivatan T, Ijzermans JN. Hepatocellular adenoma: should phenotypic classification direct management? Nat Rev Gastroenterol Hepatol. 2009;6(12):697–8.
Hale G, Liu X, Hu J, Xu Z, Che L, Solomon D, Tsokos C, Shafizadeh N, Chen X, Gill R, Kakar S. Correlation of exon 3 β-catenin mutations with glutamine synthetase staining patterns in hepatocellular adenoma and hepatocellular carcinoma. Mod Pathol. 2016;29(11):1370–80.
Marini P, Vilgrain V, Belghiti J. Management of spontaneous rupture of liver tumours. Dig Surg. 2002;19:109–13.
Rosales A, Que FG. Spontaneous hepatic hemorrhage: a single institution’s 16-year experience. Am Surg. 2016;82(11):1117–20.
Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Tilanus HW, IJzermans JN. Treatment of ruptured hepatocellular adenoma. Br J Surg. 2001;88:207–9.
Erdogan D, van Delden OM, Busch OR, Gouma DJ, van Gulik TM. Selective transcatheter arterial embolization for treatment of bleeding complications or reduction of tumor mass of hepatocellular adenomas. Cardiovasc Intervent Radiol. 2007;30:1252–8.
Erdogan D, Busch OR, van Delden OM, Ten Kate FJ, Gouma DJ, van Gulik TM. Management of spontaneous haemorrhage and rupture of hepatocellular adenomas. A single centre experience. Liver Int. 2006;26:433–8.
Huurman VA, Schaapherder AF. Management of ruptured hepatocellular adenoma. Dig Surg. 2010;27:56–60.
Landi F, De’ Angelis N, Scatton O, Vidal X, Ayav A, Muscari F, Dokmak S, Torzilli G, Demartines N, Soubrane O, Cherqui D, Hardwigsen J, Laurent A. Short-term outcomes of laparoscopic vs. open liver resection for hepatocellular adenoma: a multicenter propensity score adjustment analysis by the AFC-HCA-2013 study group. Surg Endosc. 2017;31(10):4136–44.
Stoot JH, van der Linden E, Terpstra OT, Schaapherder AF. Life-saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg. 2007;94:1249–53.
van Rosmalen BV, Coelen RJS, Bieze M, van Delden OM, Verheij J, Dejong CHC, van Gulik TM. Systematic review of transarterial embolization for hepatocellular adenomas. Br J Surg. 2017;104(7):823–35.
Deodhar A, Brody LA, Covey AM, Brown KT, Getrajdman GI. Bland embolization in the treatment of hepatic adenomas: preliminary experience. J Vasc Interv Radiol. 2011;22(6):795–9.
Kobayashi S, Sakaguchi H, Takatsuka M, Suekane T, Iwai S, Morikawa H, Enomoto M, Tamori A, Kawada N. Two cases of hepatocellular adenomatosis treated with transcatheter arterial embolization. Hepatol Int. 2009;3(2):416–20.
Smolock AR, Cristescu MM, Potretzke TA, Ziemlewicz TJ, Lubner MG, Hinshaw JL, Brace CL, Lee FT Jr. Microwave ablation for the treatment of hepatic adenomas. J Vasc Interv Radiol. 2016;27(2):244–9.
Ahn SY, Park SY, Kweon YO, Tak WY, Bae HI, Cho SH. Successful treatment of multiple hepatocellular adenomas with percutaneous radiofrequency ablation. World J Gastroenterol. 2013;19(42):7480–6.
McDaniel JD, Kukreja K, Ristagno RL, Yazigi N, Nathan JD, Tiao G. Radiofrequency ablation of a large hepatic adenoma in a child. J Pediatr Surg. 2013;48(6):E19–22.
Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular adenoma: initial experience in 10 patients. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e422–7.
Atwell TD, Brandhagen DJ, Charboneau JW, Nagorney DM, Callstrom MR, Farrell MA. Successful treatment of hepatocellular adenoma with percutaneous radiofrequency ablation. AJR Am J Roentgenol. 2005;184(3):828–31.
Fujita S, Kushihata F, Herrmann GE, Mergo PJ, Liu C, Nelson D, Fujikawa T, Hemming AW. Combined hepatic resection and radiofrequency ablation for multiple hepatic adenomas. J Gastroenterol Hepatol. 2006;21(8):1351–4.
van Vledder MG, van Aalten SM, Terkivatan T, de Man RA, Leertouwer T, Ijzermans JN. Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J Vasc Interv Radiol. 2011;22:787–93.
Scheffer HJ, Melenhorst MC, van Tilborg AA, Nielsen K, van Nieuwkerk KM, de Vries RA, van den Tol PM, Meijerink MR. Percutaneous irreversible electroporation of a large centrally located hepatocellular adenoma in a woman with a pregnancy wish. Cardiovasc Intervent Radiol. 2015;38(4):1031–5.
Chiche L, David A1, Adam R, Oliverius MM, Klempnauer J, Vibert E, Colledan M, Lerut J, Mazzafero VV, Di-Sandro S, Laurent C, Scuderi V, Suc B, Troisi R, Bachelier P, Dumortier J, Gugenheim J, Mabrut JY, Gonzalez-Pinto I, Pruvot FR, Le-Treut YP, Navarro F, Ortiz-de-Urbina J, Salamé E, Spada M, Bioulac-Sage P. Liver transplantation for adenomatosis: European experience. Liver Transpl. 2016;22(4):516–26.
Chiche L, Dao T, Salamé E, Galais MP, Bouvard N, Schmutz G, Rousselot P, Bioulac-Sage P, Ségol P, Gignoux M. Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg. 2000;231:74–81.
Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483–90.
Gordon-Burroughs S, Balogh J, Weiner MA, Monsour HP Jr, Schwartz MR, Gaber AO, Ghobrial RM. Liver transplantation in an adult with adenomatosis and congenital absence of the portal vein: a case report. Transplant Proc. 2014;46(7):2418–21.
Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004;187(2):181–91.
Noels JE, van Aalten SM, van der Windt DJ, Kok NF, de Man RA, Terkivatan T, Ijzermans JN. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54(3):553–8.
Klompenhouwer AJ, Sprengers D, Willemssen FE, Gaspersz MP, Ijzermans JN, De Man RA. Evidence of good prognosis of hepatocellular adenoma in post-menopausal women. J Hepatol. 2016;65(6):1163–70.
Acknowledgments
The author would like to thank Dr. Maxime Ronot (Department of Radiology, Beaujon Hospital, Clichy, France) and Dr. Nicolas Pote (Department of Pathology, Beaujon Hospital, Clichy, France) for providing us the radiological (Figs. 12.2, 12.3 and 12.4) and pathological (Fig. 12.1) illustrations and Dr. Roche-Lebrec for her editorial assistance and correction of the article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dokmak, S. (2018). Pathological Classification and Surgical Approach to Hepatocellular Adenomas. In: Cardona, K., Maithel, S. (eds) Primary and Metastatic Liver Tumors. Springer, Cham. https://doi.org/10.1007/978-3-319-91977-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-91977-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91976-8
Online ISBN: 978-3-319-91977-5
eBook Packages: MedicineMedicine (R0)