Abstract
Purpose of Review
Night-to-night variability in sleep patterns leads to circadian disruption and, consequently, could increase cardiometabolic risk. The purpose of this review is to summarize findings from studies published between 2015 and 2020 examining various measures of night-to-night variability in sleep in relation to metabolic syndrome (MetS), type 2 diabetes (T2D), and their risk factors. We illustrate a potential causal pathway between irregular sleep patterns and T2D, highlighting knowledge gaps along the way.
Recent Findings
Across different measures of sleep variability, irregular sleep patterns were associated with poorer cardiometabolic outcomes. Higher standard deviations (SD) across nights of sleep duration and onset or midpoint of sleep were associated with increased odds of having MetS and clusters of metabolic abnormalities as well as greater adiposity and poorer glycemic control. Conversely, greater regularity of rest-activity patterns related to lower risk for T2D. Social jetlag was associated with glycemic dysregulation, adiposity, T2D, and MetS. These associations are often observed in both metabolically healthy and unhealthy individuals; both higher SD of sleep duration and social jetlag relate to poorer glucose regulation in individuals with diabetes.
Summary
There is consistent evidence of associations of sleep variability with increased risk for adiposity, glucose dysregulation, T2D, and MetS. Although experimental evidence is needed to determine causation, there is support to recommend stabilizing sleep patterns for cardiometabolic risk prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspects of the modern lifestyle, such as artificial light exposure and demanding work schedules, have led to increased prevalence of inadequate sleep duration and sleep disturbances [1, 2]. It is likely that regularity of sleep behaviors is declining correspondingly, as high variability in sleep patterns is prevalent [3,4,5,6], especially in low-income or minority groups [7]. This has led to growing interest in a potential link between variability in sleep patterns and health. Irregular sleep/wake patterns are observed in rotating shift workers who also demonstrate elevated rates of cardiometabolic diseases [8, 9]. However, this extreme example of sleep irregularity may not apply to the general population of adults who have more regular work schedules but may still have variability in night-to-night sleep patterns.
Variability across days in duration of sleep and sleep schedules, including timing of sleep, may contribute to a mismatch between behavioral cycles and innate circadian rhythms (i.e., circadian misalignment) [10]. Given that many metabolic and endocrine functions oscillate in a circadian fashion, sleep variability-imposed circadian misalignment could cause dysregulation of these systems. Social jetlag, a chronic form of irregularity between weekend and weekday sleep/wake cycles, may also contribute to poorer cardiometabolic health outcomes. In this case, increased cardiometabolic risk may result from desynchrony between circadian clocks that internally regulate metabolic processes and social clocks that are dictated by work and school schedules [5, 6].
There is an emerging body of literature on the role of sleep regularity in cardiometabolic health. Therefore, the purpose of the present review is to summarize and discuss studies published between 2015 and 2020 that have examined various measures of night-to-night variability in sleep, including social jetlag, in relation to the risk of metabolic syndrome (MetS), type 2 diabetes (T2D), and obesity. We also evaluate evidence on sleep variability in relation to adiposity and glycemic regulation among individuals with pre-existing chronic diseases, including T2D. We use our summary of the literature to discuss clinical and public health implications of existing studies, highlight research gaps, and suggest future research to advance this field.
Associations of Night-to-Night Variability in Sleep Duration and Sleep Onset Timing with Cardiometabolic Health
To capture the regularity of sleep patterns, several studies have used multi-day wrist-worn actigraphy to calculate the standard deviation (SD) of sleep duration and sleep onset timing [11,12,13,14,15,16,17,18]. These sleep parameters are of interest since they are directly modifiable. In the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep Ancillary Study of ~ 2000 older adults, high night-to-night variability in both sleep duration and sleep onset timing, assessed from 7 days of wrist actigraphy, was associated with higher risk of cardiometabolic abnormalities after adjustment for sociodemographic, lifestyle, and sleep-related factors [11]. In cross-sectional analyses, every 1-h increase in sleep duration SD was associated with 27% higher odds of having MetS. Similar patterns were observed for sleep onset timing (i.e., bedtime); odds of having MetS increased by 23% for every 1-h increase in sleep onset SD. Those with sleep onset SD > 90 min had 45% higher odds of MetS. This association may be more marked in African Americans. Although interactions of sleep onset SD with race did not reach statistical significance, exploratory stratification showed that odds of having MetS per 1-h increase in sleep onset SD was highest in African Americans [OR: 1.49 (1.15–1.92)]. In prospective analyses within a subset of 970 participants, associations were not significant for night-to-night variability in sleep duration. However, every 1-h increase in sleep onset SD was associated with 36% higher risk of developing MetS after the sleep exam (median follow-up: 6.3 years). Notably, the associations between increased sleep onset variability persisted even after adjusting for average total sleep duration and in analyses restricted to sleep variability measured only during weekdays.
In the MESA Sleep Ancillary, clusters of metabolic abnormalities and individual components of the MetS were also studied in relation to regularity of sleep behaviors [11]. Cross-sectional analysis of sleep duration variability with individual components of MetS showed that each 1-h increase in sleep duration SD related to 21% and 20% higher odds of having central obesity and high fasting glucose, respectively. The magnitude of these associations was even greater for variability in onset of sleep timing, which was associated with 30% higher odds of having central obesity and high fasting glucose. This is noteworthy given that both of these outcomes are key risk factors for the development of T2D. Cross-sectional and prospective associations of sleep duration and sleep onset SD with 4 data-driven clusters of varying metabolic abnormalities were also examined. Only the “metabolically abnormal obese diabetes” cluster, defined as having central obesity and fasting plasma glucose > 100 mg/dL, was related to sleep duration SD and sleep onset SD. In cross-sectional analyses, every 1-h increase in sleep duration and sleep onset SD was associated with up to 45% greater odds of having metabolically abnormal obese diabetes. In the prospective analyses, for each 1-h increase in sleep duration and sleep onset SD, the odds for incidence of multiple metabolic abnormalities (metabolically abnormal obese diabetes) versus few metabolic abnormalities were almost doubled.
Indeed, investigation of sleep variability and obesity, a causal factor in insulin resistance [19], supports a role of variable sleep patterns in T2D etiology. Another study among men and women in the MESA Sleep Ancillary Study examined the relationship between SD in sleep duration (from 7 days of wrist actigraphy) and various measures of adiposity [12]. Greater sleep duration variability was associated with higher body mass index (BMI), larger waist circumference, and greater percentage of total body fat. In addition, prevalence of abdominal obesity was 5% higher in those in the highest compared to lowest quartile of sleep duration variability. Similar results were observed in a cohort of 191 older women from Tokyo [13]. Although these studies do not provide data on direct measures of glycemic control or diabetes, by demonstrating clear associations of sleep variability with excess weight and adiposity, they present a potential mechanism by which sleep variability may contribute to diabetes risk.
In the PREDIMED-Plus trial, cross-sectional associations of sleep variability with both T2D and its risk factors were studied in a group of individuals aged 55–75 years who presented with at least 3 components of MetS at baseline [14]. Sleep was measured on 8 consecutive days via wrist-worn actigraphy, and sleep variability was defined as the SD of sleep duration across nights. Each 1 h/night increase in sleep duration variability was associated with 14% higher risk for T2D in fully adjusted models. This could not, however, be explained through associations with risk factors for T2D, as associations were non-significant for fasting plasma glucose, hemoglobin A1c (HbA1c), and adiposity. On the other hand, high sleep duration SD in conjunction with sleep duration < 7 h/night (“bad” sleeper) was associated with higher fasting glucose, HbA1c, and BMI, when compared to “good” sleepers (average sleep duration ≥ 7 h/night and sleep duration SD < median). Furthermore, “bad” sleepers had higher prevalence of obesity and T2D. Thus, having irregular sleep patterns may exacerbate diabetes risk in older individuals, especially among those with habitually short sleep.
The role of sleep variability in cardiometabolic risk may be of particular significance among those with insomnia. Individuals with insomnia are reported to have an approximately 30% increased risk of developing diabetes after considering traditional risk factors [20]. Mechanisms posited to underlie increased disease risk in this population are sympathetic nervous system hyperactivity, inflammation, and abnormalities in cortisol secretion [21]. Recent recognition of the co-occurrence of increased night-to-night changes in sleep duration and sleep timing in insomnia [22] raise the additional possibility that circadian misalignment may play a role in insomnia-related cardiometabolic disease. Data from a non-pharmacologic clinical trial of 17 older adults with insomnia revealed that a higher sleep duration SD and higher sleep onset SD, measured via 7-day wrist actigraphy, were moderately correlated with higher HbA1c and higher BMI, respectively [15]. Variability in sleep behaviors was not associated with insulin resistance, but this could be due to the limited number of participants without T2D. Future research should investigate variability of sleep in a larger sample of individuals with and without insomnia to confirm associations with T2D risk and determine whether individuals with insomnia are at increased risk compared to individuals without comorbid sleep disorders.
Beyond a potential contribution of irregular sleep to risk of developing T2D, recent data suggest that increased sleep duration and timing variability are associated with poor glucose control in individuals already diagnosed with T2D [16]. In the MESA Sleep Ancillary Study, increased sleep duration variability (upper two quartiles) was associated with higher HbA1c among those with diabetes, while no association was seen in non-diabetics [17]. In a US study of 172 patients with T2D, increased night-to-night variability in sleep duration, measured with 7 days of wrist actigraphy, was the sleep characteristic most strongly associated with HbA1c, followed by sleep duration, subjective sleep quality, variability in sleep midpoint, and sleep efficiency. In multivariable-adjusted analyses, results were similar but attenuated, and total sleep duration and subjective sleep quality emerged as the strongest predictors of HbA1c.
Increased night-to-night variability in sleep duration and sleep onset has also been linked to poor glycemic control among patients living with type 1 diabetes [18]. Glycemic dysregulation in type 1 diabetes is the result of beta cell damage or dysfunction [23], and not obesity-related insulin resistance; therefore, type 1 diabetes presents a unique model of studying the role of sleep variability in glucose control, and one that could provide insight into a potential direct impact on glucose metabolism. In a study of 41 adults with type 1 diabetes from Thailand, higher sleep duration and sleep midpoint SD, computed from 5 days of wrist actigraphy, were associated with significantly higher HbA1c levels and a greater insulin requirement [18]. This relationship persisted following adjustment for neuropathic symptoms, sleep apnea risk, and sleep quality. Furthermore, sleep duration SD and sleep midpoint SD were highly correlated, indicating that those with variable sleep duration are also more likely to have variable sleep timing. These findings suggest that higher sleep variability, likely reflective of sleep curtailment alternating with sleep compensation, combined with changes in circadian timing, is associated with poor glycemic control in type 1 diabetes.
There is clearly support for a role of sleep variability in risk for the development of obesity, glucose dysregulation, and T2D. Furthermore, assessment of sleep patterns in men and women with type 1 diabetes and T2D suggest that increased variability in sleep patterns may exacerbate impaired glucose control. Individuals with chronic insomnia or short sleep duration may be at especially increased risk for cardiometabolic abnormalities if they also have high levels of night-to-night variability in sleep duration and bedtime. It is important to note, however, that these studies were all observational in nature. It could be the case that glycemic dysregulation contributes to increased variability in bedtime or sleep duration across nights, although there is not a clear plausible physiological basis for this. Nevertheless, while calculating the SD of nightly sleep metrics appears to be informative in predicting risk for metabolic disease, there is a need for clinical trials to determine the causality of this relationship and to better understand the relative importance of regularizing sleep duration alone, sleep timing alone, or both in reducing cardiometabolic risk.
Associations of the Sleep Regularity Index with Cardiometabolic Health
The Sleep Regularity Index (SRI) represents a different approach for assessing sleep/wake regularity. Introduced by Philips et al. in 2017 [10], this metric is defined as the percentage probability of a person being asleep (or awake) at any two time points 24-h apart. The SRI is particularly useful for individuals who have multiple sleep periods within the 24-h day and can capture rapid changes in sleep schedules. The SRI, calculated from 7 days of wrist actigraphy sleep data, has only been evaluated in relation to cardiometabolic risk among older adults (n = 1978; mean age: 69 years) in MESA [24]. In that study, lower SRI (i.e., more variable sleep) was associated with various risk factors for T2D, including higher BMI, HbA1c, and fasting glucose. In group comparisons, irregular sleepers displayed significantly greater BMI (29.7 vs. 27 kg/m2), HbA1c (6.0 vs. 5.7%), and fasting glucose levels (99 vs. 94 mg/dL) than regular sleepers. Individuals with MetS (71.7 vs. 76.2), T2D (68.5 vs. 75.8), and obesity (72.6 vs. 75.8) had lower SRI than those without.
Although there is limited data on the association of SRI with cardiometabolic outcomes, results of the available study suggest that it may be a good metric for capturing variability in sleep patterns. A strength of the measure is that it more precisely captures sleep/wake patterns; however, this also adds complexity to calculating the index, and may lend itself less to informing public health interventions (e.g., in comparison to messages related to need for consistent bedtimes). Still, this measure should be included in future research of sleep patterns and diabetes risk, particularly in populations with large numbers of irregular shift workers, broad age ranges, and multiple races/ethnicities, in order to confirm results in MESA and compare to other measures of sleep variability.
The Role of Social Jetlag in Cardiometabolic Health
An increasingly common form of night-to-night variability in sleep is the chronic jetlag-like phenomenon termed “social jetlag.” Social jetlag is a form of circadian disruption that results from modern day work schedules; individuals “travel back and forth” between “time zones” on workdays, characterized by socially imposed schedules for sleep-wake times, and non-work days, which are more reflective of an individual’s innate circadian rhythm [5]. Social jetlag is quantified in the literature as the difference between the midpoints of sleep intervals on work vs. non-workdays.
Studies have linked social jetlag to adverse cardiometabolic outcomes [25, 26]. In one analysis of 490 middle-aged US men and women (age range 30–54 years) working part- or full-time day shifts, a mismatch in sleep timing between workdays and non-work days was related to markers of reduced insulin sensitivity and excess adiposity [25]. Greater social jetlag, measured with 7 days of wrist actigraphy, was significantly associated with higher fasting insulin, insulin resistance, waist circumference, and BMI in linear regression models adjusted for demographics, sleep characteristics, health behaviors, and depression. Furthermore, participants with social jetlag > 1 h vs. ≤ 1 h had higher BMI (28.3 vs. 26.3 kg/m2), waist circumference (94.0 vs. 88.9 cm), fasting insulin (1.13 vs. 1.08 μU/mL), and HOMA-IR (4.07 vs. 3.72). Consistent with these findings, in a cross-sectional study of 1164 Japanese non-shift workers, aged 18–78 years, those with a difference in self-reported sleep midpoint between weekdays and weekends of ≥ 2 h vs. < 1 h had 92% higher odds of having the MetS [26] and > 2-fold increased likelihood of having a large waist circumference.
The associations of social jetlag with MetS and T2D risk may differ across the life course, as some data suggest that associations are stronger in younger and middle-aged compared to older adults [27]. This was demonstrated in a cross-sectional analysis from the New Hoorn Study, a population-based cohort of Dutch adults (n = 1585, mean age: 61 years), where social jetlag was quantified from the Munich Chronotype Questionnaire. In the total study population, those with 1–2 h and > 2 h vs. < 1 h of social jetlag had 15% and 64% higher odds of prevalent MetS, respectively. This association was mostly driven by younger participants (< 61 years), who had 29% and > 2-fold greater odds for MetS and 39% and 75% greater odds for T2D/prediabetes, with 1–2 h and > 2 h of social jetlag, respectively, compared to participants with < 1 h of social jetlag. No associations were observed in older adults.
An association between social jetlag and adiposity has been reported in a sample of 804 adolescents from Project Viva [28]. In this sample of boys and girls (aged 12 to 17 years) studied with wrist actigraphy over 7 to 10 days, 13.9% had social jetlag ≥ 2 h. In girls, each 1-h increase in social jetlag was associated with average 0.45 kg/m2 higher fat mass index in analyses adjusted for multiple potential confounders, including physical activity and TV watching. Significant associations were not observed in boys or with measures of glucose control, lipids, or blood pressure. The lack of significant associations with other components of MetS may reflect the small variation of these factors in a younger, generally healthy sample. The stronger associations in girls may reflect mediating factors such as steroid hormones, and social and environmental factors that differ by gender. These data highlight the need for future research to address sex and gender differences in physiological responses to circadian misalignment.
The strength of associations between social jetlag and measures of glycemic regulation may vary by obesity status. Social jetlag, measured from self-report on the Munich Chronotype Questionnaire, was evaluated in relation to clinically assessed metabolic phenotypes and indicators for obesity-related diseases, specifically T2D [29]. In the overall sample, greater social jetlag was associated with higher BMI, fat mass, and waist circumference and with 20% and 30% greater odds for obesity and the MetS, respectively. Moreover, social jetlag was more prevalent among metabolically unhealthy obese individuals compared with non-obese individuals. In sensitivity analyses excluding metabolically healthy individuals with obesity, individuals with metabolically unhealthy obesity and greater social jetlag had 30% higher odds of clinically elevated HbA1c, but these associations were attenuated upon adjusting for smoking and socioeconomic status.
As illustrated above, social jetlag may be particularly detrimental to cardiometabolic health among individuals with pre-existing chronic disease. Participants in a study of 792 adults in Brazil were grouped by obesity status and metabolic health: non-obese (BMI < 30 kg/m2), metabolically healthy obese (BMI ≥ 30 kg/m2 and meets < 3 criteria for MetS), and metabolically unhealthy obese (BMI ≥ 30 kg/m2 and meets criteria for ≥ 3 biomarkers for MetS) [30]. Associations of social jetlag, computed from self-reported sleep and wake times on weekdays and weekends, with cardiometabolic risk factors, were evaluated. In the overall study sample and among metabolically unhealthy patients with obesity, social jetlag was associated with having higher fasting glucose levels. In addition, individuals with social jetlag > 1 h vs. ≤ 1 h had up to 2-fold greater odds for overweight/obesity and of having a metabolically unhealthy obese phenotype, suggesting that social jetlag is associated with a higher risk of overweight and related metabolic complications in individuals with pre-existing chronic disease.
Social jetlag has also been linked to poor glycemic control among US and European adults with pre-existing type 1 diabetes or T2D. In a study from Ireland of 252 adults with T2D, greater social jetlag, measured from the Munich Chronotype Questionnaire, was associated with higher HbA1c [31]. Similarly, in a cross-sectional study of 115 Romanian adults with type 1 diabetes, those with self-reported social jetlag ≥ 1 h vs. < 1 h had significantly higher adjusted values of HbA1c (8.7% vs. 8.0%) [32]. In regression models adjusted for age, sex, diabetes duration, insulin dose, insulin regimen, and BMI, having social jetlag ≥ 1 h was associated with 0.25% higher HbA1c levels.
Assessments of social jetlag in relation to obesity and adiposity in healthy, young adult populations have yielded conflicting results. In 430 US adults aged 21–35 years, social jetlag, measured using armband actigraphy data for 4–10 days, was not associated with increased odds of having overweight or obese BMI, high waist-to-hip ratio, or high body fat percentage [33]. In contrast, a study of 534 healthy Spanish students aged 18–25 years reported a significant association between social jetlag (determined from self-reported questionnaires) and BMI: each 1-h increase in social jetlag was associated with a 0.3-point increase in BMI [34]. A number of potential explanations for differences observed between these studies could be posited. One possibility is that cultural differences, including diet and physical activity, could lead to differences in the relation between social jetlag and obesity risk. Differences could also be due to the tools used to measure sleep and markers of adiposity; one study relied on self-report [34] while the other used objective measures [33]. Regardless, based on the current available data, it remains unclear whether social jetlag is associated with increased risk of having obesity or excess adiposity.
Circadian Rest-Activity Rhythms in T2D
Circadian rest-activity rhythms represent an indirect measure of circadian rhythmicity, i.e., alignment of sleep and rest-activity patterns with circadian rhythms in a free-living community-based setting. One method of quantifying variability in these cycles across days is to calculate the Interdaily Stability Index (ISI) using multi-day wrist actigraphy. This measure has been used to assess associations of circadian rest-activity rhythms with the odds of incident MetS, T2D, and obesity [35]. In a subgroup of 1137 men and women (81.6 ± 7.5 years), higher ISI values, indicating greater stability of 24-h rest-activity patterns, were associated with 31% lower odds of having MetS. Greater stability in rest-activity patterns were also associated with reduced likelihood of having both T2D and obesity (odds ratios: 0.73 and 0.76, respectively). Pooled analysis of 578 of healthy men and women across 3 studies (age: 51.9 ± 14.9), however, did not show a significant association between BMI and actigraphic measures of inter-day variability or inter-day stability [36]. Rather, the amplitude of daily rest-activity patterns was related to BMI, with individuals in the bottom quintile of relative amplitude (least robust) having a BMI that was 2.65 kg/m2 higher than individuals in the top quintile (most robust 24-h rest-activity pattern). Taken together, there may be a beneficial role of robust circadian rest-activity rhythms in cardiometabolic health, but the influence of night-to-night stability in these rhythms on BMI warrants furthers study.
Recent data also suggest that T2D patients may exhibit a dysfunction in the rest-activity rhythm [37]. In a case-control study of 21 T2D patients and 21 controls matched by age and gender, those with T2D had significantly lower 7-day actigraphy-derived interdaily stability, indicative of greater night-to-night variability in rest-activity rhythms and less synchronization to 24-h light and dark cycles, compared to their healthy counterparts. They also exhibited significantly higher intradaily variability, indicating more fragmented rest-activity rhythms. Collectively, these data suggest that T2D is associated with more variable and fragmented sleep and rest-activity patterns. However, results warrant confirmation in larger samples and prospectively to confirm whether less robust circadian rest-activity rhythms are a predictor or a consequence of T2D or whether this is simple co-occurrence.
What Have We Learned, and What Is Still Left to Uncover?
Generalizability and Predictive Value of Current Measures of Sleep Variability
A key question that arises during the review of this literature: is one measure more representative of habitual patterns of sleep variability than others? For example, SD is a measure of total variability across nights, while social jetlag represents a more systematic variability between different sections of the week. A priority of future research should be to compare existing metrics of sleep variability to determine whether one is most generalizable across populations, or, if a single measure incorporating both overall and systematic variability is needed. In order to inform public policy, there is also a need to better understand the roles of self-reported as compared to objective sleep assessments, and whether commercially available wearable devices may provide scalable data useful for individual monitoring of sleep variability. A related question: is there a specific measure of sleep variability that is most predictive of cardiometabolic outcomes? While overall results indicate that each different component of sleep variability relates to T2D risk factors, no study compares the predictive value of various metrics of sleep-wake patterns.
Individual Differences in the Association of Sleep Variability with T2D Risk
It is also important to evaluate whether results differ based on characteristics of the population. Many of the analyses reviewed use data from large cohort studies that are well designed and often include large proportions of racial and ethnic minorities. However, a potential influence of age, race/ethnicity, socioeconomic status, or other sleep behaviors on the association of sleep variability with T2D risk warrants further investigation. Available studies tend to be in older populations, demonstrating the need for research in younger individuals, with specific consideration to developmental issues in childhood and adolescence. Additionally, the causes of sleep variability could differ by age; for example, life pressures and caregiver responsibilities that influence sleep variability may be more salient in younger compared to older adults. There are also emerging data indicating that ethnic and racial minorities and individuals from low-income households have increased sleep variability [7]. This may reflect the frequency of social and environmental stressors in these populations, including the need to work multiple jobs and/or rotating shifts. Given the known disparities in cardiometabolic diseases, there is an urgent need to better understand whether efforts at improving sleep variability may improve health and wellness in vulnerable populations. Finally, individuals with increased variability in sleep patterns may also have shorter sleep duration than those with more regular sleep. While much of the literature shows associations of increased variability with adverse metabolic parameters in analyses adjusted for average sleep duration, there is some evidence that increased variability combined with short sleep duration may have particularly negative effects [14]. It is therefore important to consider how variable sleep may interact with other aspects of sleep duration and quality as well as with disorders such as insomnia to influence cardiometabolic risk. Identifying individual differences in the association of sleep variability with cardiometabolic risk will aid in the development of tailored interventions to improve sleep and overall health.
Establishing a Causal Link Between Sleep Variability and T2D, MetS
A key gap in the literature is the determination of causality between sleep variability and cardiometabolic outcomes. As a follow-up from observational studies, there is a need for clinical trials to assess the impact of changes in sleep patterns on adiposity and glycemic regulation. We recently provided some evidence of causality by showing that women who stabilized their sleep (reduced bedtime SD) via bed and wake time prescriptions over a 6-week period had reductions in adiposity, while these measures increased in those who did not change or increased their sleep variability [38]. These data suggest that night-to-night variability in sleep could affect body composition and increase risk for insulin resistance and T2D. However, the study was not designed to answer this question; therefore, results must be replicated in a trial designed specifically to test a causal relationship between sleep variability and T2D risk factors.
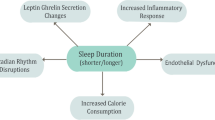
Mechanisms underlying the relation between sleep variability and cardiometabolic risk also remain largely unknown. It is likely that high variability in sleep and rest-wake activity patterns, as well as social jetlag, leads to circadian disruption. Research on endogenous circadian rhythms and circadian misalignment would support an influence of sleep variability on glucose dysregulation via circadian disruption [39, 40]. Sleep variability may impact the production or release of melatonin, which is closely linked to glucose tolerance [41, 42]. Similar to experimental circadian misalignment, increased sleep variability may reduce insulin sensitivity [39]. On the other hand, there could be behavioral mediators of the relationship. Our data [38] support epidemiologic findings of an association of irregular bedtimes with increased adiposity [12,13,14], which leads to insulin resistance [19]. Moreover, studies in adolescents show that sleep variability is linked to poorer dietary habits [43], and that diet mediates an association of sleep variability with adiposity [44]. Thus, it could be that sleep variability disrupts circadian rhythms, contributing to overeating or poor diet quality, eventually leading to increased adiposity. Changes in adiposity could mediate or moderate a relation of sleep variability with glycemic dysregulation (Fig. 1). Finally, increased sleep variability may be a general marker of disrupted family and social routines associated with variability in eating and physical activity as well as social stress. We may need interventions that focus on a holistic approach to regularizing daily routines and reducing stress.
The proposed pathway by which night-to-night variability can impact risk for T2D and MetS. Solid lines indicate well-established associations; dashed lines represent associations with limited data in adults. Variability in sleep contributes to circadian disruption, which has been associated with increased adiposity. Although not the focus of this review, such an effect could be due to changes in dietary patterns promoting positive energy balance. Increased adiposity has well-known downstream effects on insulin sensitivity and glucose metabolism. Circadian disruption can also have a direct impact on glucose regulation (independent of adiposity) via reductions in melatonin [ [41, 45, 46] not discussed herein], which influences insulin sensitivity of cells. Reductions to melatonin would therefore contribute to insulin resistance, impacting glucose metabolism. If sustained over time, the net result of this cascade of effects in response to sleep variability, then, would be T2D and risk for MetS
Conclusions
In reviewing epidemiologic studies from 2015 to 2020, we find reliable evidence of an association between irregularity in sleep patterns and heightened cardiometabolic risk. More specifically, in both cross-sectional and prospective analyses, greater variability in sleep duration and sleep onset is associated with increased risk for MetS, T2D, and obesity; these sleep exposures are also related to higher values of individual diabetes risk factors, including glycemic regulation and adiposity. Although many of these studies define sleep variability as the SD of sleep parameters measured across multiple nights, other measures, including SRI, social jetlag, and interdaily stability of rest-activity patterns, yield similar results.
Future research is needed to identify the best measure of sleep variability as well as the populations in which deleterious associations of sleep variability with cardiometabolic health are strongest. We also recommend that clinical trials be performed to assess potential causal pathways between sleep variability and glycemic regulation and determine mechanisms. These should include diverse populations, especially those at increased risk for both cardiometabolic diseases and increased sleep variability, such as ethnic minorities. Despite the need for more data in this area, current findings suggest that recommendations to stabilize sleep patterns could be useful in managing cardiometabolic risk in adults and possibly adolescents.
References
Linton SJ, Kecklund G, Franklin KA, Leissner LC, Sivertsen B, Lindberg E, et al. The effect of the work environment on future sleep disturbances: a systematic review. Sleep Med Rev. 2015 Oct 1;23:10–9.
Division of Sleep Medicine H. External factors that influence sleep | Healthy Sleep. [cited 2020 Feb 17]. Available from: http://healthysleep.med.harvard.edu/healthy/science/how/external-factors.
Dillon HR, Lichstein KL, Dautovich ND, Taylor DJ, Riedel BW, Bush AJ. Variability in self-reported normal sleep across the adult age span. J Gerontol Ser B. 2015 [cited 2020 Feb 22];70(1):46–56. Available from: https://academic.oup.com/psychsocgerontology/article/68/4/540/569189.
Tworoger SS, Davis S, Vitiello MV, Lentz MJ, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59(1):11–9.
Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;497–509.
Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–43.
Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2020;75(9):991–9.
Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. Vol. 355, BMJ (Online). BMJ Publishing Group; 2016.
Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S, et al. Meta-analysis on night shift work and risk of metabolic syndrome. Obes Rev. 2014;15(9):709–20.
Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;1:7(1).
Huang T, Redline S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: the multi-ethnic study of atherosclerosis. Diabetes Care. 2019 [cited 2019 Jun 25];dc190596. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31167888.
Ogilvie RP, Redline S, Bertoni AG, Chen X, Ouyang P, Szklo M, et al. Actigraphy measured sleep indices and adiposity: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016 Sep 1 [cited 2020 Feb 17];39(9):1701–8. Available from: https://academic.oup.com/sleep/article/39/9/1701/2708329.
Kim M, Sasai H, Kojima N, Kim H. Objectively measured night-to-night sleep variations are associated with body composition in very elderly women. J Sleep Res. 2015 [cited 2020 Feb 17];24(6):639–47. Available from: http://doi.wiley.com/10.1111/jsr.12326
Rosique-Esteban N, Papandreou C, Romaguera D, Warnberg J, Corella D, Martínez-González MÁ, et al. Cross-sectional associations of objectively-measured sleep characteristics with obesity and type 2 diabetes in the PREDIMED-Plus trial. Sleep. 2018 [cited 2020 Feb 17];41(12). Available from: https://academic.oup.com/sleep/article/doi/10.1093/sleep/zsy190/5115226.
Baron KG, Reid KJ, Malkani RG, Kang J, Zee PC. Sleep variability among older adults with insomnia: associations with sleep quality and cardiometabolic disease risk. Behav Sleep Med. 2017;15(2):144–57.
Brouwer A, Van Raalte DH, Rutters F, Elders PJM, Snoek FJ, Beekman ATF, et al. Sleep and HbA1c in patients with type 2 diabetes: which sleep characteristics matter most? Diabetes Care. 2020;43(1):235–43.
Whitaker KM, Lutsey PL, Ogilvie RP, Pankow JS, Bertoni A, Michos ED, et al. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: the multi-ethnic study of atherosclerosis. SLEEPJ [Internet]. 2018 [cited 2020 Apr 13];1–10. Available from: https://academic.oup.com/sleep/article-abstract/41/11/zsy172/5089925.
Chontong S, Saetung S, Reutrakul S. Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. J Sleep Res. 2016 [cited 2020 Feb 16];25(4):438–44. Available from: http://doi.wiley.com/10.1111/jsr.12393
Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000 Aug 15;106(4):473–81.
Leblanc ES, Smith NX, Nichols GA, Allison MJ, Clarke GN. Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting. BMJ Open Diab Res Care. 2018 [cited 2020 Apr 13];6:604. Available from: http://drc.bmj.com/
Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–44.
Sánchez-Ortuño MM, Carney CE, Edinger JD, Wyatt JK, Harris A. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep. 2011;34(4):531–9.
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82.
Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci Rep. 2018;1:8(1).
Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100(12):4612–20.
Islam Z, Akter S, Kochi T, Hu H, Eguchi M, Yamaguchi M, et al. Association of social jetlag with metabolic syndrome among Japanese working population: the Furukawa nutrition and health study. Sleep Med. 2018;51:53–8.
Koopman ADM, Rauh SP, Van’t Riet E, Groeneveld L, Van Der Heijden AA, Elders PJ, et al. The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new Hoorn study. J Biol Rhythm. 2017;32(4):359–68.
Cespedes Feliciano EM, Rifas-Shiman SL, Quante M, Redline S, Oken E, Taveras EM. Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 2019;173(11):1049–57.
Parsons MJ, Moffitt TE, Gregory AM, Goldman-Mellor S, Nolan PM, Poulton R, et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int J Obes. 2015;39(5):842–8.
Mota MC, Silva CM, Balieiro LCT, Fahmy WM, Crispim CA. Social jetlag and metabolic control in non-communicable chronic diseases: a study addressing different obesity statuses. Sci Rep. 2017;7(1).
Kelly RM, Finn J, Healy U, Gallen D, Sreenan S, McDermott JH, et al. Greater social jetlag associates with higher HbA1c in adults with type 2 diabetes: a cross sectional study. Sleep Med. 2020;66:1–9.
Rusu A, Ciobanu D, Bala C, Cerghizan A, Roman G. Social jetlag, sleep-related parameters, and glycemic control in adults with type 1 diabetes: results of a cross-sectional study. J Diabetes. 2019;11(5):394–401.
McMahon DM, Burch JB, Youngstedt SD, Wirth MD, Hardin JW, Hurley TG, et al. Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiol Int. 2019;36(4):493–509.
Rugerio Z, Cambras IP. Social jet lag associates negatively with the adherence to the Mediterranean diet and body mass index among young adults. Nutrients. 2019 [cited 2020 Feb 17];11(8):1756. Available from: https://www.mdpi.com/2072-6643/11/8/1756
Sohail S, Yu L, Bennett DA, Buchman AS, Lim ASP. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32(6):802–13.
Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, et al. Actigraphy-derived daily rest–activity patterns and body mass index in community-dwelling adults. Sleep. 2017 [cited 2020 Feb 17];40(12). Available from: https://academic.oup.com/sleep/article/doi/10.1093/sleep/zsx168/4344553.
Cavalcanti-Ferreira P, Berk L, Daher N, Campus T, Araujo J, Petrofsky J, et al. A nonparametric methodological analysis of rest-activity rhythm in type 2 diabetes. Sleep Sci. 2018;11(4):281–9.
St-Onge M, Campbell A, Zuraikat FM, Cheng B, Shah R, Berger J, et al. Impact of change in bedtime variability on body composition and inflammation: secondary findings from the Go Red for Women Strategically Focused Research Network. Int J Obes. 2020.
Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225–34.
Morris CJ, Purvis TE, Mistretta J, Scheer FAJL. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016 [cited 2020 Apr 13];101(3):1066–74. Available from: https://academic.oup.com/jcem/article-lookup/doi/10.1210/jc.2015-3924.
Owino S, Buonfiglio DDC, Tchio C, Tosini G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front Endocrinol (Lausanne). 2019;10:488.
Kim TW, Jeong JH, Hong SC. The impact of sleep and circadian disturbance on hormones and metabolism. Int J Endocrinol. 2015;2015:1–9.
He F, Bixler EO, Berg A, Imamura Kawasawa Y, Vgontzas AN, Fernandez-Mendoza J, et al. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015 [cited 2019 Jun 25];16(7):856–61. Available from: https://www.sciencedirect.com/science/article/pii/S1389945715006772?via%3Dihub.
He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015 [cited 2019 Jun 25];16(12):1489–94. Available from: https://www.sciencedirect.com/science/article/pii/S1389945715009235?via%3Dihub.
Morris CJ, Aeschbach D, Scheer FAJL. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349(1):91–104.
Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014 May 1 [cited 2020 May 11];56(4):371–81. Available from: http://doi.wiley.com/10.1111/jpi.12137
Funding
Dr. St-Onge is funded by NIH Grants R01 HL128226 and R01 HL142648 and an AHA Go Red for Women Grant 16SFRN27950012. Dr. Zuraikat is a Berrie Fellow in Diabetes Research and is supported by NIH Grant T32 HL007343. Dr. Makarem is supported by a NIH K99/R00 Pathway to Independence Award from NHLBI (Grant # K99 HL148511). Dr. Redline is supported by NIH Grant R35 HL135818. Dr. Aggarwal is funded by an AHA Go Red for Women Grant 16SFRN27960011. Dr. Jelic is funded by NIH grants R01 HL106041 and R01 HL137234 as well as by an AHA Go Red for Women Grant 16SFRN29050000.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and compiled with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity
Rights and permissions
About this article
Cite this article
Zuraikat, F.M., Makarem, N., Redline, S. et al. Sleep Regularity and Cardiometabolic Heath: Is Variability in Sleep Patterns a Risk Factor for Excess Adiposity and Glycemic Dysregulation?. Curr Diab Rep 20, 38 (2020). https://doi.org/10.1007/s11892-020-01324-w
Published:
DOI: https://doi.org/10.1007/s11892-020-01324-w