Abstract
Purpose of Review
Herein, we review the role of FXR and TGR5 in the regulation of hepatic bile acid metabolism, with a focus on how our understanding of bile acid metabolic regulation by these receptors has evolved in recent years and how this improved understanding may facilitate targeting bile acids for type 2 diabetes treatment.
Recent Findings
Bile acid profile is a key regulator of metabolic homeostasis. Inhibition of expression of the enzyme that is required for cholic acid synthesis and thus determines bile acid profile, Cyp8b1, may be an effective target for type 2 diabetes treatment. FXR and, more recently, TGR5 have been shown to regulate bile acid metabolism and Cyp8b1 expression and, therefore, may provide a mechanism with which to target bile acid profile for type 2 diabetes treatment.
Summary
Inhibition of Cyp8b1 expression is a promising therapeutic modality for type 2 diabetes; however, further work is needed to fully understand the pathways regulating Cyp8b1 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bile acids are amphipathic steroid molecules synthesized in the liver from cholesterol. Bile acids were originally thought to simply aid in the digestion and absorption of dietary lipid in the small intestine [1]; however, work over the past several decades demonstrates that bile acid metabolism and signaling are key contributors to metabolic regulation and thus are promising therapeutic targets for metabolic diseases [2]. This review will summarize our current understanding of the role of bile acid metabolism and signaling in glucose regulation and will identify the knowledge gaps that need to be addressed to enable successful targeting of bile acid signaling and metabolism for diabetes treatment.
Changes in circulating bile acid profile have been implicated in the pathogenesis of insulin resistance and type 2 diabetes (T2D) [3]. Different bile acid subtypes exhibit varying degrees of hydrophobicity which is determined by factors such as state of ionization and by the number, position, and orientation of hydroxyl groups [4]. The relative amounts of hydrophobic versus hydrophilic bile acids determine the overall hydrophobicity of the bile acid pool [4]. T2D is associated with an increase in the hydrophobicity of the circulating bile acid pool in humans [3]. Consistent with this, hydrophilic bile acid subtypes, such as tauroursodeoxycholic acid (TUDCA), have been shown to protect against inflammation and improve insulin sensitivity in rodent models and patients with T2D [5,6,7]. In contrast, hydrophobic bile acid subtypes, such as deoxycholic acid (DCA), have been shown to promote inflammation and endoplasmic reticulum stress that are associated with impaired glucose regulation [8,9,10]. These data suggest that altering bile acid profiles may be an effective approach for the treatment of T2D.
Sterol 12-α-hydroxylase (CYP8B1) is a bile acid synthetic enzyme expressed primarily in hepatocytes [1]. CYP8B1 is required for the synthesis of 12-α-hydroxylated bile acids and thereby determines systemic bile acid profiles [1]. Genetic ablation of Cyp8b1 decreases bile acid profile hydrophobicity and protects against several metabolic diseases, including obesity and T2D, in mice [11,12,13,14,15]. Furthermore, genetic ablation of Cyp8b1 in ApoE−/− mice reduces development of atherosclerotic plaque [14]. Moreover, diabetic cholesterol-fed Cyp8b1−/− mice are protected against hypercholesterolemia and cholelithiasis [15]. Finally, Cyp8b1 null mice are resistant to fatty liver development [13]. These findings suggest that Cyp8b1 may be an effective target for the treatment of T2D and many of its associated comorbidities.
Several different bile acid receptors have been implicated in metabolic regulation, including the nuclear receptor farnesoid X receptor (FXR) and the transmembrane G protein-coupled receptor, TGR5. Activation of these receptors regulates bile acid, cholesterol, lipid, and glucose metabolism and plays a role in various pathological processes, including inflammation, fibrosis, and carcinogenesis. While the mechanisms by which these receptors regulate metabolic homeostasis are incompletely understood, the impact of FXR and TGR5 is likely mediated, at least in part, via bile acid metabolism [16•, 17•, 18].
Key Steps in Bile Acid Metabolism
Primary bile acids are synthesized in the liver and then converted into secondary bile acids through interactions with the gut microbiota. The biosynthesis of primary bile acids in the liver involves a series of enzymatic reactions in which the cholesterol ring is modified; the side chain is shortened and conjugated [19] with either glycine or taurine [20]. Bile acids are stored in the gallbladder and secreted into the gastrointestinal tract in response to feeding. Primary bile acids are deconjugated and dehydroxylated in the distal intestinal lumen by gut microbes to generate the secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA) [21]. The most abundant primary bile acids in humans are cholic acid (CA) and chenodeoxycholic acid (CDCA). In rodents, the majority of CDCA is converted to muricholic acid (MCA) [22]. While a primary bile acid subtype in bears [23], UDCA is derived from CDCA in humans and rodents through gut microbial modifications [24, 25]. Detailed descriptions of the enzymes and intermediates involved in bile acid metabolism can be found in several excellent review articles [1, 19]. We provide a summary of this complex process below.
Bile acid synthesis can occur through two pathways: the classic (neutral) and alternative (acidic) pathways. The classic pathway, which occurs in the liver, accounts for the majority of bile acid synthesis [26]. Cholesterol 7-α-hydroxylase (CYP7A1) is the rate-limiting enzyme in this pathway, while CYP8B1 determines bile acid profile [27]. In the alternative pathway, cholesterol is oxidized by sterol-27-hydroxylase (CYP27A1), the rate-limiting enzyme in this pathway, followed by 7-α-hydroxylation of the oxysterol intermediates by oxysterol 7-α-hydroxylase (CYP7B1) [28, 29]. CYP27A1 and CYP7B1 are expressed in the liver and in various extrahepatic sites including vascular endothelium and macrophages [30,31,32,33,34]. Bile acid synthesis is tightly controlled to ensure the maintenance of a healthy total bile acid pool, with approximately 95% of bile acids being recycled. FXR is considered a primary regulator of this homeostatic process; however, research increasingly supports a role for TGR5 as well.
Regulation of Glucose Homeostasis and Hepatic Bile Acid Metabolism by FXR
FXR is a key regulator of hepatic bile acid metabolism [35] that plays a role in a various disease processes, including inflammatory bowel disease, colorectal cancer, and T2D [36,37,38]. FXR, which is highly expressed in the liver and gastrointestinal tract, can be activated by both free and conjugated bile acids, with CDCA having the highest affinity. The order of potency of bile acid subtypes for FXR is CDCA>LCA=DCA>CA [39, 40]. However, hydrophilic bile acids, such as UDCA and MCA, cannot activate FXR [41], and tauro-conjugated β- and α-MCA may actually serve as naturally occurring FXR antagonists [42,43,44]. More recent work in humans treated with UDCA for 3 weeks prior to bariatric surgery suggested that UDCA might also exert antagonistic effects on FXR [45].
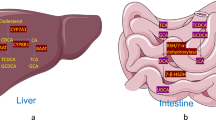
FXR is a key regulator of glucose and lipid metabolism [36, 46,47,48,49,50,51]. FXR improves glycemic control by stimulating insulin secretion from pancreatic β-cells [52], enhancing adipocyte insulin sensitivity [46] and inhibiting hepatic gluconeogenesis (Fig. 1) [53]. However, genetic ablation of FXR and FXR antagonism improve glucose regulation and liver lipid deposition [54, 55]. Furthermore, while Trabelsi et al. report that FXR inhibits secretion of the incretin hormone, glucagon-like peptide-1 (GLP-1), from intestinal L cells [56], Pathak et al. report that L cell FXR signaling increases GLP-1 secretion [17•]. Together, these conflicting reports on the role of FXR in glucose regulation suggest that further work is needed to fully understand the glucoregulatory function of FXR.
Glucoregulatory effects of FXR and TGR5. FXR-mediated glucoregulatory effects in various tissues are illustrated with purple arrows and TGR5-mediated pathways are illustrated with green arrows. Farnesoid X receptor (FXR), transmembrane G-coupled protein receptor (TGR5), glucagon-like peptide-1 (GLP-1)
Bile acid-mediated activation of FXR suppresses bile acid synthesis in a homeostatic feedback loop. Specifically, hepatocyte FXR activation upregulates small heterodimer partner (SHP), which inhibits the transcription factors hepatic nuclear factor 4α (HNF4α) and liver receptor homolog-1 (LRH-1), thus reducing their binding to the bile acid response element in the Cyp7a1 and Cyp8b1 gene promoters and inhibiting transcription of these genes [57,58,59,60,61,62,63,64,65,66]. Activation of FXR in the intestine induces fibroblast growth factor 15 (FGF15 or human orthologue FGF19) secretion, ultimately activating hepatocyte FGF receptor 4 (FGFR4) and mitogen-activated protein kinase (MAPK)/extracellular receptor kinase 1/2 (ERK1/2) [67,68,69,70]. Activation of FXR downregulates Cyp8b1 expression by upregulating SHP and FGF15/19 levels [71, 72]. However, work in tissue-specific FXR knockout mice demonstrates that treatment with an FXR agonist in mice with loss of intestinal FXR, but intact hepatocyte FXR, results in decreased Cyp8b1 expression, but not Cyp7a1 expression [69, 73•]. In contrast, treatment with an FXR agonist in mice with loss of hepatic FXR, but intact intestinal FXR, results in downregulation of both Cyp8b1 and Cyp7a1 expressions [69, 73•]. These data suggest that hepatic FXR signaling is important for suppressing Cyp8b1 expression, while intestinal FXR signaling is critical for downregulating both Cyp7a1 and Cyp8b1 expressions [69, 73•].
While FXR signaling through SHP and FGF15/19 is a critical contributor to FXR-mediated regulation of bile acid metabolism, several recent studies reveal new FXR-mediated pathways (Fig. 2, dashed lines), suggesting that there is still much to learn about FXR regulation of bile acid metabolism. For example, MAFG has been identified as an FXR target gene that transcriptionally represses Cyp8b1 expression in mice [74]. While FXR is thought to primarily exert its effects on Cyp7a1 and Cyp8b1 expressions through transcriptional pathways, recent studies have identified important FXR-dependent post-transcriptional regulators. In particular, Tarling et al. reported that FXR activation upregulates the expression of the RNA-binding protein, ZFP36L1, to rapidly degrade Cyp7a1 mRNA [75•]. ZFP36L1-dependent regulation of bile acid metabolism may also contribute to obesity, as hepatocyte-specific Zfg36l1−/− mice are resistant to diet-induced obesity [75•]. Moreover, microRNAs (miRs), such as miR-144 [76] and miR-122a [77], may be downstream mediators of FXR action. For example, Li et al. found that miR-33a is induced in a mouse model of elevated hepatic bile acid synthesis (mice overexpressing Cyp7a1) to act as a homeostatic regulator inhibiting Cyp7a1 and Cyp8b1 mRNA expressions in response to elevated hepatic bile acid synthesis [78]. Moreover, FXR activation with GW4064 induces expression of miR-122a [77], a liver-specific microRNA. MiR-122a overexpression decreases Cyp7a1, but not Cyp8b1, mRNA levels [77]. The identification of microRNAs that regulate bile acid metabolism provides a potentially viable approach to target bile acid metabolism for the treatment of T2D, as microRNAs have already been successfully applied to the treatment of liver disease in humans [79, 80]. Overall, understanding the post-transcriptional pathways involved in regulation of bile acid metabolism will be critical in rationale design of bile acid-based pharmaceutical strategies for treating metabolic disease [81,82,83].
Established and emerging pathways involved in the regulation of Cyp7a1 and Cyp8b1 expressions by TGR5 and FXR. Established pathways are illustrated with solid arrows, and newly identified regulators of Cyp7a1 and Cyp8b1 expression are illustrated with dashed arrows. Regulators of Cyp7a1 expression are illustrated with blue arrows and regulators of Cyp8b1 expression are illustrated with red arrows. Farnesoid X receptor (FXR), transmembrane G-coupled protein receptor (TGR5), Cholesterol 7-α hydroxylase (Cyp7a1), sterol 12-alpha-hydroxylase (Cyp8b1), sterol 27 hydroxylase (Cyp27a1), oxysterol 7-α hydroxylase (Cyp7b1), cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), fibroblast growth factor 19 (FGF19), FGF receptor 4 (FGFR4), small heterodimer partner (SHP)
FXR shows promise as a target for the treatment of metabolic and inflammatory disorders including T2D, primary biliary cirrhosis, nonalcoholic fatty liver disease (NAFLD), and nonalcoholic steatohepatitis [84,85,86,87,88]. In fact, an FXR agonist, obeticholic acid (Ocaliva©), has been approved by the Food and Drug Administration (FDA) to treat the rare liver disease, primary biliary cholangitis. However, FXR activation suppresses both Cyp7a1 and Cyp8b1 expressions in tandem, which can have detrimental effects on lipid metabolism as Cyp7a1 is the rate-limiting enzyme in bile acid synthesis. Specifically, inhibition of Cyp7a1 expression decreases the conversion of cholesterol to bile acids, resulting in lipid dysregulation [89, 90]. Therefore, FXR signaling has been reported to produce off-target effects, such as dyslipidemia [54, 91, 92]. For example, mice with targeted deletion of Cyp7a1 develop hypercholesterolemia and have an increased risk of developing atherosclerosis [89]. A marked decrease in bile acid synthesis and decreased Cyp7a1 expression are also associated with dyslipidemia and cholelithiasis [93, 94]. Thus, an improved understanding of the selective regulation of Cyp8b1 expression may lead to the development of bile acid-based therapeutics with fewer adverse side effects.
Regulation of Glucose Homeostasis and Hepatic Bile Acid Metabolism by TGR5
TGR5 is a transmembrane G protein-coupled receptor [95] that is ubiquitously expressed throughout the body, including endocrine glands, adipocytes, muscle, liver, and the gastrointestinal tract [96,97,98]. In the liver, TGR5 is present on liver sinusoidal endothelial cells [99], cholangiocytes [100], biliary epithelial cells [101], and Kupffer cells [102]. It has been speculated that TGR5 is not expressed on hepatocytes; however, Yang et al. reported that TGR5 is expressed in a human hepatocellular carcinoma cell line [103], and recent work reveals that TGR5 is present in canine hepatocytes [104].
The binding affinity of various bile acids to TGR5 differs as compared to FXR [95, 96]. Unlike FXR, hydrophobic bile acids have the highest affinity for TGR5, with the following rank order of potency: LCA>DCA>CDC>CA>UDCA. TGR5 activation by bile acids and synthetic agonists results in activation of the adenylyl cyclase, which in turn activates protein kinase A (PKA) signaling pathways [96]. Moreover, cell-specific signaling pathways are also activated [99, 102, 105].
Increased TGR5 signaling improves glucose regulation through several tissue-specific effects [106, 107]. TGR5 signaling in gastrointestinal enteroendocrine L cells enhances secretion of GLP-1 [107]. Recent work reveals that L cell TGR5 is located on the basolateral L cell membrane [108, 109]. TGR5 signaling in immune cells decreases inflammatory cytokine secretion and TGR5 signaling on adipocytes increases energy expenditure by promoting the beiging of white adipose tissue in mice [110,111,112]. Therefore, TGR5 may exert its metabolic effects, in part, by decreasing inflammation and promoting mitochondrial biogenesis [96, 113, 114]. However, TGR5 activation leads to unwanted side effects, including pruritus [115, 116], cholesterol gallstone formation [117], and cholestasis [118, 119]. Together, these data suggest that identifying and targeting downstream effectors of TGR5 may be a more effective approach with less risk for adverse side effects than directly targeting TGR5.
Several studies point to an important role for TGR5 in bile acid metabolism and the maintenance of a healthy bile acid profile [17•, 120]. Indeed, Pean et al. reported that genetic ablation of TGR5 leads to increased hydrophobicity of the biliary, circulating, and hepatic bile acid pools and revealed a protective effect of TGR5 during liver regeneration in mice [18]. Donepudi et al. report similar shifts in the gallbladder bile acid profile in Tgr5−/− compared to Tgr5+/+ mice in free-fed and fasted conditions [120], in parallel with decreased Cyp7b1 and Cyp27a1 mRNA levels. Thus, TGR5 can upregulate the alternative pathway of bile acid synthesis, which likely contributes to TGR5-dependent decreases in bile acid profile hydrophobicity [120]. Pathak et al. reported that TGR5 is a downstream target of FXR that is required for the effect of L cell FXR signaling to promote GLP-1 secretion [17•]. Specifically, this study identified an FXR response element on the human TGR5 gene promoter, which is highly conserved in the mouse Tgr5 gene [17•]. Thus, interactions between TGR5 and FXR may have implications for hepatic bile acid metabolism; however, further work is needed to comprehensively define the underlying molecular mechanisms.
Role of Bile Acids in Glucoregulatory Improvement After Bariatric Surgery
Bariatric surgery, including Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG), is the most effective long-term treatment for obesity and results in high rates of T2D remission, even prior to weight loss [121, 122]. One well-established mechanism by which bariatric surgery improves glucose regulation is by increasing bile acid signaling [16•, 123, 124]. A reoccurring finding between vastly different bariatric procedures is increased circulating bile acid concentrations, in both humans and rodent models [16•, 124,125,126,127,128,129,130]. Therefore, bariatric surgery provides a useful model system with which to understand bile acid metabolism and how it may be targeted for T2D treatment.
The mechanisms by which bariatric surgery increases circulating bile acid concentrations remain incompletely defined, but appear to be dependent on anatomic re-arrangement of the gastrointestinal tract; Roux-en-Y gastric bypass, but not laparoscopic adjustable gastric banding, increases circulating bile acid concentrations in humans [131]. Furthermore, diverting bile to the distal gut increases circulating bile acid concentrations and improves glucose regulation, suggesting an important role for the distal gastrointestinal tract [132, 133]. Finally, studies in rodents have suggested that increased bile acid reabsorption may contribute to increased circulating bile acid concentrations after bariatric surgery. One study reports an increase in ileal apical sodium–bile acid transporter (ASBT) expression after VSG in mice [134]. Other studies report gut hypertrophy after various types of bariatric surgery in rodents, which likely enhances gut absorptive capacity [134,135,136]. However, further work is needed to fully define the mechanisms responsible for increased circulating bile acid concentrations after bariatric surgery.
FXR and TGR5 Contribute to Improved Glucose Regulation After Bariatric Surgery
Several studies in whole body TGR5 and FXR knockout mouse models demonstrate that increased bile acid receptor signaling contributes to the metabolic benefits of bariatric surgery [16•, 123, 124]. Using a whole body FXR knockout mouse model, Ryan et al. reported that FXR contributes to body weight loss and improved glucose regulation after VSG [124]. More recently, TGR5 has been shown to contribute to improved glucose regulation after VSG in mice [16•, 123]. TGR5-dependent improvement in glycemic control after VSG was associated with a TGR5-dependent decrease in the hydrophobicity of the circulating bile acid pool. This beneficial bile acid profile shift was associated with a TGR5-dependent reduction in hepatic CYP8B1 protein expression, with no effect on hepatic CYP7A1 expression [16•]. Ding et al. confirmed that TGR5 contributes to the glucoregulatory benefits of VSG, in part due to increased GLP-1 secretion after VSG [123]. By contrast, McGavigan et al. did not observe a TGR5-dependent increase in GLP-1 secretion after VSG. These discrepancies could be a result of differences in surgical model, strain, and/or the timing of experiments. Notably, all of the published work assessing the role of TGR5 and FXR in the benefits of bariatric surgery has been performed in whole body knockout mouse models which are prone to development of compensatory pathways. Therefore, further work is needed in inducible and tissue-specific knockout models to gain a deeper understanding of the mechanisms by which TGR5 and FXR contribute to improved glucose regulation after bariatric surgery.
In contrast, the role of TGR5 in Roux-en-Y gastric bypass-mediated improvements in glucose regulation is unclear, with one study reporting that TGR5 does not contribute to weight loss or improved glucose regulation after Roux-en-Y gastric bypass in mice [137]. This suggests that VSG and Roux-en-Y gastric bypass activate different signaling pathways to exert their beneficial metabolic effects. However, Zhai et al. observed increased ileal TGR5 expression after Roux-en-Y gastric bypass in mice and present data to suggest that TGR5 contributes to elevated GLP-1 production after Roux-en-Y gastric bypass, although this was not directly assessed in vivo [138].
Overall, bariatric surgery increases bile acid concentrations to increase TGR5 and FXR signaling and improve glucose regulation. However, the exact molecular mechanisms and tissue site(s) of action by which enhanced TGR5 and FXR signaling improve glucose regulation after bariatric surgery are unknown.
Conclusion
Targeting bile acid profile by inhibiting Cyp8b1 expression is a promising therapeutic modality for T2D. The role of FXR in the regulation of bile acid synthesis and Cyp8b1 expression is well documented; however, recent work suggests that TGR5 may also regulate Cyp8b1 expression. Furthermore, recent studies reveal nontraditional pathways in the regulation of bile acid metabolism, including key post-transcriptional mechanisms. Overall, our understanding of the regulation of bile acid metabolism by FXR and TGR5 is rapidly evolving; however, there remain significant gaps in our understanding of these processes. In particular, it will be important to define the pathway(s) by which Cyp8b1 expression is selectively regulated to enable pharmaceutical targeting of this promising therapeutic target.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91. https://doi.org/10.1152/physrev.00010.2008.
Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7(8):678–93. https://doi.org/10.1038/nrd2619.
Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62(12):4184–91. https://doi.org/10.2337/db13-0639.
Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30(5):719–30.
Mahmoud AA, Elshazly SM. Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats. PLoS One. 2014;9(9):e106993. https://doi.org/10.1371/journal.pone.0106993.
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. https://doi.org/10.1126/science.1128294.
Shima KR, Ota T, Kato KI, Takeshita Y, Misu H, Kaneko S, et al. Ursodeoxycholic acid potentiates dipeptidyl peptidase-4 inhibitor sitagliptin by enhancing glucagon-like peptide-1 secretion in patients with type 2 diabetes and chronic liver disease: a pilot randomized controlled and add-on study. BMJ Open Diabetes Res Care. 2018;6(1):e000469. https://doi.org/10.1136/bmjdrc-2017-000469.
Cariou B, Chetiveaux M, Zair Y, Pouteau E, Disse E, Guyomarc'h-Delasalle B, et al. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond). 2011;8(1):48. https://doi.org/10.1186/1743-7075-8-48.
Chung SJ, Lee CH, Lee HS, Kim ST, Sohn UD, Park ES, et al. The role of phosphatidylcholine and deoxycholic acid in inflammation. Life Sci. 2014;108(2):88–93. https://doi.org/10.1016/j.lfs.2014.05.013.
Zaborska KE, Lee SA, Garribay D, Cha E, Cummings BP. Deoxycholic acid supplementation impairs glucose homeostasis in mice. PLoS One. 2018;13(7):e0200908. https://doi.org/10.1371/journal.pone.0200908.
Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, Chan RB, et al. Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. Am J Physiol Endocrinol Metab. 2017;313(2):E121–33. https://doi.org/10.1152/ajpendo.00409.2016.
Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, Schuster G, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110(8):1191–200. https://doi.org/10.1172/JCI16309.
Murphy C, Parini P, Wang J, Bjorkhem I, Eggertsen G, Gafvels M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochim Biophys Acta. 2005;1735(3):167–75. https://doi.org/10.1016/j.bbalip.2005.06.001.
Slatis K, Gafvels M, Kannisto K, Ovchinnikova O, Paulsson-Berne G, Parini P, et al. Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice. J Lipid Res. 2010;51(11):3289–98. https://doi.org/10.1194/jlr.M009308.
Wang J, Gafvels M, Rudling M, Murphy C, Bjorkhem I, Einarsson C, et al. Critical role of cholic acid for development of hypercholesterolemia and gallstones in diabetic mice. Biochem Biophys Res Commun. 2006;342(4):1382–8. https://doi.org/10.1016/j.bbrc.2006.02.108.
• McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, et al. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226–34. https://doi.org/10.1136/gutjnl-2015-309871 This study identifies a role for TGR5 in the regulation of hepatic CYP8B1 expression.
• Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292(26):11055–69. https://doi.org/10.1074/jbc.M117.784322 This study provides the first description of an interation between TGR5 and FXR.
Pean N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58(4):1451–60. https://doi.org/10.1002/hep.26463.
Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. https://doi.org/10.1146/annurev.biochem.72.121801.161712.
He D, Barnes S, Falany CN. Rat liver bile acid CoA:amino acid N-acyltransferase: expression, characterization, and peroxisomal localization. J Lipid Res. 2003;44(12):2242–9. https://doi.org/10.1194/jlr.M300128-JLR200.
Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–8. https://doi.org/10.1097/MOG.0000000000000057.
Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51(11):3230–42. https://doi.org/10.1194/jlr.M007641.
Hagey LR, Crombie DL, Espinosa E, Carey MC, Igimi H, Hofmann AF. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J Lipid Res. 1993;34(11):1911–7.
Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. part I. Dig Dis Sci. 1982;27(8):737–61.
Fedorowski T, Salen G, Tint GS, Mosbach E. Transformation of chenodeoxycholic acid and ursodeoxycholic acid by human intestinal bacteria. Gastroenterology. 1979;77(5):1068–73.
Ferdinandusse S, Houten SM. Peroxisomes and bile acid biosynthesis. Biochim Biophys Acta. 2006;1763(12):1427–40. https://doi.org/10.1016/j.bbamcr.2006.09.001.
Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–93.
Norlin M, Wikvall K. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med. 2007;7(2):199–218.
Wu Z, Martin KO, Javitt NB, Chiang JY. Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs and gene CYP7B1. J Lipid Res. 1999;40(12):2195–203.
Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264(14):8222–9.
Preuss I, Ludwig MG, Baumgarten B, Bassilana F, Gessier F, Seuwen K, et al. Transcriptional regulation and functional characterization of the oxysterol/EBI2 system in primary human macrophages. Biochem Biophys Res Commun. 2014;446(3):663–8. https://doi.org/10.1016/j.bbrc.2014.01.069.
Reiss AB, Martin KO, Rojer DE, Iyer S, Grossi EA, Galloway AC, et al. Sterol 27-hydroxylase: expression in human arterial endothelium. J Lipid Res. 1997;38(6):1254–60.
Schwarz M, Lund EG, Lathe R, Bjorkhem I, Russell DW. Identification and characterization of a mouse oxysterol 7alpha-hydroxylase cDNA. J Biol Chem. 1997;272(38):23995–4001..
Shanahan CM, Carpenter KL, Cary NR. A potential role for sterol 27-hydroxylase in atherogenesis. Atherosclerosis. 2001;154(2):269–76.
Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–93.
Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51(4):771–84. https://doi.org/10.1194/jlr.M001602.
De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, et al. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci. 2004;49(6):982–9.
Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590(3):441–6. https://doi.org/10.1113/jphysiol.2011.222133.
Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5.
Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53.
Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol. 2013;86(11):1517–24. https://doi.org/10.1016/j.bcp.2013.08.015.
Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. https://doi.org/10.1038/ncomms3384.
Parseus A, Sommer N, Sommer F, Caesar R, Molinaro A, Stahlman M, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66(3):429–37. https://doi.org/10.1136/gutjnl-2015-310283.
Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–35. https://doi.org/10.1016/j.cmet.2013.01.003.
Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62(6):1398–404. https://doi.org/10.1016/j.jhep.2014.12.034.
Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281(16):11039–49. https://doi.org/10.1074/jbc.M510258200.
Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127(5):1497–512.
Fiorucci S, Rizzo G, Donini A, Distrutti E, Santucci L. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med. 2007;13(7):298–309. https://doi.org/10.1016/j.molmed.2007.06.001.
Mencarelli A, Renga B, D'Amore C, Santorelli C, Graziosi L, Bruno A, et al. Dissociation of intestinal and hepatic activities of FXR and LXRalpha supports metabolic effects of terminal ileum interposition in rodents. Diabetes. 2013;62(10):3384–93. https://doi.org/10.2337/db13-0299.
Swanson HI, Wada T, Xie W, Renga B, Zampella A, Distrutti E, et al. Role of nuclear receptors in lipid dysfunction and obesity-related diseases. Drug Metab Dispos. 2013;41(1):1–11. https://doi.org/10.1124/dmd.112.048694.
Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–11. https://doi.org/10.1073/pnas.0506982103.
Dufer M, Horth K, Wagner R, Schittenhelm B, Prowald S, Wagner TF, et al. Bile acids acutely stimulate insulin secretion of mouse beta-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61(6):1479–89. https://doi.org/10.2337/db11-0815.
Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13(6):729–38. https://doi.org/10.1016/j.cmet.2011.03.019.
Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125(1):386–402. https://doi.org/10.1172/JCI76738.
Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. https://doi.org/10.1038/ncomms10166.
Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. https://doi.org/10.1038/ncomms8629.
Becker-Andre M, Andre E, DeLamarter JF. Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun. 1993;194(3):1371–9.
Chiang JY, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275(15):10918–24.
Galarneau L, Pare JF, Allard D, Hamel D, Levesque L, Tugwood JD, et al. The alpha1-fetoprotein locus is activated by a nuclear receptor of the Drosophila FTZ-F1 family. Mol Cell Biol. 1996;16(7):3853–65.
Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26.
Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–15.
Nitta M, Ku S, Brown C, Okamoto AY, Shan B. CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7alpha-hydroxylase gene. Proc Natl Acad Sci U S A. 1999;96(12):6660–5.
Stroup D, Chiang JY. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1). J Lipid Res. 2000;41(1):1–11.
Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276(45):41690–9. https://doi.org/10.1074/jbc.M105117200.
Lee YK, Moore DD. Dual mechanisms for repression of the monomeric orphan receptor liver receptor homologous protein-1 by the orphan small heterodimer partner. J Biol Chem. 2002;277(4):2463–7. https://doi.org/10.1074/jbc.M105161200.
Yang Y, Zhang M, Eggertsen G, Chiang JY. On the mechanism of bile acid inhibition of rat sterol 12alpha-hydroxylase gene (CYP8B1) transcription: roles of alpha-fetoprotein transcription factor and hepatocyte nuclear factor 4alpha. Biochim Biophys Acta. 2002;1583(1):63–73.
Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–91. https://doi.org/10.1101/gad.1083503.
Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25. https://doi.org/10.1016/j.cmet.2005.09.001.
Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56(3):1034–43. https://doi.org/10.1002/hep.25740.
Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49(1):297–305. https://doi.org/10.1002/hep.22627.
Kerr TA, Saeki S, Schneider M, Schaefer K, Berdy S, Redder T, et al. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell. 2002;2(6):713–20.
Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2(6):721–31.
• Xu Y, Li F, Zalzala M, Xu J, Gonzalez FJ, Adorini L, et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology. 2016;64(4):1072–85. https://doi.org/10.1002/hep.28712 This study provides important in vivo tissue-specific information on FXR regulation of hepatic bile acid metabolism.
de Aguiar Vallim TQ, Tarling EJ, Ahn H, Hagey LR, Romanoski CE, Lee RG, et al. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015;21(2):298–311. https://doi.org/10.1016/j.cmet.2015.01.007.
• Tarling EJ, Clifford BL, Cheng J, Morand P, Cheng A, Lester E, et al. RNA-binding protein ZFP36L1 maintains posttranscriptional regulation of bile acid metabolism. J Clin Invest. 2017;127(10):3741–54. https://doi.org/10.1172/JCI94029 This manuscript identifies an important post-transcriptional regulator of bile acid metabolism.
de Aguiar Vallim TQ, Tarling EJ, Kim T, Civelek M, Baldan A, Esau C, et al. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ Res. 2013;112(12):1602–12. https://doi.org/10.1161/CIRCRESAHA.112.300648.
Song KH, Li T, Owsley E, Chiang JY. A putative role of micro RNA in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J Lipid Res. 2010;51(8):2223–33. https://doi.org/10.1194/jlr.M004531.
Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58(3):1111–21. https://doi.org/10.1002/hep.26427.
Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. The N Engl J Med. 2013;368(18):1685–94. https://doi.org/10.1056/NEJMoa1209026.
Schmidt MF. miRNA targeting drugs: the next blockbusters? Methods Mol Biol. 2017;1517:3–22. https://doi.org/10.1007/978-1-4939-6563-2_1.
Baker DM, Wang SL, Bell DJ, Drevon CA, Davis RA. One or more labile proteins regulate the stability of chimeric mRNAs containing the 3′-untranslated region of cholesterol-7alpha -hydroxylase mRNA. J Biol Chem. 2000;275(26):19985–91. https://doi.org/10.1074/jbc.M002351200.
Noshiro M, Nishimoto M, Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J Biol Chem. 1990;265(17):10036–41.
Pandak WM, Stravitz RT, Lucas V, Heuman DM, Chiang JY. Hep G2 cells: a model for studies on regulation of human cholesterol 7alpha-hydroxylase at the molecular level. Am J Phys. 1996;270(3 Pt 1):G401–10. https://doi.org/10.1152/ajpgi.1996.270.3.G401.
Fiorucci S, Baldelli F. Farnesoid X receptor agonists in biliary tract disease. Curr Opin Gastroenterol. 2009;25(3):252–9. https://doi.org/10.1097/MOG.0b013e328324f87e.
Fiorucci S, Distrutti E, Ricci P, Giuliano V, Donini A, Baldelli F. Targeting FXR in cholestasis: hype or hope. Expert Opin Ther Targets. 2014;18(12):1449–59. https://doi.org/10.1517/14728222.2014.956087.
Fiorucci S, Mencarelli A, Cipriani S, Renga B, Palladino G, Santucci L, et al. Activation of the farnesoid-X receptor protects against gastrointestinal injury caused by non-steroidal anti-inflammatory drugs in mice. Br J Pharmacol. 2011;164(8):1929–38. https://doi.org/10.1111/j.1476-5381.2011.01481.x.
Fiorucci S, Mencarelli A, Distrutti E, Zampella A. Farnesoid X receptor: from medicinal chemistry to clinical applications. Future Med Chem. 2012;4(7):877–91. https://doi.org/10.4155/fmc.12.41.
Sepe V, Distrutti E, Fiorucci S, Zampella A. Farnesoid X receptor modulators (2011 - 2014): a patent review. Expert Opin Ther Pat. 2015;25(8):885–96. https://doi.org/10.1517/13543776.2015.1045413.
Erickson SK, Lear SR, Deane S, Dubrac S, Huling SL, Nguyen L, et al. Hypercholesterolemia and changes in lipid and bile acid metabolism in male and female cyp7A1-deficient mice. J Lipid Res. 2003;44(5):1001–9. https://doi.org/10.1194/jlr.M200489-JLR200.
Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110(1):109–17. https://doi.org/10.1172/JCI15387.
Fiorucci S, Cipriani S, Baldelli F, Mencarelli A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res. 2010;49(2):171–85. https://doi.org/10.1016/j.plipres.2009.11.001.
Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30(11):570–80. https://doi.org/10.1016/j.tips.2009.08.001.
Kern F Jr. Epidemiology and natural history of gallstones. Semin Liver Dis. 1983;3(2):87–96. https://doi.org/10.1055/s-2008-1040675.
Salen G, Nicolau G, Shefer S, Mosbach EH. Hepatic cholesterol metabolism in patients with gallstones. Gastroenterology. 1975;69(3):676–84.
Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714–9.
Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–40. https://doi.org/10.1074/jbc.M209706200.
Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191(1):197–205. https://doi.org/10.1677/joe.1.06546.
Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 2010;22(7):814–25, e227–8. doi:https://doi.org/10.1111/j.1365-2982.2010.01487.x, e228.
Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45(3):695–704. https://doi.org/10.1002/hep.21458.
Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391(7):785–9. https://doi.org/10.1515/BC.2010.077.
Khurana S, Raufman JP, Pallone TL. Bile acids regulate cardiovascular function. Clin Transl Sci. 2011;4(3):210–8. https://doi.org/10.1111/j.1752-8062.2011.00272.x.
Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. https://doi.org/10.1016/j.bbrc.2008.04.171.
Yang JI, Yoon JH, Myung SJ, Gwak GY, Kim W, Chung GE, et al. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem Biophys Res Commun. 2007;361(1):156–61. https://doi.org/10.1016/j.bbrc.2007.07.001.
Giaretta PR, Suchodolski JS, Blick AK, Steiner JM, Lidbury JA, Rech RR. Distribution of bile acid receptor TGR5 in the gastrointestinal tract of dogs. Histol Histopathol. 2018:18025. https://doi.org/10.14670/HH-18-025.
Keitel V, Haussinger D. TGR5 in the biliary tree. Dig Dis. 2011;29(1):45–7. https://doi.org/10.1159/000324127.
Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. https://doi.org/10.1038/msb.2008.50.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. https://doi.org/10.1016/j.cmet.2009.08.001.
Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156(11):3961–70. https://doi.org/10.1210/en.2015-1321.
Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Moller E, Andersen DB, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84–95. https://doi.org/10.1016/j.molmet.2018.03.007.
Carino A, Cipriani S, Marchiano S, Biagioli M, Scarpelli P, Zampella A, et al. Gpbar1 agonism promotes a Pgc-1alpha-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci Rep. 2017;7(1):13689. https://doi.org/10.1038/s41598-017-13102-y.
Velazquez-Villegas LA, Perino A, Lemos V, Zietak M, Nomura M, Pols TWH, et al. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. 2018;9(1):245. https://doi.org/10.1038/s41467-017-02068-0.
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9. https://doi.org/10.1038/nature04330.
Perino A, Pols TW, Nomura M, Stein S, Pellicciari R, Schoonjans K. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Invest. 2014;124(12):5424–36. https://doi.org/10.1172/JCI76289.
Pols TW, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14(6):747–57. https://doi.org/10.1016/j.cmet.2011.11.006.
Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123(4):1513–30. https://doi.org/10.1172/JCI64551.
Lieu T, Jayaweera G, Zhao P, Poole DP, Jensen D, Grace M, et al. The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice. Gastroenterology. 2014;147(6):1417–28. https://doi.org/10.1053/j.gastro.2014.08.042.
Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398(3):423–30. https://doi.org/10.1042/BJ20060537.
Briere DA, Ruan X, Cheng CC, Siesky AM, Fitch TE, Dominguez C, et al. Novel small molecule agonist of TGR5 possesses anti-diabetic effects but causes gallbladder filling in mice. PLoS One. 2015;10(8):e0136873. https://doi.org/10.1371/journal.pone.0136873.
Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25(6):1066–71. https://doi.org/10.1210/me.2010-0460.
Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2017;65(3):813–27. https://doi.org/10.1002/hep.28707.
Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308(11):1122–31. https://doi.org/10.1001/2012.jama.11164.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. The N Engl J Med. 2014;370(21):2002–13. https://doi.org/10.1056/NEJMoa1401329.
Ding L, Sousa KM, Jin L, Dong B, Kim BW, Ramirez R, et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64(3):760–73. https://doi.org/10.1002/hep.28689.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8. https://doi.org/10.1038/nature13135.
Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10(3):e0122273. https://doi.org/10.1371/journal.pone.0122273.
Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–64. https://doi.org/10.2337/dc12-2255.
Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17(9):1671–7. https://doi.org/10.1038/oby.2009.102.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9. https://doi.org/10.1210/en.2011-2145.
Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Kakela P, Paakkonen M, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22(9):1473–80. https://doi.org/10.1007/s11695-012-0673-5.
Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660–8. https://doi.org/10.1002/oby.20522.
Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–12. https://doi.org/10.1210/jc.2012-3736.
Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51. https://doi.org/10.1210/en.2012-2069.
Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. https://doi.org/10.1038/ncomms8715 https://www.nature.com/articles/ncomms8715#supplementary-information.
Myronovych A, Salazar-Gonzalez RM, Ryan KK, Miles L, Zhang W, Jha P, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity (Silver Spring). 2014;22(11):2301–11. https://doi.org/10.1002/oby.20890.
Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J, et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut. 2014;63(8):1238–46. https://doi.org/10.1136/gutjnl-2013-304583.
Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G652–60. https://doi.org/10.1152/ajpgi.00221.2010.
Hao Z, Leigh Townsend R, Mumphrey MB, Gettys TW, Yu S, Munzberg H, et al. Roux-en-Y gastric bypass surgery-induced weight loss and metabolic improvements are similar in TGR5-deficient and wildtype mice. Obes Surg. 2018. https://doi.org/10.1007/s11695-018-3297-6.
Zhai H, Li Z, Peng M, Huang Z, Qin T, Chen L, et al. Takeda G protein-coupled receptor 5-mechanistic target of rapamycin complex 1 signaling contributes to the increment of glucagon-like peptide-1 production after Roux-en-Y gastric bypass. EBioMedicine. 2018;32:201–14. https://doi.org/10.1016/j.ebiom.2018.05.026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Karolina E. Zaborska and Bethany P. Cummings declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Zaborska, K.E., Cummings, B.P. Rethinking Bile Acid Metabolism and Signaling for Type 2 Diabetes Treatment. Curr Diab Rep 18, 109 (2018). https://doi.org/10.1007/s11892-018-1092-3
Published:
DOI: https://doi.org/10.1007/s11892-018-1092-3