Abstract
The atmospheric chemistry and health implications of pollutants are important scientific concerns in the rural atmosphere. The current study investigates the estimation of seasonal and diurnal variability of VOCs, ozone, and NOx in the rural area located in a tropical region of India during the year 2013–2014. Results showed that most of the targeted VOCs were higher in winter followed by summer and autumn. The diurnal variability of aromatic hydrocarbons showed similar pattern with different amplitudes as maxima and minima during morning (07:00–10:00 h) or evening (16:00–19:00 h) and daytime (10:00–16:00 h), respectively. The sum of aromatic VOCs are found to be in the range from 27.3 to 87.9 μg/m3. In addition to this, O3 and NOx were observed as 45.04 ± 15.19 μg/m3 and 12.41 ± 3.49 μg/m3, respectively, during the observation period. The estimated VOC/NOx ratios (ranged from 3.4 to 3.7) indicated that the selected rural area was VOC limited in terms of ozone sensitivity. The sources of the VOCs have been explained by characteristic ratios, correlation, and principal component analysis. Further, ozone-forming potential (OFP) of the targeted aromatic VOCs has been evaluated using maximum incremental reactivity which suggested toluene (benzene) contributed the largest (lowest) in the ozone formation. Exposure assessment in terms of lifetime cancer and non-cancer risks lies within the acceptable range of USEPA guidelines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
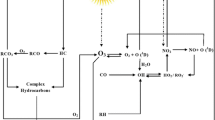
Volatile organic compounds (VOCs) are a diverse group of species comprising of non-methane, oxygenated, and halogenated hydrocarbons which are released from natural as well as anthropogenic sources (Kuo et al. 2014). They play a critical role in chemical and/or photochemical reactions, formation/destruction of ozone (O3), secondary organic aerosols (SOAs), and air-borne toxic chemical formation (Baudice et al. 2016; Kim et al. 2008; Singh et al. 2016). O3 is considered as secondary pollutant which is formed through a series of chemical reactions between VOCs and oxides of nitrogen (NOx) in the presence of solar radiation (Kumar et al. 2014a, 2014b; Ras et al. 2009). The product of VOC concentrations and the OH reaction coefficient is often called the reactivity of VOC to determine the O3 formation (Ran et al. 2009). The residence time of natural CH4 is increased by 15% due to reactivity of VOCs with OH radicals. SOA is formed by the reactions between VOCs with hydroxyl (OH) and/or nitrate (NO3) radicals by nucleation and condensation processes; although, the formation of SOA from VOCs are not clearly understood (Hallquist et al. 2009; Ramanathan et al. 2007; Sarkar et al. 2014).
The release of VOCs in the environment from biogenic (e.g., terrestrial plants) and anthropogenic sources (industrial, transport, evaporative emissions, waste water treatment plants, and solvent usage) are largely dependent on source strength and meteorological variable (Kansal 2009; Nguyen et al. 2009; Pagans et al. 2006; Yang et al. 2016). Global emissions of VOCs are estimated approximately in the range of 1200 to 1600 TgC/year into the atmosphere (Bon et al. 2011). However, the relative emission varies from region to region depending upon the level of anthropogenic activities, climate, and vegetation cover. Anthropogenic VOCs emissions have significant role in the air pollution scenario of high population density.
Exposure to VOCs has also a significant concern to human beings besides the role in atmospheric chemistry and radiative balance. VOCs can have detrimental impacts on public health and welfare in terms of short-term and long-term exposure (Oiamo et al. 2015; Ramírez et al. 2012; Sánchez et al. 2014). Adverse impact ranges from sensory irritation, respiratory illness, and impairment in liver-kidney to carcinogenic effects such as lung, blood (leukemia and non-Hodgkin lymphoma), liver, kidney, and biliary tract cancer (Chang and Chen 2008; Huang et al. 2011; Saral et al. 2009). Inhalation is the major exposure route because of their relatively low boiling points and high vapor pressures (Du et al. 2014). As a matter of toxicity, the US Clean Air Act (1990) separates 97 compounds as VOCs among 188 hazardous air pollutants where some of the VOCs are reported to be carcinogenic (benzene, CHCl3, CCl4) and mutagenic (α-pinine) (Demir et al. 2012; IARC 2006).
The understanding of VOCs in the ambient atmosphere is still complex because of its ubiquitous nature, formation, transformation processes, and emission pattern. In the last few decades, the qualitative and quantitative assessment of VOCs have been focused in the urban areas across the globe (Alghamdi et al. 2014; Filella and Peñuelas 2006; Kos et al. 2014; Strandberg et al. 2014; Tang et al. 2009; Toro et al. 2015; Yang et al. 2016). In Indian context, no studies pertain to the estimation of VOCs along with O3 and NOx in the rural atmosphere till date. The present study mainly focused with the following objectives: (1) seasonal and diurnal variability of VOCs, O3, and NOx in the rural atmosphere of tropical India; (2) identification of emission sources using various statistical tools; and (3) estimation of ozone-forming potential and theoretical health risk assessment.
Study site and sampling
Ambient measurements of VOCs, O3, and NOx were performed in the rural area (28.81°N, 77.00°E) located in the west of the capital city Delhi, India (shown in Fig. 1). The location is characterized by subtropical climate with hot and humid summer and short and intense winter. Sampling was conducted for three seasons, i.e., summer, autumn, and winter, during the year 2013–2014. There are small shops serving the daily needs of village population, houses, and one or two lane roads having very low to moderate traffic of vehicles. The village is located approximately 35 km from the main commercial area of the Delhi city. From the economic activity point, around 60–70% of the area is under crop cultivation. The village is connected to the nearby small towns and Delhi city connected by bituminous roads. The sampling area was free from the influence of any emission source in its immediate vicinity and any other kinds of obstacles (e.g., high building and trees) in the surrounding areas.
Volatile organic compounds
Air samples of VOCs were collected and analyzed using National Institute of Occupational Safety and Health (NIOSH) methods 1003 and 1501. Twelve VOCs, namely, benzene (B), toluene (T), ethylbenzene (EtB), m/p-xylene (m/p-X), o-xylene (o-X), styearene (S), 12,4-trimethylbenzene (1,2,4-TMB), 1,3,5-trimethylbenzene (1,3,5-TMB), chloroform (CHL), carbon tetrachloride (CTC), trichloroethylene (TCE), and tetrachloroethene (PERC) have been examined. Their role in atmospheric chemistry and toxicity were the main selection criteria of these VOCs (Ramírez et al. 2012; Zhou et al. 2011). The VOCs were sampled from the atmosphere by drawing air (indigenous portable sampler) with flow rate of 100 ml/min through Orbo™-32 charcoal sampling tubes. The dimension of Orbo™-32 charcoal tube comprises 7 cm in length × 6 mm o.d. which was acquired from Supelco. Three-hour averaged samples were measured at four time periods between 07:00 to 19:00 h. The samples were collected in the four time intervals, i.e., morning period (07:00 to 10:00 h), daytime (10:00 to 13:00 h and 13:00 to 16:00 h), and evening (16:00 to 19:00 h). Then, the Orbo™-32 charcoal tubes were rapidly sealed with Teflon tape to prevent any further contamination. Afterwards, the tubes were labeled, wrapped with aluminum foil, and kept at <4 °C until analysis.
Analytical procedure is initiated by transferring the activated charcoal to 2-ml amber-colored glass vial, mixed with 1 ml of low-benzene CS2 as an extraction solvent (99% purity with less than 0.001% benzene, purchased from Supelco), and ultrasonicated bath for 30 min. The extracted samples were analyzed with gas chromatograph (GC-450, Bruker) coupled with capillary column Equity-1 (60 m, 0.25 mm ID, and 1.0 μm film thickness) and FID detector. The oven temperature was set for 40 °C (hold time 6 min), which was then raised to 200 °C at a rate of 6 °C/min (hold time 6 min).The identification and quantification of targeted compounds were achieved by their retention time and peak area in relation to calibration VOC standards (JMHW VOC mix, 1000 mg/ml each in methanol, procured from Supelco) under the specified chromatographic conditions.
Ozone and oxides of nitrogen (NOx)
Surface ozone was monitored with automatic ozone analyzer (Model EC 9810 series O3) which employs photometric detection of the specific absorption of UV light by ozone. It is a microprocessor-controlled analyzer that uses the Beer-Lambert law for measuring the concentrations of O3 in ambient air. However, NOx (NO + NO2) was monitored continuously using an ambient analyzer (Model EcotechSernious 40). The analyzer works on the principle that nitric oxide (NO) and ozone (O3) react to produce a characteristic luminescence with intensity linearly proportional to the NO concentration. The O3 and NOx levels were monitored continuously for 24 h during the sampling campaign. Hourly averaged data were derived from the original 1-min average interval data. Instrument maintenance (monthly and annually) was carried out as per manufacturer guidelines, and calibration was performed just before the sampling campaign. Particulate filter was used to prevent particles entering into the instruments from the ambient air and replaced once at the interval of 2 weeks. Further, an inverted Teflon funnel was fitted at the tube entrance to avoid dust and rainwater from entering the tube and measuring instruments. The instrument meets the technical specifications given by USEPA.
Exposure assessment
Using the Integrated Risk Information Systems of USEPA 1997, the exposure assessment parameters such as lifetime cancer risk (LCR) and hazard quotient (HQ) have been calculated using the following equations:
where CDI, SF, and RfD represent the chronic daily intake (mg/kg/day), slope factor (mg/kg/day)−1, and reference dose (mg/kg/day) of the chemical. HQ denotes the non-cancer health hazard by individual chemical while hazard index (HI) represents the total non-cancer health hazard by all targeted chemicals. Equation (3) has been used to evaluate the CDI of each compound:
where CA is the VOC concentration (μg/m3), IR is an inhalation rate (m3/h), ET is the exposure time (h/day), EF is exposure frequency (day/year), ED is the exposure duration (year), BW is a body weight (kg), AT is averaging time (year), and 1000 is the conversion factor (μg/mg). For CDI calculation, Inhalation rates of 0.83 and 0.87 m3/h while body weights of 70 and 36 kg for adults and children were used, respectively. Other parameters such as ET, EF, ED, and AT were assumed to be 24 h/day, 350 d/year, 30 years and 70 years, respectively. The slope factors (SFs) and reference dose (RfD) are documented in Table 1.
Statistical analyses
In addition to descriptive statistics, various other statistical tools have been conducted using SPSS (version 16.0.; SPSS Inc., Chicago, IL, USA) and MATLAB (R2011b; MathWorks, Natick, MA, USA) software. In order to see the seasonal differences of VOCs, O3, and NOx, paired-sample t test, Wilcoxan rank sum test, Friedman test, and Mann-Whitney test have been carried out. Further, Pearson correlation and principal component analysis were also used to examine the sources of VOCs. A significance value of 0.05 was used in all statistical testing.
Results and discussion
Seasonal and diurnal variability of VOCs
A total number of 84 samples were collected during the entire monitoring period which means 28 samples in each season (sampling begins in May, 2013 and ends in January, 2014). Eight species of aromatics and four halogenated hydrocarbons were measured. The highest mean concentration of ∑VOCs was identified in winter (65.9 ± 28.6 μg/m3) followed by summer (57.2 ± 19.6 μg/m3) and autumn (43.3 ± 16.6 μg/m3). An analysis of variance (ANOVA) test indicated that the observed levels of ∑VOCs were significantly (p < 0.05) different during the three seasons. The distinctive feature of the seasonal variations could be an account of factors such as distribution and strength of emission sources, seasonal variability of hydroxyl (OH) radicals, and the prevailing meteorological conditions. Higher temperature and solar radiation in the summer is associated with high losses of VOCs by photochemical degradation which leads to formation of simpler molecules such as CO, CO2, and other intermediates (Lai et al. 2013). The other reasons for the lower levels in summer could be attributed to well dispersive dilution and mixing of the pollutants (Monod et al. 2001). The higher mixing depth results increased convection phenomenon correspondingly decreases the levels of VOCs during the summer (Filella and Peñuelas 2006). However, the highest VOCs concentration in winter could be due to calm conditions and high atmospheric stability that is commonly encountered during the winter months in the area, restricting the dilution of pollutants (Dumanoglu et al. 2014).
The comparative variability of individual VOC has been displayed in Fig. 2 for the three seasons. It is clearly observed that the levels of aromatics were higher in contrast of halogenated hydrocarbons during the entire studied period. Most of the targeted VOCs were noticed higher in winter with few exceptions whereas lower levels were observed during either autumn or summer (Table 2). Toluene was found to be highest among aromatics as 34.1/19.3/22.8 μg/m3 during summer/autumn/winter, respectively. Next to toluene, the aromatics followed the order as m/p-xylene > benzene > ethylbenzene > styrene > o-xylene >1,3,5-TMB > 1,2,4-TMB. The levels of m/p-xylene, benzene, ethylbenzene, and styrene were observed to be 3.1/5.6/10.2, 3.8/6.0/8.4, 3.0/1.9/5.7, and 3.7/2.4/3.9 μg/m3 during summer/autumn/winter, respectively. The emitted aromatic VOCs could be emitted from vehicular emissions, solvent usage, and other human activities (Alghamdi et al. 2014; Yan et al. 2017). Specifically, toluene is noticed higher during summer which could be due to transport of pollutants from nearby commercial and industrial activities to rural sampling site. Toluene levels were also reported higher during summer time by Yang et al. (2016), Miller et al. (2012), and Nguyen et al. (2009). On the other hand, the halogenated VOCs showed lower values in the range of nd to 4.11 μg/m3, nd to 5.24 μg/m3, nd to 3.15 μg/m3, and nd to 5.14 μg/m3 for chloroform, carbon tetrachloride, trichloroethene, and tetrachloroethene, respectively, during the monitoring period. The possible sources of halogenated VOCs are from rural area’s own sources like building materials or products such as chlorine bleach household products, paints and adhesives used in home, industrial solvents, pesticidal fumigants, and chlorinated tap water. Apart from this, the other sources of VOCs could be transported pollutant from the nearby region. Similar results have been also reported by Cai et al. (2010), de Blas et al. (2013), and Zhu et al. (2016). Table 3 compares the observation of the present study with the previous research across the world.
In order to understand the sources, transport, and chemical formation/destruction of the air pollutants, the study of diurnal variability of the pollutants are necessary. Factors such as human activities, nearby local traffic volume, and flow throughout the day and meteorological parameters explain the diurnal variability of the air pollutants. Olumayede and Okuo (2012) stated that it is imperative to know the variability of VOCs at the different times of the day. The average diurnal variation of aromatic and halogenated VOCs at the rural area is depicted in Fig. 3.
The diurnal variability for the aromatic compounds showed similar variation trends with minimal values that appeared in daytime period and higher concentrations in the morning/evening during all seasons. Apart from their primary source strength, their variations mainly corresponded to the diurnal course of meteorological conditions. The accumulation of air pollutants in the morning could be explained by the presence of calm meteorological conditions. However, the elevation of planetary boundary layer (PBL) during daytime enhanced the dispersion and dilution of the pollutants (Zhang et al. 2012). Along with it, photochemical destruction could also be the reason for the lower levels of VOCs in daytime (Tan et al. 2012; Tang et al. 2007). The highest and lowest concentration of OH radicals exhibited during morning/evening and daytime where major sinks of VOCs are its reactions with OH radical. Subsequently, the levels of VOCs generally showed maximum values in morning/evening and minimum in daytime. The higher vehicular emissions from the traffic nearby urban areas during the morning/evening rush hours and agricultural vehicles used in agricultural field explain the higher levels of VOCs. On the other hand, the variability of halogenated VOCs does not follow as that of aromatic VOCs.
Seasonal variability of O3 and NOx
Figure 4 represents the seasonal and diurnal variability of O3 and NOx at the rural site using hourly concentrations averaged over the sampling duration. The meteorological variables, boundary layer processes, and human activities principally influence the variability in the levels of O3 and NOx (Reddy et al. 2012). The mean concentration of O3 was found to be highest in summer (56.41 ± 14.21 μg/m3) followed by winter (43.62 ± 10.83 μg/m3) and autumn (35.10 ± 12.40 μg/m3) for averaging time of 24 h. Higher solar radiation and temperature during summer could be the cause of more photochemical ozone production. Lower O3 levels were recorded in winter which could be attributed to the shorter daylight hours and larger solar zenith angle (Wang et al. 2013). On the other hand, the levels of NOx experienced highest levels in winter (15.85 ± 2.57 μg/m3) and lowest during autumn (9.25 ± 1.56 μg/m3). The suitable meteorological conditions and more use of fossil fuels could be the cause of higher NOx emissions in winter. However, lower levels of NOx during summer/autumn might be due to more photo-oxidation reaction and stronger vertical turbulence. Taking into account the adverse health effects, the World Health Organization (WHO) and Central Pollution Control Boards (CPCB), India, have established air quality guidelines for both O3 and NO2. The permissible limit of O3 (100 μg/m3 8-h average) and NO2 (40 μg/m3 annual average) is prescribed by WHO and CPCB, India. The observed values for O3 and NO2 during all seasons are lower as compared to the recommended guidelines.
The diurnal variations of O3 and NOx during the three seasons were more or less similar with different amplitudes (Fig. 4). It indicates that O3 concentrations were observed maximum (minimum) during the afternoon (evening or early morning) hours. It is noted that O3 levels start increasing after the sunrise coinciding with the increasing solar radiation, and it reaches its peak value around 14:00 to 15:00 h. Thereafter, it decreases gradually and reaches minimum values in the night due to absence of solar radiation. This pattern of O3 and NOx is universally accepted and also reported in rural areas of previous studies in Indian context (Debaje and Kakade 2009; Hassan et al. 2013; Naja and Lal 2002; Reddy et al. 2012). Duenas et al. (2004) reported that the lower levels of O3 could also be due to its reaction with NO (sink) present in the atmosphere. However, NOx showed a more or less opposite diurnal trend to that of O3 which is characterized by low (high) concentrations during the day (night or early morning).
O3 photochemical sensitivity
The ratios of mean values of ambient VOCs (μg/m3) to NOx (μg/m3) have been used to examine the O3 photochemical sensitivity in the rural environment (Cerón-Bretón et al. 2015). The levels of O3 in the afternoon are dependent on the absolute concentrations and ratios of VOCs and NOx. Therefore, the estimation of VOCs to NOx ratios in the morning was used in order to achieve better understanding of effective control strategies of precursors of ozone. The levels of O3, VOCs, and NOx are compared during the observation period (Fig. 5). In the present study, VOCs to NOx ratios were noticed as 3.6, 3.7, and 3.4 for summer, autumn, and winter, respectively. It indicates that the O3 formation regime in rural areas was VOC-limited during all the three seasons. Since the measurement of all potential VOCs present in the ambient environment has not been carried, the observed VOCs to NOx ratios can be underestimated. Although, the results indicated that in order to reduce the ozone contamination would result after the control of VOCs emissions (Avery 2006; Cerón-Bretón et al. 2015; Kang et al. 2004).
OFP of VOCs
To estimate the contribution of individual VOC to photochemical O3 formation, the ozone-forming potential (OFP) has been calculated using maximum incremental reactivity (MIR) values reported by Carter (1994). The OFP is evaluated as the product of the concentration of VOC and the MIR coefficient (dimensionless, gram of O3 produced per gram of VOC). Apart from the reactivity of VOC, the other factors viz. levels of NOx, solar intensity, and meteorological parameters also play a decisive role in the photochemical formation of O3. Figure 6 illustrates the OFP of aromatic compounds for the rural area during the three seasons. In general, most of the compounds have higher contribution in O3 formation during winter in contrast to other seasons. Toluene (benzene) contributed the highest (lowest) among the targeted VOCs as 91.9 (1.6), 52.2 (2.5), and 61.5 g O3 g VOC−1 (3.5) during summer, autumn, and winter, respectively. In addition to this, m/p-xylene and o-xylene have also significant roles in total ozone formation. Many studies reported that BTEX has a significant role for tropospheric ozone formation in ambient atmosphere of Foshan, Yokohama, Beijing, Barcelona, and Jeddah (Alghamdi et al. 2014; Duan et al. 2008; Filella and Peñuelas 2006; Tan et al. 2012; Tiwari et al. 2010).
Sources of VOCs
To estimate the emission sources, two different characteristic ratios of toluene to benzene (T/B) and xylene to benzene (X/B) are used in the present study. The levels of highly reactive organic compounds are decreased due to photochemical degradation during the daytime period while less reactive compounds are accumulated. Benzene and toluene are the stable compounds because of its low reactivity which have 12.5 and 2.0 days of atmospheric lifetime (Prinn et al. 1987). In contrast, xylene has a lifetime of 7.8 h which means they get converted very fast into other atmospheric components. If the ratio of T/B fall in the range of 1.5–4.3, it could be reflected as indicator of vehicular emissions while higher values indicate some additional sources nearby (Alghamdi et al. 2014; Liu et al. 2008, Niu et al. 2012). However, X/B ratio implies the age of air mass and indicates the evidence of transport. The higher values indicate the fresh air mass, local sources, and the low photochemical reactivity of VOCs while lower values signify the old/aged air mass.
Figure 7 illustrates the characteristic ratios of T/B and X/B during the studied seasons. It indicates that the mean T/B ratio during summer is considerably highest at 9.5 which could have some additional sources in contrast to that during autumn (3.4) and winter (2.8). Apart from vehicular emissions, additional sources could be evaporative emissions and painting and cooking processes. The seasonal variability in values of T/B could be attributed to differences in the emissions, photochemistry, and meteorology. The observed T/B ratios in the current study were nearly similar to those found in several previous studies (Ho et al. 2004; Niu et al. 2012). In addition to this, the mean values of X/B showed lower as 1.4, 1.1, and 1.7 for summer, autumn, and winter, respectively. It reveals the high photochemical reactivity, diffusion, and dispersion of the pollutants to the rural area from nearby sources.
Pearson correlation analysis has also been performed to the targeted compounds in order to identify the possible emission sources (Table 4). Correlation coefficients (r) explain that the majority of aromatic VOCs showed good positive moderate to strong correlation with one other. Except toluene, benzene is significantly positively correlated with other aromatics (r > 0.60). In addition to this, the correlation of ethylbenzene with benzene (0.64), m/p-xylene (0.61), o-xylene (0.75), and styrene (0.78) was found to be significantly higher. The other halogenated hydrocarbons did not show any significant correlation with each other and aromatics also. The variability in the correlation among the aromatics could be due to the difference in composition of emission sources (vehicular and solvent usages) and meteorological variables. Further, the differential decay rates of the organic compounds with oxidants such as OH and NO3 also have a role in correlation variability. This result is highly consistent with the studies performed previously worldwide which stated strong correlation among aromatic hydrocarbons (Choi et al. 2009; Parra et al. 2006; Singh et al. 2015).
PCA, a multivariate statistical tool, has also been performed using varimax rotation to identify the emission sources in the present work. The analysis categorized the huge scattered dataset into clustered groups as principal components (PCs) based on the similarities of the variation of the different VOCs (Lü et al. 2009). Eigenvalues of more than 1.0 are selected for the interpretation. Table 5 documents the loading of the factors, fractions of the variance explained by each factor, total variance, and communalities. Three PCs are extracted to explain 70.12% of the total variance where PC-1, PC-2, and PC-3 account for 37.28, 19.74, and 12.99%, respectively. PC-1 comprises with benzene, m/p-xylene, o-xylene, ethylbenzene, 1,2,4-TMB, 1,3,5-TMB, and PERC. However, PC-2 accounted for toluene, o-xylene, ethylbenzene, and styrene while PC-3 is only associated with chloroform and carbon tetrachloride. It may be inferred that vehicle exhaust, solvent usage and degreasing solvents, and industrial sources act as indicators for PC-1, PC-2, and PC-3, respectively.
Exposure assessment
Exposure assessment via inhalation pathway has been evaluated for the two population groups (adults and children) using the USEPA guidelines. The three calculations, namely, chronic daily intake (CDI), hazard quotient (HQ), and lifetime cancer risk (LCR) for each component are documented in Table 6. The order of estimated CDI (mg/kg/day) to observed dataset was found to be in the range from 1.0 × 10−05 to 1.0 × 10−03. The estimated HQs ranged from 11.76 × 10−04 to 9.36 × 10−02 and from 2.40 × 10−03 to 19.06 × 10−02 for adults and children, respectively, which inferred that none of them exceed the threshold value. Further, hazard indices (HI = ∑HQs) also did not exceed the permissible value (1.0) for both population groups. Although, many researchers have reported that values of HQ greater than 0.1 can be considered as potential concern (Kumar et al. 2013). However, the range of estimated LCR is observed to be from 2.24 × 10−07 to 5.98 × 10−05 and from 4.56 × 10−07 to 12.18 × 10−05 for adults and children, respectively. The sum of individual LCR for both population groups were noticed to be higher than the recommended guideline values (1.0 × 10−05) of WHO; however, it lies in the acceptable range (1.0 × 10−04 to 1.0 × 10−06) of USEPA (Kumar et al. 2014a, 2014b; Ramírez et al. 2012). Results showed that the all estimated risk parameters were higher for children as compared to adults.
Conclusions
Measurement of VOCs, O3, and NOx were carried out extensively to see the seasonal and diurnal variability in the rural area of tropical India during 2013–2014. The sum of total targeted VOCs (∑VOCs) were observed to be 65.9 ± 28.6/57.2 ± 19.6/43.3 ± 16.6 μg/m3 during winter/summer/autumn, respectively, in which variation could be due to distribution of emission sources, seasonal change of OH radicals, and meteorological variability. Toluene had the highest level among the selected VOCs. After examining the diurnal variability, morning/evening and daytime showed the maximum and minimum levels, respectively, for most of the VOCs. In addition to this, O3 was highest during summer (56.41 μg/m3) followed by winter (43.62 μg/m3) and autumn (35.10 μg/m3) while NOx followed the order as winter > summer > autumn. The observed ratios of VOC to NOx clearly indicated the rural area is VOC limited. Toluene to benzene ratios showed an average value of 5.23, concluding that sources are mainly vehicular exhaust, while low xylene-to-benzene ratios explain the old/aged air masses. Significant association has been noticed among aromatic compounds after performing Pearson correlation analysis. Principal component analysis explained the major sources could be vehicle exhaust, solvent usage, and degreasing solvents. Further, toluene and xylene had the highest and lowest contribution in ozone formation. In the concern of lifetime cancer risk and non-cancer risks, the both population groups (adult and children) lie within the acceptable range of USEPA guidelines.
The present study highlights the seasonal and diurnal variability of VOCs, O3, and NOx in the rural ambient air of subtropical India. The observed results for origin of VOCs, its photochemistry and theoretical health risk assessment, are useful in improving the efficiencies and efficacies of future policies to maintain air quality. In order to effectively control measures of VOCs and O3, some important steps need to be taken which includes minimizing the release of the pollutants at the source itself by using effective and efficient technological measures. In addition to this, the number air quality monitoring networks should be increased to identify the possible sources present in nearby areas as well as implementation of proper land use planning. Indeed, our study is limited to few VOCs; therefore, year-round continuous measurements of more VOCs are required to improve better understanding for atmospheric chemistry in rural areas. Further research is also warranted to gain mutual interaction among trace gases along with various statistical and modeling studies.
References
Alghamdi MA, Khoder M, Abdelmaksoud AS, Harrison M, Hussein T (2014) Seasonal and diurnal variations of BTEX and their potential for ozone formation in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Air Qual Atmos Health 7:467–480
Avery RJ (2006) Reactivity-based VOC control for solvent products: more efficient ozone reduction strategies. Environ Sci Technol 40:4845–4850
Baudice A, Gros V, Sauvage S, Locoge N, Sanchez O, Kalogridis C (2016) Seasonal variability and source apportionment of volatile organic compounds (VOCs) in the Paris megacity (France). Atmos Chem Phys Diss 185:1–51
Bon DM, Ulbrich IM, De Gouw JA, Warneke C, Kuster WC, Alexander ML, Baker A (2011) Measurements of volatile organic compounds at a suburban ground site (T1) in Mexico City during the MILAGRO 2006 campaign: measurement comparison , emission ratios , and source attribution. Atmos Chem Phys 11:2399–2421
Cai CJ, Geng FH, Tie XX, Yu Q, Li P, Zhou GQ (2010) Characteristics of ambient volatile organic compounds (VOCs) measured in shanghai. China, Sensors 10:7843–7862
Carter WPL (1994) Development of ozone reactivity scales for volatile organic compounds. J Air Waste Manage Assoc 44:881–899
Cerón-Bretón JG, Cerón-Bretón RM, Kahl JDW, Ramírez-Lara E, Guarnaccia C, Aguilar-Ucán CA, Montalvo-Romero C, Anguebes-Franseschi F, López-Chuken U (2015) Diurnal and seasonal variation of BTEX in the air of Monterrey, Mexico: preliminary study of sources and photochemical ozone pollution. Air Qual Atmos Health 8:469–482
Chang CT, Chen BY (2008) Toxicity assessment of volatile organic compounds and polycyclic aromatic hydrocarbons in motorcycle exhaust. J Hazard Mat 153:1262–1269
Choi SW, Park SW, Lee CS, Kim HJ, Bae S, Inyang HI (2009) Patterns of VOC and BTEX concentration in ambient air around industrial sources in Daegu, Korea. J Environ Sci Health Part-A 44:99–107
de Blas M, Navazo M, Alonso L, Durana N, Iza J (2013) Trichloroethylene, tetrachloroethylene and carbon tetrachloride in an urban atmosphere: mixing ratios and temporal patterns. Intl J Environ Anal Chem 93(2):228–244
Debaje SB, Kakade AD (2009) Surface ozone variability over western Maharashtra. India J Hazard Mater 161:686–700
Demir S, Saral A, Ertürk F (2012) Effect of diurnal changes in VOC source strengths on performances of receptor models. Environ Sci Pollut Res 19:1503–1514
Du Z, Mo J, Zhang Y (2014) Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China. Environ Int 73:33–45
Duan J, Tan J, Yang L, Wu S, Hao J (2008) Concentration , sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos Res 88:25–35
Duenas C, Fernandez MC, Canete S, Carretero J, Liger E (2004) Analyses of ozone in urban and rural sites in Malaga (Spain). Chemosphere 56:631–639
Dumanoglu Y, Kara M, Altiok H, Odabas M, Elbir T (2014) Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmos Environ 98:168–178
Filella IÃ, Peñuelas J (2006) Daily, weekly and seasonal time courses of VOC concentrations in a semi-urban area near Barcelona. Atmos Environ 40:7752–7769
Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, Dommen J (2009) The formation , properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys 9:5155–5236
Hassan IA, Basahi JM, Iqbal MI, Tutki MH (2013) Spatial distribution and temporal variation in ambient ozone and its associated NOx in the atmosphere of Jeddah City, Saudi Arabia. Aeros Air Qual Res 13:1712–1722
Ho KF, Lee SC, Guo H, Tsai WY (2004) Seasonal and diurnal variations of volatile organic compounds (VOCs) in the atmosphere of Hong Kong. Sci Total Environ 322:155–166
Huang Y, Sai S, Ho H, Ho KF, Lee SC, Yu JZ, Louie PKK (2011) Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J Hazard Mat 186:344–351
IARC (2006) Monographs on the evaluation of carcinogenic risks to humans. Complete list of agents evaluated and their classification
Kang DW, Aneja VP, Mathur R, Ray JD (2004) Observed and modeled VOC chemistry under high VOC/NOx conditions in the Southeast United States national parks. Atmos Environ 38:4969–4974
Kansal A (2009) Sources and reactivity of NMHCs and VOCs in the atmosphere: a review. J Hazard Mat 166:17–26
Kim KH, Shon ZH, Kim MY, Sunwoo Y, Jeon EC, Hong JH (2008) Major aromatic VOC in the ambient air in the proximity of an urban landfill facility. J Hazard Mater 150:754–764
Kos G, Kanthasami V, Adechina N, Ariya PA (2014) Volatile organic compounds in Arctic snow: concentrations and implications for atmospheric processes. Environ Sci Proc Imp 16:2592–2603
Kumar A, Singh BP, Punia M, Singh D, Kumar K, Jain VK (2013) Assessment of indoor air concentrations of VOCs and their associated health risks in the library of Jawaharlal Nehru University, New Delhi. Environ Sci Pollu Res 21:2240–2248
Kumar A, Singh BP, Punia M, Singh D, Kumar K, Jain VK (2014a) Determination of volatile organic compounds and associated health risk assessment in residential homes and hostels within an academic institute, New Delhi. Indoor Air 24:474–483
Kumar A, Singh D, Singh BP, Singh M, Kumar K, Jain VK (2014b) Spatial and temporal variability of surface ozone and nitrogen oxides in urban and rural ambient air of Delhi-NCR, India. Air Qual Atmos Health 8:391–399
Kuo CP, Liao HT, Chou CCK, Wu CF (2014) Source apportionment of particulate matter and selected volatile organic compounds with multiple time resolution data. Sci Total Environ 472:880–887
Lai C, Chuang K, Chang J (2013) Source apportionment of volatile organic compounds at an international airport. Aero Air Qual Res 13:689–698
Li L, Xie S, Zeng L, Wu R, Li J (2015) Characteristics of volatile organic compounds and their role in ground-level ozone formation in the Beijing-Tianjin-Hebei region, China. Atmos Environ 113:247–254
Liu Y, Shao M, Lu S, Chang C, Wang J, Chen G (2008) Volatile organic compound (VOC) measurements in the Pearl River Delta (PRD) region, China. Atmos Chem Phys 8:1531–1545
Lü H, Cai Q, Wen S, Chi Y, Guo S, Sheng G, Fu J, Antizar-Ladislao B, Lü H, Cai Q, Wen S (2009) Carbonyl compounds in the ambient air of hazy days and clear days in Guangzhou, China. Atmos Res 94:363–372
Miller L, Xu X, Mannion AG, Brook J, Wheeler A (2012) Multi-season, multi-year concentrations and correlations amongst the BTEX group of VOCs in an urbanized industrial city. Atmos Environ 61:305–315
Monod A, Sive BC, Avino P, Chen T, Blake DR, Rowland FS (2001) Monoaromatic compounds in ambient air of various cities: a focus on correlations between the xylenes and ethylbenzene. Atmos Environ 35:135–149
Naja M, Lal S (2002) Surface ozone and precursor gases at Gadanki (13.5°N, 79.2°E), a tropical rural site in India. J Geophys Res 107:4197
Nguyen HT, Kim KH, Kim MY (2009) Volatile organic compounds at an urban monitoring station in Korea. J Hazard Mat 161:163–174
Niu Z, Zhang H, Xu Y, Liao X, Xu L, Chen J (2012) Pollution characteristics of volatile organic compounds in the atmosphere of Haicang District in Xiamen City, Southeast China. J Environ Monit 14:1145–1152
Oiamo TH, Johnson M, Tang K, Luginaah IN (2015) Assessing traffic and industrial contributions to ambient nitrogen dioxide and volatile organic compounds in a low pollution urban environment. Sci Total Environ 529:149–157
Olumayede EG, Okuo JM (2012) Distribution, temporal and diurnal behaviors of total volatile organic compounds over the urban atmosphere of southwestern Nigeria. J Environ Sc Eng A 1:785–796
Pagans E, Font X, S’anchez A (2006) Emission of volatile organic compounds from composting of different solid wastes: abatement by biofiltration. J Hazard Mat 131:179–186
Parra MA, González L, Elustondo D, Garrigó J, Bermejo R, Santamaría JM (2006) Spatial and temporal trends of volatile organic compounds (VOC) in a rural area of northern Spain. Sci Total Environ 370:157–167
Prinn R, Cunnold D, Rasmussen R, Simmonds P, Alyea F, Crawford A, Fraser P, Rosen R (1987) Atmospheric trends in methylchloroform and the global average for the hydroxyl radical. Science 238:945–950
Ramanathan V, Ramana MV, Roberts G, Kim D, Corrigan C, Chung C, Winker D (2007) Warming trends in Asia amplified by brown cloud solar absorption. Nature 448:575–578
Ramírez N, Cuadras A, Rovira E, Borrull FR, Marcé M (2012) Chronic risk assessment of exposure to volatile organic compounds in the atmosphere near the largest Mediterranean industrial site. Environ Int 39:200–209
Ran L, Zhao C, Geng F, Tie X, Tang X, Peng L, Zhou G, Yu Q, Xu J, Guenther A (2009) Ozone photochemical production in urban Shanghai , China: analysis based on ground level observations. J Geo Res 114:1–14
Ras MR, Marcé RM, Borrull F (2009) Characterization of ozone precursor volatile organic compounds in urban atmospheres and around the petrochemical industry in the Tarragona region. Sci Total Environ 407:4312–4319
Reddy BSK, Kumar KR, Balakrishnaiah G, Gopal KR, Reddy RR, Sivakumar V, Lingaswamy AP, Arafath SM, Umadevi K, Kumari SP, Ahammed YN, Lal S (2012) Analysis of diurnal and seasonal behavior of surface ozone and its precursors (NOx) at a semi-arid rural site in southern India. Aero Air Qual Res 12:1081–1094
Sánchez NS, Alarcón ST, Santonja RT, Pórcel JL (2014) New device for time-averaged measurement of volatile organic compounds (VOCs). Sci Total Environ 485–486:720–725
Saral A, Demir S, Yıldız S (2009) Assessment of odorous VOCs released from a main MSW landfill site in Istanbul-Turkey via a modelling approach. J Hazard Mat 168:338–345
Sarkar C, Chatterjee A, Majumdar D, Ghosh SK, Srivastava A, Raha S (2014) Volatile organic compounds over eastern Himalaya, India: temporal variation and source characterization using positive matrix factorization. Atmos Chem Phys Discuss 14:32133–32175
Sauvage S, Plaisance H, Locoge N, Wroblewski A, Coddeville P, Galloo JC (2009) Long term measurement and source apportionment of non-methane hydrocarbons in three French rural areas. Atmos Environ 43:2430–2441
Singh D, Kumar A, Singh BP, Anandam K, Singh M, Mina U, Kumar K, Jain VK (2015) Spatial and temporal variability of VOCs and its source estimation during rush/non-rush hours in ambient air of Delhi, India. Air Qual Atmos Health 9:483–493
Singh D, Kumar A, Kumar K, Singh B, Mina U, Singh BB, Jain VK (2016) Statistical modeling of O3 , NOx , CO , PM2.5, VOCs and noise levels in commercial complex and associated health risk assessment in an academic institution. Sci Total Environ 572:586–594
Strandberg B, Rynell KB, Sallsten G (2014) Evaluation of three types of passive samplers for measuring 1,3-butadiene and benzene at workplaces. Environ Sci Proc Imp 16:1006–1014
Tan JH, Guo SJ, Ma YL, Yang FM, He KB, Yu YC, Wang JW, Shi ZB, Chen GC (2012) Non-methane hydrocarbons and their ozone formation potentials in Foshan. China, Aero Air Qual Res 12:387–398
Tang JH, Chan LY, Chan CY, Lia YS, Chang CC, Liu SC, Wu D, Li YD (2007) Characteristics and diurnal variations of NMHCs at urban, suburban, and rural sites in the Pearl River Delta and a remote site in South China. Atmos Environ 41:8620–8632
Tang JH, Chan LY, Chang CC, Liu S, Li YS (2009) Characteristics and sources of non-methane hydrocarbons in background atmosphere of eastern, southwestern and southern China. J Geophy Res 114:D03304
Tiwari V, Hanai Y, Masunaga S (2010) Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual Atmos Health 3:65–75
Toro AR, Seguel RJ, Raul GEMS, Manuel ALG (2015) Ozone, nitrogen oxides, and volatile organic compounds in a central zone of Chile. Air Qual Atmos Health 8:545–557
USEPA (1997) Air risk assessment work plan. Air and Radiation Division, Tristate, EPA, Washington
United States Environmental Protection Agency (USEPA) (1998) Integrated risk information system, www.epa.gov. US Environmental Protection Agency, Washington, DC
Wang T, Wong CH, Cheung TF, Blake DR, Arimoto R, Baumann K, Tang J, Ding GA, Yu XM, Li YS, Streets DG, Simpson IJ (2004) Relationships of trace gases and aerosols and the emission characteristics at Linan, a rural site in eastern China, during spring 2001. J Geophys Res 109:D19S05
Wang Y, Hu B, Tang G, Ji D, Zhang H, Bai J, Wang X, Wang Y (2013) Characteristics of ozone and its precursors in northern China: a comparative study of three sites. Atmos Res 132–133:450–459
Yan Y, Peng L, Li R, Li Y, Li L, Bai H (2017) Concentration, ozone formation potential and source analysis of volatile organic compounds (VOCs) in a thermal power station centralized area: a study in Shuozhou. China, Environ Poll 223:295–304
Yang M, Wang Y, Chen J, Li H, Li Y (2016) Aromatic hydrocarbons and halocarbons at a mountaintop in southern China. Aerosol Air Qual Res 16:478–491
Zhang Y, Mu Y, Liu J, Mellouki A (2012) Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China. J Environ Sci 24:124–130
Zhang Y, Wang X, Barlett SIJ, Blake DR, Fu X, Zhang Z, Ding X (2013) Source attributions of hazardous aromatic hydrocarbons in urban, suburban and rural areas in the Pearl River Delta (PRD) region. J Hazard Mater 250–251:403–411
Zhou J, You Y, Bai Z, Hu Y, Zhang J, Zhang N (2011) Environment health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci Total Environ 409:452–459
Zhu Y, Yang L, Chen J, Wang X, Xue L, Shao M, Lu S, Wang W (2016) Characteristics of ambient volatile organic compounds and the influence of biomass burning at a rural site in northern China during summer 2013. Atmos Environ 124:156–165
Acknowledgements
The study is supported by Research Grant (Senior Research Fellowship) from Council for Scientific and Industrial Research (CSIR) and Department of Science and Technology (DST) New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Singh, D., Anandam, K. et al. Dynamic interaction of trace gases (VOCs, ozone, and NOx) in the rural atmosphere of sub-tropical India. Air Qual Atmos Health 10, 885–896 (2017). https://doi.org/10.1007/s11869-017-0478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-017-0478-8