Abstract
Purpose
One of the aspects of lifestyle affecting weight and metabolic factors is the sleep pattern that is most neglected. Sleep pattern includes the quality and quantity of sleep throughout the day. According to studies, a high prevalence of disorders in sleep pattern is seen in people, especially obese. The goal of this review is to highlight the link between sleep pattern and anthropometric indicators and metabolic risk factors.
Method
We performed a search of journals and articles by utilizing the Institute for Scientific Information database available under the banner of the Web of Science, SCOPUS, and Google Scholar database through keywords such as: Sleep quality, Sleep duration, Obesity, Fasting blood sugar, Lipid profile. Next, we manually reviewed the contents of these articles.
Results
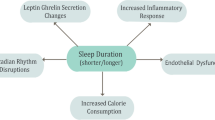
Bedtime, shift work, sleep duration, daytime sleep, and sleep quality can be considered as important factors in human health. All of this indicates the importance of circadian rhythms in the body. Given the accumulating evidence for profound effects of sleep pattern, one important area of investigation is to scrutinize its roles in weight, body composition, appetite, and metabolic variables modulation. There are also several mechanisms for expressing these effects.
Conclusion
There is ample evidence to suggest that enough sleep and early bedtime exert several beneficial effects on health. In contrast, insufficient and untimely sleep is known to cause obesity and metabolic disorders. In light of this, management of sleep has become a priority in recent years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sleep schedule is influenced by multiple factors such as homeostatic processes, environmental factors such as working hours and social factors. Sleep consists of two components including duration of sleep as the quantitative component, and the people reports of the depth and feeling of restfulness on awakening as the qualitative component [1]. An accumulating body of evidence suggests that sleep deprivation can lead to impaired glucose tolerance and increased appetite, blood pressure, and cardiovascular risk factors [2]. Furthermore, researchers believe that insufficient sleep duration causes neurological and physiological changes. According to the results of a longitudinal study, the amount of visceral fat in people whose sleep duration is less than 6 h and/or more than 9 h, is significantly higher than others [3]. It has also been revealed that the pathways of insulin secretion in people with insufficient sleep (4.5 h) are down regulated in comparison with those with adequate sleep (8.5 h) [4]. People with short and/or long sleep duration clearly show the components of the metabolic syndrome, and, therefore, it can be concluded that the relationship between sleep duration and metabolic syndrome is a U-shaped relation [5]. Imaging results from different areas of the brain show that sleep deprivation is associated with lower activity of appetite-sensitive brain areas in the frontal and insular cortex and higher amygdala activity. Sleep deprivation, even overnight, can affect energy expenditure and metabolism; As it reduces post-absorption energy expenditure and increases morning levels of ghrelin, cortisol, and norepinephrine [6, 7].
In addition to sleep quantity, poor sleep quality can also lead to impaired ability to think, concentrate, deal with stress, and weakened the immune system [3]. On the other hand, the prevalence of sleep disorders in Iranian adults has been reported between 15 and 42% and in parallel with that the overall prevalence of obesity and metabolic syndrome is on the rise [8]. Disturbance in the normal sleep pattern, both quantitatively and qualitatively, is known as sleep disorders. There are some types of sleep disorders. One of the major types of sleep disorders is circadian rhythm disorders. It refers to problems with the sleep–wake cycle, so that make people unable to sleep and wake at the right times [9]. Since about a third of a person's life is spent in sleep, sleep disorders can threaten people's health, both directly and indirectly [3].
Given the evidence that poor sleep quality and sleep deprivation are all associated with the risk of obesity, some researchers suggest that modifying sleep disorders, both quantitatively and qualitatively, can regulate the body's central clock system and help with weight management [10].
Studies have not yet reached the definitive conclusion about body metabolic responses to the sleep patterns and research is ongoing. Accordingly, the content of this review provides a summary on the current body of knowledge on the differential effects of quality and quantity of sleep on anthropometric parameters, body composition, serum lipid and glycemic profile. It also refers to the putative mechanisms underlying this association and reflects the controversies on this topic.
2 Association Between Sleep Pattern and Anthropometric Indicators
Poor sleep quality alters the circadian rhythm, which increases body fat mass through various abnormalities such as eating disorders and hormonal imbalance [11]. On the other hand, sleep deprivation can impair hormone regulating sleep such as orexin, monoamine, acetylcholine, and GABA and melatonin [12,13,14]. In support of this, some studies have reported an association between sleep deficiency and higher fat mass in middle-aged people [15,16,17], adolescents [16], and students [17]. Various mechanisms may explain the association between poor sleep quality with increased waist circumference, weight gain, and obesity [18, 19]. Previous studies have shown that both acute and chronic sleep deprivation and poor sleep quality can lower serum leptin levels and increase ghrelin levels [20, 21] [22]. Leptin, reduces food intake and increases energy expenditure, while ghrelin, as a strong appetite stimulant, lead to polyphagia [23]. So, changes in serum level of leptin and ghrelin induced by sleep disorders, lead to obesity [24]. Leptin levels rise during sleep [25,26,27]. One of the reason for this elevation is leptin response to daytime meal ingestion [26]. On the other hand, sleep deprivation increases the activity of sympathetic nervous system and activation of sympathetic nervous system, which in turn, inhibits leptin release [28]. So, it is possible that sleep deprivation leads to decrease in leptin levels. So, sleep plays an important role in energy balance. In a study published in 2018, Jurado-Fasoli and colleagues examined the relationship between sleep quality, anthropometric parameters, and body composition in sedentary middle-aged people. Their results revealed that sleep quality score (based on Pittsburgh sleep quality index or PSQI questionnaire) is inversely related to lean body mass and directly related to fat mass [29]. Regarding the effect of poor sleep quality on the reduction of muscle mass, several mechanisms can be proposed. For instance, increased secretion of cortisol as a catabolic hormone [30], which is known as a wasting stimulant and inhibitor of muscle protein synthesis [31]; disruption of the physiological rhythm of the anabolic hormone testosterone and the consequent decrease in muscle synthesis; alteration in the peak of growth hormone secretion, as an anabolic hormone [32]; decreased the concentration of insulin-like growth factor-1 (IGF-1) [22], which plays a crucial role in stimulating the synthesis of muscle proteins [33]; increased proinflammatory cytokines [32], which leads to muscle atrophy [33]; and increased insulin resistance [34], which is a limiting factor in protein synthesis in muscle cells [35]. In addition, sleep disorders affect neurohormones, increase calorie intake and cause fatigue [36, 37]. All of these factors may lead to muscle mass reduction. Furthermore, some researchers claim that in an environment where food is readily available, insufficient sleep can be a way to increase the chance of eating and, consequently, weight gain, especially if most of waking hours spend in a sedentary state like watching TV with a snack [38]. So, disorders in sleep patterns may change the body composition, in favor of accumulation of fat mass.
3 Association Between Sleep Pattern and Metabolic Factors
Most studies in this area have been performed on patients with diabetes or other chronic diseases [39,40,41]. For example, Byberg and colleagues in 2012, examined the association between quantity and quality of sleep with glucose homeostasis alterations. According to the results, each hour increase in sleep duration was associated with a 0.3% decrease in HbA1c and a 25% reduction in the risk of impaired glucose regulation. In addition, every 1-point increase in sleep quality was associated with a 2% increase in insulin sensitivity index (0.120) [42]. One of the important mechanisms is that short sleep duration along with oxidative stress increases inflammation by inducing inflammatory mediators such as C-reactive protein (CRP), increases cortisol, activates the sympathetic system, and raises catecholamine levels [43]. Sleep restriction leads to insulin resistance due to increased levels of free fatty acids and subsequent inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-17, and CRP [44]. According to the Whitehall II study, each hour of sleep deprivation per night causes an average of 8.1% increase in CRP and 4.5% increase in IL-6. These results are averaged across measures both at baseline and after 5 years of follow-up [45]. Based on what was observed in experimentally sleep-restricted women, another possible mechanism by which sleep deprivation induces insulin resistance is a decrease in levels of glucagon-like peptide-1, which in turn increases glucose-induced insulin secretion following nutrients ingestion [46].
Table 1 shows some studies investigating the association of sleep quantity and quality with anthropometric and metabolic factors in different population.
4 Association Between Bedtime, Meal Time, Circadian Rhythm and Weight Changes, and Metabolic Factors
Both metabolic and hedonic mechanisms regulate body weight [58]. Sleeping late leads to a hedonic overeating in which hyperphagia is mainly due to the response to food stimuli and exposure to an obesogenic environment not a disturbance in satiety signals. To obviate this problem, in addition to modifying sleep time, the environmental factors such as food availability and accessibility should be controlled and behavioral training should be addressed [59].
Although several studies have reported an association between bedtime and obesity [60,61,62], the effect of bedtime on the body's circadian system is not yet well understood and there is still no consensus [63]. For example, a 2019 study by Mi et al. found no significant relationship between sleeping and waking hours with circadian rhythm [47]. Circadian rhythm refers to rhythmic fluctuations in physiological processes that enable living organisms to adapt to the environment and optimize their metabolism [64]. However, some researches have shown that untimely eating can cause an imbalance between the circadian rhythm and peripheral clock, and disrupt the body's normal metabolism. Animal studies show that consuming a high-fat diet during the inactive (dark) phase leads to a significant weight gain compared to consuming the same amount of food in the active phase (light). Fat mass gain was significantly higher in mice fed a high-fat diet during the rest phase in comparison with mice fed HFDs during the active phase [65]. Consistent with these results, another study showed that fat mass gain was higher in mice that fed normal chow during the rest phase than in those that fed during active phase [66]. The possible reason for these observations is that the imbalance of energy intake and energy expenditure results in deleterious metabolic effects of the rest phase TRF [65,66,67]. In fact, not only what is consumed, but also when consumed can determine body weight. The results of a study by Laermans et al. show that the rate of weight loss in obese and overweight people who eat lunch later is significantly lower than people who eat lunch earlier. This is while they have similar calorie intake and energy expenditure, sleep duration and appetite hormone levels [10]. In 2013, Garaulet et al. examined the effect of meal time on weight loss in 420 adults with a mean age of 42 years and an average BMI of 31.4 kg/m2 following a 20-week weight loss program. Participants were divided into two groups: early consumers (before 3 o'clock) and late consumers (after 3 o'clock) based on lunch time. The amount of energy intake and energy expenditure, the level of appetite hormones and the duration of sleep were determined. Findings of this study showed that people who ate their lunch later lost less weight and their weight loss rate was slower in these 20 weeks (p = 0.002). Energy intake, diet composition, appetite-related hormone levels and sleep duration were similar between the two groups. Participants in the late-consumer group had lower-calorie breakfasts and were more likely to skip breakfast. Overall, this study showed that eating lunch late may reduce weight loss success. Therefore, new therapeutical approaches should not only focus on calorie intake, but also on meal time [68].
The association between abnormal short-term feeding with peripheral clock disorders resulting in obesity, hyperphagia, physical inactivity, and metabolic disorders was studied by Yukiyasumoto et al. The variables were compared between mice fed only during the rest phase and mice fed only during the active phase. The diets of both groups were similar and high in fat. After 1 week, it was found that overeating and weight gain were more common in mice fed only during the rest phase than in mice that fed only in the active phase and showed a more significant increase in serum leptin levels which indicates leptin resistance. Secretion of neuropeptide Y and agouti-dependent protein were also significantly higher in inactive phase mice. Serum insulin peak was higher in inactive phase mice, although the two groups did not express significant differences in insulin resistance and HOMA-IR index. Increased expression of lipogenic genes including Scd1, Acaca and Fasn in inactive phase mice prompted hepatic free fatty acids, triglycerides and cholesterol accumulation. Overall, the results of this experiment showed that eating in the inactive phase causes obesity, overeating, leptin resistance, metabolic disorders, and hepatic steatosis which all are due to inconsistency with the body clock [69]. Some researches show that calorie intake after 8 pm, after adjusting the effect of bedtime and sleep duration, is still associated with a higher BMI [70]. Some researchers also claim that night shift workers have significantly higher post-absorption-glucose, insulin, triglycerides, and fat mass and lower insulin sensitivity [71]. This suggests that food provides metabolic adaptation only if consumed in the active phase. Although the mechanisms of the relationship between untimely eating habit and obesity are not yet well understood, one possible mechanism may be related to changes in serum leptin levels. This means that untimely eating leads to overeating, which is partly due to the malfunctioning of appetite regulation systems. Just as the body's circadian clock controls the expression of the leptin gene as an appetite suppressant hormone, disruption of the circadian rhythm leads to a decrease in serum leptin throughout the day. The body's circadian rhythms, metabolism, and nutrition are closely intertwined [66, 72]. The major genes expressed in adipose tissue follow a circadian rhythmic pattern. Therefore, observing circadian order in the expression pattern of these genes has a significant effect on adipose tissue [68]. Therefore, eating according to this rhythm has a significant effect on adipose tissue by regulating the expression of these genes. Overall, understanding the potential physiological mechanisms involved in the development of metabolic disorders resulting from untimely eating is critical to providing effective therapeutical approaches.
5 Conclusions
As a whole, proper and timely sleep, with sufficient duration and quality has significant effects on health. This is one of the ways to achieve and maintain a healthy weight and body composition and reduce metabolic risk factors such as lipid and glycemic profile disorders. It is worth noting that oversleep and under sleep both increase the risk of metabolic syndrome. Taking into consideration the health benefits, it is recommended that adequate and timely sleep be considered. The major rationale for changing a person’s sleep pattern is to blunt manifestations of the metabolic abnormalities. Lifestyle modification is the first step in improving the quantity and quality of sleep. Having a proper schedule for daily activities, postponing work to early morning instead of late night, meditating before bed, eating early dinner and sleeping at a certain time can help improve the quantity and quality of sleep and thus reduce the risk of metabolic disorders. Information provided in this review, can have significant clinical implications to human health and behavioral therapies for the prevention and treatment of obesity and metabolic abnormalities. Further studies are required, however, to determine the precise mechanisms involved in the effects of sleep pattern on body composition, energy metabolism, and metabolic syndrome.
References
Lemma S, Gelaye B, Berhane Y, et al. Sleep quality and its psychological correlates among university students in Ethiopia: a cross-sectional study. BMC Psychiatry. 2012;12:237.
Paine S, Gander P, Travier N. The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years). J Biol Rhythms. 2006;21(1):68–76.
Barceló A, Barbé F, Llompart E, de la Peña M, Durán-Cantolla J, Ladaria A, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171(2):183–7.
Chaput JP, Bouchard C, Tremblay A. Change in sleep duration and visceral fat accumulation over 6 years in adults. Obesity (Silver Spring, Md). 2014;22(5):E9-12.
Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–57.
Ohkuma T, Fujii H, Iwase M, Ogata-Kaizu S, Ide H, Kikuchi Y, et al. U-shaped association of sleep duration with metabolic syndrome and insulin resistance in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Metabolism: Clin Experimental. 2014;63(4):484–91.
Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93(6):1229–36.
Mokarrar MH, Afsharmanesh A, Afshari M, Mohammadi F. Prevalence of sleep disorder among medical students in an Eastern University in Iran. Iran-J-Health-Sci. 2017;5(1):49–54.
Sleep Disorders. https://medlineplus.gov/sleepdisorders.html. Page last updated: 2021
Laermans J, Depoortere I. Chronobesity: role of the circadian system in the obesity epidemic. Obesity Rev. 2016;17(2):108–25.
Potter GDM, Skene DJ, Arendt J, Cade JE, Grant PJ, Hardie LJ. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. 2016;37(6):584–608.
Chittora R, Jain A, Suhalka P, Sharma C, Jaiswal N, Bhatnagar M. Sleep deprivation: neural regulation and consequences. Sleep Biol Rhythms. 2015;13(3):210–8.
Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81.
Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9(1):11–24.
Rahe C, Czira ME, Teismann H, Berger K. Associations between poor sleep quality and different measures of obesity. Sleep Med. 2015;16(10):1225–8.
Ferranti R, Marventano S, Castellano S, Giogianni G, Nolfo F, Rametta S, et al. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Science. 2016;9(2):117–22.
Kahlhöfer J, Karschin J, Breusing N, Bosy-Westphal A. Relationship between actigraphy-assessed sleep quality and fat mass in college students. Obesity (Silver Spring, Md). 2016;24(2):335–41.
Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers AK, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. 2017;1(4):00019.
Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166(16):1775–82.
Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15(9):851–4.
Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–50.
Banks S, Dinges D. Behavioral and physiological consequences of sleep restriction. JCSM. 2007;3(5):519–28.
Flier JS, Maratos-Flier E. The stomach speaks–ghrelin and weight regulation. N Engl J Med. 2002;346(21):1662–3.
Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62.
Sinha M, Ohannesian J, Heiman M, Kriauciunas A, Stephens T, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–7.
Schoeller D, Cella L, Sinha M, Caro JG. Entrainment of the diurnal rhythm of plasma leptin to meal timin. J Clin Invest. 1997;100:1882–7.
Simon C, Gronfier C, Schlienger J, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–9.
Rayner D, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20.
Jurado-Fasoli L, Amaro-Gahete FJ, De-la OA, Dote-Montero M, Gutiérrez Á, Castillo MJ. Association between sleep quality and body composition in sedentary middle-aged adults. Medicina (Kaunas). 2018;54(5):91.
Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91(1):36–41.
Peeters GM, van Schoor NM, van Rossum EF, Visser M, Lips P. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol. 2008;69(4):673–82.
Banks S, Dinges D. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–28.
Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 2008;23:160–70.
Pyykkönen A, Isomaa B, Pesonen A, Eriksson J, Groop L, Tuomi T, et al. Subjective sleep complaints are associated with insulin resistance in individuals without diabetes: the PPP-Botnia Study. Diabetes Care. 2012;35(11):2271–8.
Gordon B, Kelleher A, Kimball S. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol. 2013;45(10):2147–57.
Patel S, Malhotra A, White D, Gottlieb D, Hu F. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–54.
Weinger M, Ancoli-Israel S. Sleep deprivation and clinical performance. JAMA. 2002;287(8):955–7.
Sivak M. Sleeping more as a way to lose weight. Obesity Rev. 2006;7(3):295–6.
Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50(11):2298–304.
Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–7.
Tare A, Lane JM, Cade BE, Grant SF, Chen TH, Punjabi NM, et al. Sleep duration does not mediate or modify association of common genetic variants with type 2 diabetes. Diabetologia. 2014;57(2):339–46.
Gozashti MHM, Eslami NM, Radfar MHM, Pakmanesh HM. Sleep pattern, duration and quality in relation with glycemic control in people with type 2 diabetes mellitus. Iran J Med Sci. 2016;41(6):531–8.
Koren D, Dumin M, Gozal D. Role of sleep quality in the metabolic syndrome. Diabetes Metab Syndr Obes. 2016;9:281–310.
Donga E, van Dijk M, van Dijk J, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95(6):2963–8.
Ferrie J, Kivimaki M, Akbaraly T, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178(6):956–61.
St-Onge M, O’Keeffe M, Roberts A, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–10.
Mi SJ, Kelly NR, Brychta RJ, Grammer AC, Jaramillo M, Chen KY, et al. Associations of sleep patterns with metabolic syndrome indices, body composition, and energy intake in children and adolescents. Pediatr Obes. 2019;14(6):e12507.
Kim K, Shin D, Jung G, Lee D, Park S. Association between sleep duration, fat mass, lean mass and obesity in Korean adults: the fourth and fifth Korea National Health and Nutrition Examination Surveys. J Sleep Res. 2017;26(4):453–60.
Lin P, Chang K-T, Lin Y-A, Tzeng I, Chuang H-H, Chen J-Y. Association between self-reported sleep duration and serum lipid profile in a middle-aged and elderly population in Taiwan: a community-based, cross-sectional study. BMJ. 2017;7(10):e015964.
Baoying H, Hongjie C, Changsheng Q, Peijian W, Qingfei L, Yinghua L, et al. Association of napping and night-time sleep with impaired glucose regulation, insulin resistance and glycated haemoglobin in Chinese middle-aged adults with no diabetes: a cross-sectional study. BMJ Open. 2014;4(7):e004419.
Ford E, Li C, Wheaton A, Chapman D, Perry G, Croft J. Sleep duration and body mass index and waist circumference among US adults. Obesity (Silver Spring, Md). 2014;22(2):598–607.
Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31(5):645–52.
Geovanini G, Lorenzi-Filho G, de Paula L, Oliveira C, de Oliveira AR, Beijamini F, et al. Poor sleep quality and lipid profile in a rural cohort (The Baependi Heart Study). Sleep Med Disord. 2019;57:30–5.
Barikani A, Javadi M, Rafiei S. Sleep quality and blood lipid composition among patients with diabetes. Int J Endocrinol Metab. 2019;17(3):81062.
Khorasani M, Mohammadpoorasl A, Javadi M. The association between sleep quality and metabolic factors and anthropometric measurements. Biotechnol Health Sci. 2016;3(4):25–31.
Zhu B-Q, Li X-M, Wang D, Yu X-F. Sleep quality and its impact on glycaemic control in patients with type 2 diabetes mellitus. Int J Nurs Sci. 2014;1(3):260–5.
Jennings J, Muldoon M, Hall M, Buysse D, Manuck S. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–23.
Yu YH, Vasselli JR, Zhang Y, Mechanick JI, Korner J, Peterli R. Metabolic vs hedonic obesity: a conceptual distinction and its clinical implications. Obesity Rev. 2015;16(3):234–47.
Chaput J-P, St-Onge M-P. Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation? Front Endocrinol (Lausanne). 2014;5:116.
Kim RH, Kim KI, Kim JH, Park YS. Association between sleep duration and body composition measures in Korean Adults: The Korea National Health and Nutrition Examination Survey 2010. Korean J Fam Med. 2018;39(4):219–24.
Garaulet M, Esteban Tardito A, Lee Y, Smith C, Parnell L, Ordovas J. SIRT1 and CLOCK 3111T>C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int J Obes. 2005;2012(36):1436–41.
Scott E, Carter A, Grant P. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes. 2005;2008(32):658–62.
Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, et al. Meal timing regulates the human circadian system. Current Biol. 2017;27(12):1768-75.e3.
Tayfun Güldür T, Otlu G. Circadian rhythm in mammals: time to eat & time to sleep. Biol Rhythm Res. 2017;48(2):243–61.
Arble D, Bass J, Laposky A, et al. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring, Md). 2009;17(11):2100–2.
Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes. 2013;37(6):843–52.
Olsen MK, Choi MH, Kulseng B, Zhao C-M, Chen D. Time-restricted feeding on weekdays restricts weight gain: a study using rat models of high-fat diet-induced obesity. Physiol Behav. 2017;173:298–304.
Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–11.
Yasumoto Y, Hashimoto C, Nakao R, Yamazaki H, Hiroyama H, Nemoto T, et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism. 2016;65(5):714–27.
Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–9.
Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171(3):557–64.
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–61.
Author information
Authors and Affiliations
Contributions
ZY collaborated in searching and writing the manuscript. SR designed the study, wrote and revised the manuscript. Both authors, approved the version to be published. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical Approval
Ethical approval is not required for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yari, Z., Rabiei, S. Association Between Sleep Pattern, Anthropometric Indicators, and Metabolic Risk Factors. Sleep Vigilance 6, 7–13 (2022). https://doi.org/10.1007/s41782-021-00177-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41782-021-00177-x