Abstract
Past measurements of volatile organic compound (VOC) concentrations from Middle Eastern countries are very few, and this study assesses the concentrations and processes affecting benzene, toluene, ethylbenzene and xylenes (BTEX) in the atmosphere of an urban background area of Jeddah, a coastal city in Saudi Arabia, and their potential for ozone formation. The measurements were carried out for a year (from December 2011 to November 2012) and include hourly BTEX and meteorological parameters. The annual average concentrations of BTEX species were 0.41 ppb for benzene, 1.40 ppb for toluene, 0.49 ppb for ethylbenzene, 1.56 ppb for m,p-xylene and 0.94 ppb for o-xylene. The annual mean benzene level (0.41 ppb, ∼1.31 μg m−3) did not exceed the annual threshold level (5 μg m−3) set by the European Union but still represents a small risk to human health. BTEX showed a seasonal variation, with higher concentrations during the spring and lower concentrations during the autumn. The diurnal variation of BTEX concentrations followed a commonly observed pattern, with two peaks associated with high traffic volumes. m,p-Xylene was the largest contributor to ozone formation potential followed by o-xylene, toluene and benzene. The significantly positive correlation between BTEX compounds as well as the ratio of toluene/benzene (average = 4.03) suggested that vehicle emissions were the major source of BTEX during the whole investigated period. m,p-Xylene-to-ethylbenzene ratios showed an annual mean of 3.18 with little variability during the different seasons indicating that the photochemical age in the study area is relatively young due to the continual fresh emissions experienced in Jeddah city.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the increasing rate of urbanisation and industrialization worldwide, especially in developing countries, large amounts of volatile organic compounds (VOCs) are released into the atmosphere annually (Gee and Sollars 1998; Tonooka et al. 2001; Khoder 2007). Hydrocarbons are the main group of VOCs in the atmosphere and can originate from both natural and anthropogenic sources. Large quantities of VOCs in urban areas are emitted from gasoline and diesel-powered motor vehicles, fuel storage and fuel combustion, biomass burning, residential heating, natural gas, liquefied petroleum gas, solvent usage in industrial processes, industrial emissions and biogenic sources (Lai et al. 2005; Latella et al. 2005; Guenther et al. 2006; Williams and Koppmann 2007; Guo et al. 2007; Geng et al. 2007; Yokelson et al. 2008; Lanz et al. 2008; Badol et al. 2008; Duan et al. 2008; Kansal 2009; Demir et al. 2011). In addition to combustion emissions, VOC can be released into the atmosphere through evaporation during storage and filling operations and from gasoline stations, as many VOCs exist in the fuel formulations (Schifter et al. 2002). No emissions data are available for Saudi Arabia, but in the UK, VOC emissions from transport account for less than 9 % of all VOC emissions to the atmosphere in 2011, compared with 35 % in 1990 (NAEI 2012). Other major sources are solvent and product use (44.5 %) and fossil fuel extraction/distribution (19.8 %) in 2011 (NAEI 2012). Li et al. (2011) evaluated the impacts upon benzene, toluene, ethylbenzene and the xylenes (BTEX) emissions of combustion (85 %), evaporation (small contribution) and industrial sources (less than evaporation) in New York State. Evaporative emissions occur from the use of solvents, gasoline evaporation and gasoline spills (Na and Kim 2001; Na et al. 2003).
The levels of VOCs in the ambient air are related to the fuels used, vehicle types and ages, flow rates and speed of traffic as well as road and environmental conditions in the city (Paul 1997). Passenger cars moving at a speed below 50 km h−1 have emissions of organic pollutants markedly greater than at higher speed (60–130 km h−1) (Heeb et al. 1999).
Among VOC pollutants, the BTEX group is an important component of ambient air (Hsieh et al. 2011a). They constitute up to 60 % of nonmethane VOCs (Lee et al. 2002). Special attention has been paid to BTEX species, especially to benzene, due to their adverse effects on human health. It is established that benzene is a human carcinogen (US EPA 2012; WHO 2000). Exposure to aromatic hydrocarbons such as toluene and xylene may cause sensory irritation symptoms or respiratory diseases (Otto et al. 1990; Delfino et al. 2003). Many VOCs are reported to be toxic, carcinogenic or mutagenic (Duce et al. 1983; Edgerton et al. 1989; Sweet and Vermette 1992; Kostiainen 1995; Mukund et al. 1996). They can also cause harm to ecosystems by changing the atmospheric chemistry via photochemical reactions which increase the formation of secondary air pollutants such as ozone (O3), peroxyacyl nitrates (PANs), photochemical smog and organic aerosols (Atkinson 2000; Cerqueira et al. 2003; Derwent et al. 2003; Bernstein et al. 2008). Vehicle tailpipe emission and fuel evaporation tend to be the dominant source of BTEX in urban regions (Lan and Minh 2013). Motor vehicle exhaust and motor vehicle refuelling operations typically account for the highest benzene exposures especially in urban areas (Cocheo et al. 2000; Suh et al. 2000).
The fate of VOCs is affected by a number of physical and chemical processes leading to their transformation or their removal from the atmosphere. Chemical processes leading to degradation of VOCs are photolysis, reaction with the hydroxyl radical (OH) during daytime, reaction with NO3 radical at night, reaction with O3 and the reaction with Cl atoms in coastal and maritime areas (Atkinson and Arey 2003). As VOCs with a high molecular weight can be adsorbed on atmospheric particles, dry and wet deposition are also important scavenging mechanisms for some VOC (Karl et al. 2010). Mixing processes, which are closely related to meteorological conditions, tend to redistribute pollutants through advective and convective transport on both regional and long-range scales (Borbon et al. 2004). Therefore, VOC concentrations vary across time and space.
BTEX compounds take part in photochemical processes, even in areas distant from primary emissions. They have a high photochemical ozone creation potential in the atmosphere. The main process of chemical removal of BTEX from the atmosphere is through reaction with the OH radical during daytime. Typical atmospheric lifetimes with respect to reaction with OH radical are 225 h (benzene), 50 h (toluene), 40 h (ethylbenzene), 20 h (o-xylene), 12 h (m-xylene) and 19 h (p-xylene) for a 24-h average OH concentration of 1 × 106 cm−3. The xylenes (m,p-xylene plus o-xylene) are reported to be the dominant contributors to ozone formation among BTEX (Na et al. 2005; Yassaa et al. 2006). BTEX ratios can be useful as a tool to investigate photochemical processes (Yassaa et al. 2006). The ratio between m,p-xylene and ethylbenzene (m,p-X/E) is used to investigate the extent of atmospheric photochemical reactivity and is a useful tool for estimating the photochemical age of an air mass (Nelson and Quigley 1984; Monod et al. 2001; Hsieh and Tsai 2003; Hsieh et al. 2011b).
The recent rapid increase in urbanisation, industrialisation and human activities has had important impacts on air quality in Jeddah city. As a result, the emissions of BTEX are expected to have significantly increased. Data on atmospheric BTEX concentration in Jeddah are scarce. Therefore, the objectives of this study are to (1) assess the concentrations of BTEX compounds in an urban background area of Jeddah, (2) study the diurnal and seasonal variations of these pollutants, (3) use BTEX ratios to characterise major sources and photochemical age and (4) evaluate the ozone formation potential of BTEX species. This will help in establishing effective strategies on air pollution mitigation.
Materials and methods
Study area
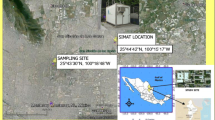
Jeddah is the second largest city in the country; it lies on the coast of the Red Sea and is the major urban centre of western Saudi Arabia. Currently, it has a population of 3.4 million (CDSI 2009) (Fig. 1). However, it is likely that the true figure would be much greater if the population census included illegal unregistered immigrants. Jeddah’s rapid growth has had a significant impact on its environment and resources, mainly from pollution and degradation. Emissions in Jeddah are most notably from stationary sources and road traffic. The main stationary sources in the city include: an oil refinery, sea port activities, desalination plant, a power-generation plant and two industrial zones in the north and south of the city. On a daily basis, more than 1.40 million vehicles are running in the streets of Jeddah city using mainly unleaded gasoline and diesel (Khodeir et al. 2012). In addition, massive demolition and construction projects are taking place all over the city. Construction of a new airport, central train station and railway, new stadium and many bridges and tunnels are just examples.
The sampling site is located on King Abdulaziz University campus, situated in an urban background area (Jamea district), (21° 29′ 12.8″ N, 39° 15′ 6.0″ E), which is located in the southeast part of Jeddah. Most of the local air pollutant emissions arise from the surrounding traffic activities. The sampling site is located in an area of intensively trafficked roads with the ring road at about 1.7 km, and from one of the busiest main roads in the area is only about 133 m distant (Fig. 1).
The general climate of Jeddah city is warm and moderate in winter, with high temperature and high solar radiation in the summer season. Rainfall is generally sparse. During the period of the present study, the average temperatures were 25.26 °C in winter, 31.60 °C in spring, 35.10 °C in summer, 31.33 °C in autumn and 30.82 °C over the whole year. The average relative humidity was 55.10, 46.70, 38.60, 53.50 and 48.46 % in winter, spring, summer, autumn and the whole year, respectively. The average wind speeds were 2.88, 3.13, 3.12, 2.54 and 2.92 m s−1 in winter, spring, summer, autumn and the whole year, respectively. Wind roses showing the seasonal and annual variations in wind direction at the sampling site are presented graphically in Fig. 2.
Monitoring of BTEX
Sampling was carried out at a height of 3.5 m from ground level in the period from December 2011 to November 2012. BTEX in ambient air were monitored in a semi-continuous mode by means of a Syntech Spectras BTEX analyser GC 955, type 600 (Syntech Spectras, Groningen, the Netherlands) equipped with a 13-m capillary column AT5, ID 0.32 mm, film 1 μm and PID detector operating at 10.6 eV. The oven temperature ramped from 40 to 120 °C. Carrier gas flow was in the range 1.8–3.5 mL min−1. Pre-concentration is achieved by flushing the sample tubing by drawing sample gas through it with a pump. Then the pump is switched off, and with the help of an indirect piston system, a volume of 35-mL sample gas is pre-concentrated on a Tenax column. The sensitivity for aromatic hydrocarbons is down to 150 ppt (0.4 μg m−3), and the resolution time was 15 min. The instrument response was corrected based on a regular calibration with a standard gas mixture. The analyser is placed in a trailer at a height of 0.7 m from ground level. The sampling line starts with a shielded stainless steel probe of length 1.0 m and ID 25 mm joined from the end with a glass header of length 0.4 m and ID 25 mm. A PTFE (Teflon) hose of length 3 m and ID 6 mm is extended from the glass header to the sampling port. A Teflon filter membrane of 5 μm is placed at the sampling port. The flow rate on the header is 12 L min−1 and the sampling flow rate is 1.5 L min−1. The captured data were filtered to exclude anomalies and transformed to daily averages and prepared for statistical treatment.
Meteorological parameters
Air temperature, relative humidity, wind speed and direction and atmospheric pressure were measured continuously using compact weather station model WS600-UMB (Lufft, Fellbach, Germany), simultaneously with measurements of atmospheric pollutant concentrations. The station sensor was located at 6.5 m height above ground level, without interference from local buildings.
Results and discussion
BTEX level in the study area
The annual average concentrations of BTEX during the period of study are graphically presented in Fig. 3. From this figure, it can be seen that m,p-xylene and toluene were the most abundant BTEX compounds in the study area. The daily average concentrations ranged from 0.09 to 1.10 ppb (with a mean value of 0.41 ± 0.21 ppb) for benzene, 0.18 to 4.46 ppb (with a mean value of 1.40 ± 0.75 ppb) for toluene, 0.12 to 1.62 ppb (with a mean value of 0.49 ± 0.26 ppb) for ethylbenzene, 0.25 to 1.90 ppb (with a mean value of 0.94 ± 0.44 ppb) for o-xylene and 0.38 to 2.98 ppb (with a mean value of 1.56 ± 0.63 ppb) for m,p-xylene during the period of study. The annual mean benzene concentration (0.41 ppb ∼ 1.31 μg m−3) did not exceed the annual threshold concentration (5 μg m−3) set by the European Union (European Commission 2013). Benzene is a carcinogenic compound causing leukaemia. The US EPA (2005), through cancer risk analysis, estimates that an individual exposed to benzene levels between 0.13 and 0.45 μg m−3 for 70 years has a cancer risk probability of 1/1,000,000. Exposure levels between 1.3 and 4.5 μg m−3 raise the risk to 1/100,000 and between 13 and 45 μg m−3, the risk of getting cancer, especially leukaemia, rises to 1/1,000. Using the unit risk factor from the WHO (2000), for a city such as Jeddah with a population of about 3.4 million and average benzene levels of 1.31 μg m−3, about 27 additional cases of leukaemia would be expected in the city over a 70-year period.
The average concentrations of atmospheric BTEX compounds in the present study in comparison with those reported from other cities in the world are shown in Table 1. From this table, it can be concluded that the mean measured concentrations were within the range of those detected in other cities of the world. Generally, this variation in average concentrations among the locations across the world can be attributed to the difference in the traffic density, industrial activities, fuel composition and combustion, solvent usage in industrial processes, intensity of human activities, land use patterns and chemical removal of the BTEX from the atmosphere. In addition, the profile of BTEX compounds at every location is influenced by the local conditions such as differences in climate, geography, industrial activity, vehicle age and fuels used.
Monthly and seasonal variations of BTEX concentrations
The seasonal cycle characteristics of BTEX are valuable for understanding important processes in atmospheric transport and chemistry. In this study, winter is defined as December–February, spring as March–May, summer as June–August and autumn as September–November. The monthly and seasonal variations of BTEX compound concentrations during the period of study are graphically presented in Fig. 4. The maximum monthly 24-h average concentration of BTEX compounds were found in May for benzene, toluene and o-xylene, February, March and May for ethylbenzene and April and May for m,p-xylene, whereas the minimum concentration of these pollutants was observed in January for benzene, August for toluene and November for ethylbenzene, o-xylene and m,p-xylene. Monthly 24-h average concentrations varied from 0.22 ppb (January) to 0.70 ppb (May) for benzene, 0.80 ppb (August) to 2.20 ppb (May) for toluene, 0.20 ppb (November) to 0.73 ppb (February) for ethylbenzene, 0.37 ppb (November) to 1.54 ppb (May) for o-xylene and 0.80 ppb (November) to 2.16 ppb (April and May) for m,p-xylene. From Fig. 4, it can be seen that the highest concentrations of benzene, toluene, ethylbenzene and m,p-xylene were found in spring, whereas the lowest levels were detected in the autumn season. For o-xylene, the highest level was found in summer, whereas the lowest concentration was found in autumn. The ∑BTEX concentrations were 4.46, 6.17, 5.17 and 2.96 ppb during winter, spring, summer and autumn, respectively. This pattern is highly suggestive of evaporative processes as a major source (see below).
Determinants of airborne concentrations include source strength (i.e. emissions), dispersion processes and sink processes. Exhaust released from the tailpipes of motor (especially gasoline powered) vehicles during combustion, liquid fuel arising from spillage, leakage and vehicle operations, and vapour emitted from headspace emissions at refuelling stations and bulk terminals and from vehicles are the pathways for emission of BTEX in the atmosphere from gasoline vehicle fuel (Watson et al. 2001; Choi and Ehrman 2004; Zhang et al. 2013). Evaporative emissions (e.g. solvent usage, gasoline evaporation from the headspace of a fuel tank, and evaporation from gasoline spillage while filling a tank) are expected to be greater in the summer than in the winter because of increased vapour pressure (Na et al. 2005). The distinguishing difference between benzene and TEX in terms of usage is that TEX is used in solvents, while benzene is not (Na et al. 2004).
The advection of air pollutants from highly polluted areas could increase the BTEX mixing ratios in the study area. In north Jeddah, there are vehicular service stations and many small workshops for maintenance and repair of cars. These workshops make extensive use of solvents. They are the primary components of coatings, adhesives, paints and cleaning agents. This leads to increased emissions of toluene, ethylbenzene and the xylenes (TEX), which are the primary constituents of many solvent formulations (Guo et al. 2004a; Choi et al. 2011; Borban et al. 2002; Chan et al. 2006). In the present study, the wind rose derived from hourly meteorological data from our Met station in the study area (Fig. 2), indicates that the dominant wind direction during the period of highest BTEX concentration (spring) was from a northerly direction. The wind rose for the winter is, however, similar suggesting that this is not a major factor.
Air temperatures are high throughout the year in Jeddah, with the highest temperatures in May to September (Alghamdi et al. 2014). Concentrations of NO x were notably higher in May 2012 than in other months in the period March 2012 to February 2013, reflecting an exceptionally high volume of traffic or poor dispersion conditions (Alghamdi et al. 2014). The combination of high temperatures and high vehicle exhaust emissions explains the observed maximum in BTEX in May and is consistent with the overall seasonal pattern.
In the present study, the average concentrations of BTEX compounds in daytime and nighttime were similar, except in summer where the nighttime average concentrations of these pollutants were higher than the daytime. The daytime/nighttime concentration ratios were 0.67, 0.61. 0.72, 0.93 and 0.73 for BTEX, respectively, during the summer season. Considering the high temperatures in daytime during the summer season in Jeddah city and the official days off for schools and colleges, most of the people stay home, and consequently the density of traffic during daytime is greatly decreased. Therefore, low concentrations of BTEX are observed in daytime. Conversely, after sunset the weather becomes more suitable for going out for shopping and travelling, and the traffic continues to flow until about midnight on weekdays. The traffic continues after midnight on Fridays and even longer until early morning during Ramadan (20th July to 18th August). This leads to a decrease in daytime/nighttime ratio in the concentrations of traffic-generated pollutants. It is also reflected in the lower summer concentrations.
Diurnal variation of BTEX concentrations
The study of diurnal variations of air pollutants can provide valuable information about the sources, transport and chemical formation/destruction of such pollutants. The diurnal variations in the concentrations of BTEX compounds during the period of study are graphically presented in Fig. 5. From this figure, concentrations of BTEX compounds were typically low at midday and high during rush hour periods (morning and evening), and they were higher in the morning than in the evening, indicating the local elevated vehicular emissions during the morning rush hour during a period of relatively high atmospheric stability. This trend is very similar to the diurnal trends found in other urban areas (Ho et al. 2004; Yang et al. 2005; Filella and Peñuelas 2006; Tang et al. 2007). In the present study, the hourly concentrations of BTEX compounds increased from 0600 to 0900 hours in spring, autumn and winter and from 0000 to 0300 hours in summer (Fig. 5), and then decreased due to the reduction of traffic volume and enhanced atmospheric mixing. Moreover, the high temperature and solar radiation intensity during the midday period will lead to increasing photochemical reactions and consequently increased chemical loss of BTEX compounds. During midday, VOC concentrations decrease, which may be due to photochemical reactions or to an increase in the mixing depth (Yang et al. 2005) or a reduction in emissions. In the present study, the diurnal variations in the concentrations of all BTEX compounds in each individual season showed a similar pattern, indicating that these compounds in the atmosphere of the study area have a similar sources and dispersion mechanisms. Even at a relatively high midday hydroxyl radical concentration of 5 × 106 cm−3, the lifetimes of BTEX range from around 24 h (benzene) to 3 h (m-xylene), and hence only a modest impact of photochemical decay would be anticipated.
BTEX correlations and ratios
The correlation coefficients between BTEX species during the period of study are presented in Table 2. Significant positive correlation coefficients (p < 0.01) were found between the concentrations of all BTEX species. This is consistent with previous studies that have observed strong correlations amongst BTEX in the United States, Greater Cairo, India and Canada (Pankow et al. 2003; Khoder 2007; Hoque et al. 2008; Miller et al. 2010; Miller et al. 2012). In the present study, correlations between benzene, toluene, ethylbenzene and m,p-xylene species were highest (r > 0.68). These high correlations suggest that the species were originating from common sources. The lower but significant correlations (r < 0.59) were mainly seen between o-xylene and the other BTEX species, suggesting an additional source of o-xylene within the study area.
The inter-species BTEX concentration ratios and their seasonal and diurnal variations are used as indicators of BTEX sources and photochemical processing occurring between their emission and sampling reflecting ageing in the troposphere due to their reaction with the OH radical (Parrish et al. 2007; Zalel et al. 2008). These ratios depend on sources, composition, climatic conditions as well as the age of air parcels. BTEX compounds in urban areas are emitted mainly from road vehicles. Benzene is a marker of vehicle exhaust (Hong et al. 2006) and evaporative emissions. It originates predominantly from this source, whereas TEX species, especially toluene which is also emitted from motor vehicles has other sources, particularly from evaporation of solvents used in inks, paint etc. (Yuan et al. 2010). Therefore, the toluene to benzene (T/B) ratio has been commonly used as an indicator of sources. These, and other ratios are expressed on a mass ratio (μg m−3/μg m−3) basis. T/B values of 1.5–4.3 are considered an indicator of traffic emissions (Hoque et al. 2008; Liu et al. 2009), with many studies reporting T/B values below 3 as characteristic of traffic emissions worldwide (Perry and Gee 1995; Brocco et al. 1997; Heeb et al. 2000; Monod et al. 2001; Chan et al. 2002; Hsieh et al. 2006; Kumar and Tyagi 2006; Khoder 2007; Truc and Oanh 2007; Hoque et al. 2008; Liu et al. 2009; Matysik et al. 2010). In the present study, the average T/B ratios were higher than most of those found to be characteristic of traffic emissions worldwide and vary little with season. T/B ratios were 4.26, 3.99, 4.22, 3.58 and 4.03 during winter, spring, summer, autumn and the full period of study, respectively, (Fig. 6). The (T/B) ratios found in the present study were similar to those found in many cities such as in Sydney (4.04), Ankara (4.3), China (3.85), Windsor (4.3) and within Belgium (3.8–4.4) (Nelson and Quigley 1982; Buczynska et al. 2009; Miller et al. 2010; Tong et al. 2013; Yurdakul et al. 2013) and higher than those found in some other cities including Bari (2.0), Beijing (1.5–2.2), Rome (2.80), Izmir (1.87–2) and Santiago (2.01) (Brocco et al. 1997; Hartmann et al. 1997; Muezzinoglu et al. 2001; Liu et al. 2009; Caselli et al. 2010). Conversely, our results are lower than those found in Bangkok (10.22), Hong Kong (7.74), and Osaka (7.19) (Tsujino and Kuwata 1993; Gee and Sollars 1998; Lee et al. 2002). The differences of (T/B) ratios among these cities may reflect a difference between their vehicle types, fuel composition and industrial activities.
The m,p-xylene-to-ethylbenzene ratio (m,p-X/E ratio) has been used to evaluate the age of air parcels and as indicator for the age of the VOCs in the atmosphere (Guo et al. 2004a,b; Elbir et al. 2007; Hsieh et al. 2011b). Both m- and p-xylene are more reactive towards the OH radical than ethylbenzene and a low m,p-X/E ratio suggests an aged air parcel. Ratios of 3.8, 3.8 and 4.4 in fresh emissions at a gasoline station, in a tunnel and in an underground garage, respectively were reported by Kuntasal (2005). Relatively constant ratios ranging from 2.8 to 4.6 with a mean value of 3.5 due to traffic exhaust emissions have been reported by Monod et al. (2001). The variation of the m,p-X/E ratio in the course of this study is depicted in Fig. 6. From this figure, it can be seen that the average m,p-X/E ratios show a relatively small difference between the different seasons, ranging from 2.74 in winter to 3.92 in autumn with an average of 3.18 during the period of study, suggesting rather little photochemical ageing. The m,p-X/E ratios in the present study were very similar to those found in other major urban areas such as Munich with a ratio of 3.4 (Rappenglueck and Fabian 1999), Sydney with a ratio of 3.0 (Nelson et al. 1983), the centre of Algiers with a ratio of 2.9 (Kerbachi et al. 2006), Bari with a ratio of 3.9 (Caselli et al. 2010) and in various cities of the UK with a ratio of 2.9 (Derwent et al. 2000). It is consistent with the source in local emissions and quite long atmospheric lifetimes of the VOCs.
Ranking of BTEX compounds with respect to ozone formation potential and reactivity with OH
The individual BTEX compounds play differing roles in photochemical smog formation (Carter 1990) including production of ozone (Carter 1994). Ozone formation potential (OFP) is a widely used metric for describing the maximum ozone formation capacity in cities where ozone formation is VOC sensitive. It can be evaluated using the maximum incremental reactivity (MIR) developed by Carter (1994). MIR is popular in the assessment of OFP of various VOC compounds (Hung-Lung et al. 2007). Carter's MIR is the amount of ozone formed when 1 g of VOC is added to an initial VOC–NO x mixture under relatively high NO x conditions, indicating how much a compound may contribute to the ozone formation in the air mass (Carter 1994). The reactivity of VOC with OH radical determines the ability of the hydrocarbon to form higher oxidised products such as ketones, acids, aldehydes, organic peroxy radicals etc. The MIR coefficients were taken from Atkinson (1997) and Carter (1990, 1994) and rate constants of BTEX-OH reactions from Jenkin et al. (2003). The ranking of the BTEX species according to their concentrations, OFP and reaction with OH during the different seasons is given in Table 3. Based on the MIR scale, m,p-xylenes were the biggest contributors to ozone formation followed by o-xylene and toluene, whereas benzene was the lowest contributor during the different seasons and period of study. This is an agreement with Na et al. (2005) and Grosjean et al. (1998), who found that m,p-xylene and o-xylene were the dominant contributor to ozone formation among BTEX at Seoul and Porto Alegre, respectively. In the present study, the highest m,p-xylene contribution was found in spring, followed by summer and the lowest in autumn. Reaction of BTEX with OH radical, which leads to the formation of oxidised products, followed a similar pattern to that found in the contribution of BTEX to ozone formation during the different seasons and period of study. The pattern was m,p-xylenes > o-xylene > toluene > ethylbenzene > benzene. In addition, the highest m,p-xylene contribution was also found in spring, followed by summer and the lowest in autumn. Derwent et al. (1998) have also produced a scale for VOC contribution to ozone production referred to as Photochemical Ozone Creation Potential (POCP). Individual values are benzene (21.8), toluene (63.7), ethylbenzene (73.0), o-xylene (105.3), m-xylene (110.8) and p-xylene (101.0). Although showing less large ratios between compounds than the MIR values (Table 3), they rank in the same order and also lead to the conclusion that the xylenes contribute most from the BTEX compounds to ozone formation.
An analysis of ozone data from the same location in Jeddah (Alghamdi et al. 2014) has found that Jeddah has a “NO x -saturated” atmosphere in which ozone concentrations are suppressed relative to rural concentrations. In such an atmosphere, VOC such as BTEX make a minor contribution to ozone destruction, but net ozone formation does not occur. The contribution to ozone creation will be in the downwind urban plume, as the urban emissions are diluted and NO2/NO x ratios increase allowing ozone formation to exceed ozone destruction.
Summary
BTEX compounds were measured from December 2011 to November 2012. Seasonal and diurnal variation of BTEX concentrations and their potential for ozone formation have been evaluated and discussed. It was found that m,p-xylene was most abundant amongst the BTEX compounds, followed by toluene, o-xylene, ethylbenzene and benzene during the period of study. ∑BTEX showed a seasonal variation, with higher concentrations during the spring, followed by summer, winter and autumn. Diurnal variations in the concentrations of BTEX compounds during different seasons were found to follow a similar pattern and had two daily peaks linked to traffic density. A different diurnal profile in the summer season is reflective of a different pattern of road traffic activity at this time of year. The average toluene / benzene concentration ratio suggested that vehicle emissions are the major source of BTEX in Jeddah city. This is confirmed by the significant positive correlation between the concentrations of BTEX compounds in the study area. m,p-Xylene-to-ethylbenzene ratios showed little variability between the different seasons and indicate that the photochemical age in the study area is relatively young due to the continual fresh emissions experienced in Jeddah city. Ozone formation potentials for BTEX species were estimated using MIR. Xylenes were the dominant contributor to ozone formation. Benzene had the lowest potential for the formation of ozone. Finally, the annual average benzene level in the study did not exceed the annual limit value set by the European Union but still represents a risk to human health. For a city such as Jeddah with a population of about 3.4 million and average benzene levels of 1.31 μg m−3, about 27 additional cases of leukaemia would be expected in the city over a 70-year period, using the unit risk factor of 6 × 10−6 (μg m−3)−1 recommended by the WHO (2000).
References
Alghamdi MA, Khoder M, Harrison RM, Hyvärinen A-P, Hussein T, Al-Jeelani H, Abdelmaksoud AS, Goknil M., Shabbaj II, Almehmadi FM, Lihavainen H, Kulmala M (2014) Temporal variations of O3 and NO x in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Atmos. Environ 94:205–214
Atkinson R (1997) Gas-phase tropospheric chemistry of volatile organic compounds 1. Alkanes and alkenes. J Phys Chem 26:215–290
Atkinson R (2000) Atmospheric chemistry of VOCs and NO x . Atmos Environ 34:2063–2101
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103:4605–4638
Badol C, Locoge N, Galloo JC (2008) Using a source–receptor approach to characterize VOC behaviour in a French urban area influenced by industrial emissions part II: source contribution assessment using the Chemical Mass Balance (CMB) model. Sci Tot Environ 389:429–440
Baltrėnas P, Baltrėnaitė E, Šereviĉienė V, Pereira P (2011) Atmospheric BTEX concentrations in the vicinity of the crude oil refinery of the Baltic region. Environ Monit Asses 182:115–127
Barletta B, Meinardi S, Simpson IJ, Khwaja HA, Blake D, Rowland FS (2002) Mixing ratios of volatile organic compounds (VOCs) in the atmosphere of Karachi, Pakistan. Atmos Environ 36:3429–3443
Bernstein JA, Alexis N, Bacchus H, Bernstein IL, Fritz P, Horner E, Li N, Mason S, Nel A, Oullette J (2008) The health effects of nonindustrial indoor air pollution. J Allergy Clin Immun 121:585–591
Borban A, Locoge N, Veillerot M, Galloo JC, Guillermo R (2002) Characterization of NMHCs in a French urban atmosphere: overview of the main sources. Sci TotEnviron 292:177–191
Borbon A, Coddeville P, Locoge N, Galloo JC (2004) Characterising sources and sinks of rural VOC in eastern France. Chemosphere 57:931–942
Brocco D, Fratarcangeli R, Lepore L, Petricca M, Ventrone I (1997) Determination of aromatic hydrocarbons in urban air of Rome. Atmos Environ 31:557–566
Buczynska AJ, Krata A, Stranger M, Flavia Locateli Godoi A, Kontozova Deutsch V, Bencs L, Naveau I, Roekens E, Van Grieken R (2009) Atmospheric BTEX concentrations in an area with intensive street traffic. Atmos Environ 43:311–318
Carter WPL (1990) A detailed mechanism for the gas-phase atmospheric reaction of organic compounds. Atmos Environ 24A:481–518
Carter WPL (1994) Development of ozone reactivity scales for volatile organic compounds. J Air Waste Manage 44:881–899
Caselli M, de Gennaro G, Marzocca A, Trizio L, Tutino M (2010) Assessment of the impact of the vehicular traffic on BTEX concentration in ring roads in urban areas of Bari (Italy). Chemosphere 81:306–311
CDSI (2009) Central department of statistics and information: http://www.cdsi.gov.sa/2010-07-31-07-00-05/cat_view/31-/138----/342---1431-2010
Cerqueira M, Pio C, Gomes P, Matos J, Nunes T (2003) Volatile organic compounds in rural atmospheres of central Portugal. Sci Total Environ 313:49–60
Chan CY, Chan LY, Wang XM, Liu YM, Lee SC, Zou SC, Sheng GY, Fu JF (2002) Volatile organic compounds in roadside microenvironments of metropolitan Hong Kong. Atmos Environ 36:2039–2047
Chan LY, Chu KW, Zou SC, Chan CY, Wang XM, Barletta B, Blake DR, Guo H, Tsai WY (2006) Characteristics of nonmethane hydrocarbons (NMHCs) in industrial, industrial urban, and industrial suburban atmospheres of the Pearl River Delta (PRD) region of south China. J Geophys Res 111:D11304
Choi YJ, Ehrman SH (2004) Investigation of sources of volatile organic carbon in the Baltimore area using highly time-resolved measurements. Atmos Environ 38:775–791
Choi E, Choi K, Yi SM (2011) Non-methane hydrocarbons in the atmosphere of a Metropolitan City and a background site in South Korea: sources and health risk potentials. Atmos Environ 45:7563–7573
Cocheo V, Sacco P, Boaretto C, De Saeger E, Ballesta PP, Skov H et al (2000) Urban benzene and population exposure. Nature 404:141
CPCB (2002) Benzene in air and its effect on human health. Central Pollution Control Board, Parivesh News Letter, 1-32
Delfino RJ, Gong H, Linn WS, Hu Y, Pellizzari ED (2003) Respiratory symptoms and peak expiratory flow in children with asthma in relation to volatile organic compounds in exhaled breath and ambient air. J Expo Sci Env Epid 13:348–363
Demir S, Saral A, Ertürk F, Kuzu SL, Goncaloğlu BI, Demir G (2011) Effect of diurnal changes in VOC source strengths on performances of receptor models. Environ Sci Pollut Res Int 19:1503–1514
Derwent RG, Jenkin ME, Saunders SM, Pilling MJ (1998) Photochemical ozone creation potentials for organic compounds in northwest Europe calculated with a master chemical mechanism. Atmos Environ 32:2429–2441
Derwent RG, Davies TJ, Delaney M, Dollard GJ, Field RA, Dumitrean P et al (2000) Analysis and interpretation of the continuous hourly monitoring data for 26 C2–C8 hydrocarbons at 12 United Kingdom sites during 1996. Atmos Environ 34:297–312
Derwent R, Jenkin M, Saunders S, Pilling M, Simmonds P, Passant N, Dollard G, Dumitrean P, Kent A (2003) Photochemical ozone formation in north west Europe and its control. Atmos Environ 37:1983–1991
Duan J, Tan J, Yang L, Wu S, Hao J (2008) Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos Res 88:25–35
Duce R, Mohnen VA, Zimmerman PR, Grosjean D, Cautreels WJ, Chatfield R, Jaenicke R, Ogren JA, Pellizzari ED, Wallace GT (1983) Organic material in global troposphere. Rev Geophys Space Phys 21:921–952
Edgerton SA, Holdren MW, Smith DL (1989) Inter-urban comparison of ambient volatile organic compound concentrations in US cities. J Air Pollut Control Assoc 39:729–732
Elbir T, Cetin B, Çetin E, Bayram A, Odabasi M (2007) Characterization of volatile organic compounds (VOCs) and their sources in the air of İzmir, Turkey. Environ Monit Assess 133:149–160
European Commission, Environment (2013) http://www.ec.Europa.eu/environment /air/quality/standards.htm. Accessed 23 May 2013
Filella I, Peñuelas J (2006) Daily, weekly and seasonal relationships among VOCs, NO x and O3 in a semi-urban area near Barcelona. J Atmos Chem 54:189–201
Gee IL, Sollars CJ (1998) Ambient air levels of volatile organic compounds in Latin American and Asian cities. Chemosphere 36:2497–2506
Geng F, Zhao C, Tang X, Lu G, Tie X (2007) Analysis of ozone and VOCs measured in Shanghai: a case study. Atmos Environ 41:989–1001
Grosjean E, Rasmussen RA, Grosjean D (1998) Ambient levels of gas phase pollution in Porto Alegre, Brazil. Atmos Environ 32:3371–3379
Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C (2006) Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos Chem Phys 6:3181–3210
Guo H, Lee SC, Loure PKK, Ho KF (2004a) Characterization of hydrocarbons, halocarbons and carbonyls in the atmosphere of Hong Kong. Chemosphere 57:1363–1372
Guo H, Wang T, Louie PKK (2004b) Source apportionment of ambient non-methane hydrocarbons in Hong Kong: application of a principal component analysis/absolute principal component scores (PCA/APCS) receptor model. Environ Pollut 129:489–496
Guo H, So K, Simpson I, Barletta B, Meinardi S, Blake D (2007) C1–C8 volatile organic compounds in the atmosphere of Hong Kong: overview of atmospheric processing and source apportionment. Atmos Environ 41:1456–1472
Hartmann R, Voght U, Baumbach G, Seyfioglu R, Muezzinoglu A (1997) Results of emission and ambient air measurements of VOC in Izmir. Environ Res Forum 7–8:107–112
Heeb NV, Forss AM, Bach C (1999) Fast and quantitative measurement of benzene, toluene and C2-benzenes in automotive exhaust during transient engine operation with and without catalytic exhaust gas treatment. Atmos Environ 33:205–215
Heeb NV, Forss AM, Bach C, Reimann S, Herzog A, Jäckle HW (2000) A comparison of benzene, toluene and C2-benzene mixing ratios in automotive exhaust and in the suburban atmosphere during the introduction of catalytic converter technology to the Swiss Car Fleet. Atmos Environ 34:3103–3116
Ho KF, Lee SC, Guo H, Tsai WY (2004) Seasonal and diurnal variations of volatile organic compounds (VOCs) in the atmosphere of Hong Kong. Sci Tot Environ 322:155–166
Hong YJ, Jeng HA, Gau YY, Lin C, Lee IL (2006) Distributions of volatile organic compounds in ambient air of Kaohsiung, Taiwan. Environ Monit Assess 119:43–56
Hoque RR, Khillare PS, Agarwal T, Shridhar V, Balachandran S (2008) Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Sci Tot Environ 392:30–40
Hsieh CC, Tsai JH (2003) VOC concentration characteristics in Southern Taiwan. Chemosphere 50:545–556
Hsieh LT, Yang HH, Chen HW (2006) Ambient BTEX and MTBE in the neighborhoods of different industrial parks in Southern Taiwan. J Hazard Mater 128:106–115
Hsieh LT, Tsai CH, Chang JE, Tsao MC (2011a) Decomposition of methyl tert-butyl ether by adding hydrogen in a cold plasma reactor. Aerosol Air Qual Res 11:265–281
Hsieh LT, Wang YF, Yang HH, Mi HH (2011b) Measurements and correlations of MTBE and BETX in traffic tunnels. Aerosol Air Qual Res 11:763–775
Hung-Lung C, Jiun-Horng T, Shih-Yu C, Kuo-Hsiung L, Sen-Yi M (2007) VOC concentration profiles in an ozone non-attainment area: a case study in an urban and industrial complex metroplex in southern Taiwan. Atmos Environ 41:1848–1860
Jenkin ME, Saunders SM, Wagner V, Pilling MJ (2003) Protocol for the development of the master chemical mechanism, MCM v3 (part B): tropospheric degradation of aromatic volatile organic compounds. Atmos Chem Phys 3:191–193
Kansal A (2009) Sources and reactivity of NMHCs and VOCs in the atmosphere: a review. J Hazard Mater 166:17–26
Karl T, Harley P, Emmons L, Thornton B, Guenther A, Basu C, Turnipseed A, Jardine K (2010) Revisiting the dry depositional sink of oxidized organic vapors to vegetation. Geophys Res Abstr 12 (EGU2010-6255, 2010, EGU General Assembly 2010)
Kerbachi R, Boughedaouib M, Bounouac L, Keddama M (2006) Ambient air pollution by aromatic hydrocarbons in Algiers. Atmos Environ 40:3995–4003
Khamdan SAA, Al Madany IM, Buhussain E (2009) Temporal and spatial variations of the quality of ambient air in the Kingdom of Bahrain during 2007. Environ Monit Assess 154:241–252
Khodeir M, Shamy M, Alghamdi M, Zhong M, Sun H, Costa M, Chen L, Maciejczyk P (2012) Source apportionment and elemental composition of PM2.5 and PM10 in Jeddah City, Saudi Arabia. Atmos Pollut Res 3:331–340
Khoder MI (2007) Ambient levels of volatile organic compounds in the atmosphere of Greater Cairo. Atmos Environ 41:554–566
Kinney PL, Chillrud SN, Ramstrom S, Ross J, Spengler JD (2002) Exposure to multiple air toxics in New York City. Environ Health Perspect 110:539–546
Kostiainen R (1995) Volatile organic compounds in the indoor air of normal and sick houses. Atmos Environ 29:693–702
Kumar A, Tyagi SK (2006) Benzene and toluene profiles in ambient air of Delhi as determine by active sampling and GC analyses. J Scie Ind Res 65:252–257
Kuntasal ÖO (2005) Organic composition of particles and gases in the Ankara atmosphere. Unpublished Ph.D. thesis, METU, Ankara
Lai CH, Chen KS, Ho YT, Peng YP, Chou YM (2005) Receptor modeling of source contributions to atmospheric hydrocarbons in urban Kaohsiung, Taiwan. Atmos Environ 39:4543–4559
Lan TTN, Minh PA (2013) BTEX pollution caused by motorcycles in the megacity of HoChiMinh. J Environ Sci 25:348–356
Lanz VA, Buchmann B, Hueglin C, Locher R, Reimann S, Staehelin J (2008) Factor analytical modeling of C2–C7 hydrocarbon sources at an urban background site in Zurich (Switzerland): changes between 1993–1994 and 2005–2006. Atmos Chem Phys Discuss 8:907–955
Latella A, Stani G, Cobelli L, Duane M, Junninen H, Astorga C, Larsen BR (2005) Semicontinuous GC analysis and receptor modelling for source apportionment of ozone precursor hydrocarbons in Bresso, Milan, 2003. J Chromatogr A 1071:29–39
Lee SC, Chiu MY, Ho KF, Zou SC, Wang X (2002) Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong. Chemosphere 48:375–382
Li R, Kalenge S, Hopke PK, Leboulf R, Rossner A, Benedict A (2011) Source appointment of benzene downwind of a major point source. Atmos Pollut Res 2:138–143
Liu J, Mu Y, Zhang Y, Zhang Z, Wang X, Liu Y, Sun Z (2009) Atmospheric levels of the BTEX compounds during the 2008 Olympic Games in the urban area of Beijing. Sci Tot Environ 408:109–116
Matysik S, Ramadan AB, Schlink U (2010) Spatial and temporal variation of outdoor and indoor exposure of volatile organic compounds in Greater Cairo. Atmos Pollut Res 1:94–101
Miller L, Lemke LD, Xu X, Molaroni SM, You H, Wheeler AJ, Booza J, Grgicak-Mannion A, Krajenta R, Graniero P, Krouse H, Lamerato L, Raymond D, Reiners J Jr, Weglicki L (2010) Intra-urban correlation and spatial variability of air toxics across as international airshed in Detroit, Michigan (USA) and Windsor, Ontario (Canada). Atmos Environ 44:1162–1174
Miller L, Xu X, Grgicak-Mannion A, Brook J, Wheeler A (2012) Multi season, multi-year concentrations and correlations amongst the BTEX group of VOCs in an urbanized industrial city. AtmosEnviron 61:305–315
Monod A, Sive BC, Avino P, Chen T, Blake DR, Rowland FS (2001) Monoaromatic compounds in ambient air of various cities: a focus on correlations between the xylenes and ethylbenzene. Atmos Environ 35:135–149
Moschonas N, Glavas S (1996) C3–C10 hydrocarbons in the atmosphere of Athens, Greece. Atmos Environ 30:2769–2772
Muezzinoglu A, Odabasi M, Onat L (2001) Volatile organic compounds in the air of Izmir, Turkey. Atmos Environ 35:753–760
Mukund R, Kelly TJ, Spicer CW (1996) Source attribution of ambient air toxics and other VOCs in ColombusOhio. Atmos Environ 30:3457–3470
Na K, Kim YP (2001) Seasonal characteristics of ambient volatile organic compounds in Seoul, Korea. Atmos Environ 35:2603–2614
Na K, Kim YP, Moon KC (2003) Diurnal characteristics of volatile organic compounds in the Seoul atmosphere. Atmos Environ 37:733–742
Na K, Kim YP, Moon I, Moon KC (2004) Chemical composition of VOC major emission sources in the Seoul atmosphere. Chemosphere 55:585–594
Na K, Moon KC, Kim YP (2005) Source contribution to aromatic VOC concentration and ozone formation potential in the atmosphere of Seoul. Atmos Environ 39:5517–5524
NAEI (2012) Emissions of Air Quality Pollutants, 1970–2011. National Atmospheric Emissions Inventory
Nelson PF, Quigley SM (1982) Non-methane hydrocarbons in the atmosphere of Sydney, Australia. Environ Sci Technol 16:650–655
Nelson PF, Quigley SM (1984) The hydrocarbon composition of exhaust emitted from gasoline fueled vehicles. Atmos Environ 18:79–87
Nelson PF, Quigley SM, Smith MY (1983) Sources of atmospheric previous term hydrocarbons next term in Sydney: a quantitative determination using a source reconciliation technique. Atmos Environ 17:439–449
Otto D, Molhave L, Rose G, Hudnell HK, House D (1990) Neurobehavioral and sensory irritant effects of controlled exposure to a complex mixture of volatile organic compounds. Neuro Toxicol Teratol 12:649–652
Pankow JF, Luo W, Bender DA, Isabelle LM, Hollingsworth JS, Chen C, Asher WE, Zogorski JS (2003) Concentration and co-occurrence correlations of 88 volatile organic compounds (VOC) in the ambient air of 13 semi-rural tourban locations in the United States. AtmosEnviron 37:5023–5046
Parrish DD, Stohl A, Forster C, Atlas EL, Blake DR, Goldan PD, Kuster WC, de Gouw JA (2007) Effects of mixing on evolution of hydrocarbon ratios in the troposphere. J Geophys Res 112(D10):34–50
Paul J (1997) Improved air quality on Turkish roads: fuels and exhaust gas treatment. Environl Res Forum 7–8:145–152
Perry R, Gee IL (1995) Vehicle emissions in relation to fuel composition. Sci Tot Environ 169:149–156
Pratt GC, Palmer K, Wu CY, Oliaei F, Hollerach C, Fenske MJ (2000) An assessment of air toxics in Minnesota. Environ Health Perspect 108:815–825
Rappenglueck B, Fabian P (1999) Nonmethane previous term hydrocarbons next term (NMHC) in the greater Munich area, Germany. Atmos Environ 33:3843–3857
Schifter I, Magdaleno M, Díaz L, Krüger B, León J, Palmerín ME, Casas R, Melgarejo A, López-Salinas E (2002) Contribution of the gasoline distribution cycle to volatile organic compound emissions in the metropolitan area of Mexico City. J Air Waste Manage Assoc 52:535–541
Suh HH, Bahadori T, Vallarino J, Spengler JD (2000) Criteria air pollutants and toxic air pollutants. Environ Health Perspect 108:625–633
Sweet CW, Vermette SJ (1992) Toxic volatile organic compounds in urban air in Illinois. Environ Sci Technol 26:165
Tang JH, Chan LY, Chan CY, Li YS, Chang CC, Liu SC et al (2007) Characteristics and diurnal variations of NMHCs at urban, suburban and rural sites in the Pearl River Delta and a remote site in South China. Atmos Environ 41:8620–8632
Tong L, Liao X, Chen J, Xiao H, Xu L, Zhang F, Niu Z, Yu J (2013) Pollution characteristics of ambient volatile organic compounds (VOCs) in the southeast coastal cities of China. Environ Sci Pollut Res 20:2603–2615
Tonooka Y, Kannari A, Higashino H, Murano K (2001) NMVOCs and CO emission inventory in East Asia. Water Air Soil Pollut 130:1–4
Truc VTQ, Oanh NTK (2007) Roadside BTEX and other gaseous air pollutants in relation to emission sources. Atmos Environ 41:7685–7697
Tsujino Y, Kuwata K (1993) Sensitive flame ionization detector for the determination of trace of atmospheric hydrocarbons by capillary gas chromatography. J Chromatogr 642:383–388
US EPA (2005) Guidelines for carcinogenic risk assessment. Risk Assessment Forum, EPA/630/P-03/001F, March 2005
US EPA (2012) Benzene. http://www.epa.gov/ttn/atw/hlthef/benzene.html
Watson JG, Chow JC, Fujita EM (2001) Review of volatile organic compound source apportionment by chemical mass balance. Atmos Environ 35:1567–1584
WHO (2000) Air quality guidelines for Europe, 2nd edn. World Health Organisation, Copenhagen
Williams J, Koppmann R (2007) Volatile organic compounds in the atmosphere: an overview. In: Volatile organic compounds in the atmosphere. Blackwell Publishing Ltd., Oxford, UK
Yamamoto N, Okayasu H, Murayama S, Mori S, Hunahashi K, Suzuki K (2000) Measurement of volatile organic compounds in the urban atmosphere of Yokohama, Japan, by an automated gas chromatographic system. Atmos Environ 34:4441–4446
Yang KL, Ting CC, Wang JL, Wingenter OW, Chan CC (2005) Diurnal and seasonal cycles of ozone precursors observed from continuous measurement at an urban site in Taiwan. Atmos Environ 39:3221–3230
Yassaa N, Brancaleoni E, Frattoni M, Ciccioli P (2006) Isomeric Analysis of BTEXs in the atmosphere using b-cyclodextrin capillary chromatography coupled with thermal desorption and mass spectrometry. Chemosphere 63:502–508
Yokelson RJ, Christian TJ, Karl TG, Guenther A (2008) The tropical forest and fire emissions experiment: laboratory fire measurements and synthesis of campaign data. Atmos Chem Phys 8:3509–3527
Yuan B, Shao M, Lu S, Wang B (2010) Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos Environ 44:1919–1926
Yurdakul S, Civan M, Tuncel G (2013) Volatile organic compounds in suburban Ankara atmosphere, Turkey: sources and variability. Atmos Res 120–121:298–311
Zalel A, Yuval BMD (2008) Revealing source signatures in ambient BTEX concentrations. Environ Pollut 156:553–562
Zhang J, Sun Y, Wu F, Sun J, Wang Y (2013) The characteristics, seasonal variation and source apportionment of VOCs at Gongga Mountain, China. Atmos Environ in press
Acknowledgements
This study was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University (KAU), Jeddah, under grant no. 1220/430. The authors therefore acknowledge with thanks DSR and KAU for their technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• One year of continuously collected BTEX data is analysed.
• Concentrations are highest in spring and lowest in autumn.
• The main source is road traffic
• The potential for ozone formation is analysed.
Rights and permissions
About this article
Cite this article
Alghamdi, M.A., Khoder, M., Abdelmaksoud, A.S. et al. Seasonal and diurnal variations of BTEX and their potential for ozone formation in the urban background atmosphere of the coastal city Jeddah, Saudi Arabia. Air Qual Atmos Health 7, 467–480 (2014). https://doi.org/10.1007/s11869-014-0263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-014-0263-x