Abstract
Background
The aim of the present study was to evaluate whether different Nd:YAG laser applications as an adjunct to scaling and root planning (SRP) improve the healing response to periodontal therapy in smokers with periodontitis.
Methods
This clinical trial included eighty systemically healthy smokers with periodontitis. Patients were randomly allocated to a treatment group: SRP alone (group 1), SRP+low-level laser therapy (LLLT) with Nd:YAG laser (group 2), SRP+pocket debridement with ND:YAG laser (group 3), and SRP+combined pocket debridement and LLLT with Nd:YAG laser (group 4). Gingival index (GI), plaque index (PI), bleeding on probing (%), probing depth (PD), and clinical attachment level (CAL) were recorded, and gingival crevicular fluid (GCF) samples for metalloproteinase-8 (MMP-8) levels were collected at baseline, 1 month and 3 months after treatment.
Results
There were no significant differences between the treatment groups for the GI, PI, and BOP (%) parameters and MMP-8 levels at any time points (p > 0.05). For moderately deep pockets, PD and CAL reductions were significantly greater in all test groups compared to group 1 (p ˂ 0.05). For deep pockets, these reductions were significantly greater in group 2 and group 4 compared to group 1 (p ˂ 0.05). PD and CAL reductions were generally similar between test groups (p > 0.05) except PD reduction between baseline and 3 months in deep pockets (p ˂ 0.05).
Conclusions
The findings of this clinical trial suggest that Nd:YAG laser applications may be beneficial on the healing response of smokers to non-surgical therapy compared to SRP alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is defined as a multifactorial inflammatory disease initiated by dental plaque and results in periodontal supporting tissues destruction and eventually tooth loss [1]. Smoking is confirmed as a risk factor for periodontitis considering the substantial body of evidence that demonstrates the relationship between the destruction of periodontium and cigarette smoking. Compared to non-smokers, smokers exhibit more attachment loss [2, 3], a higher number of deep periodontal pockets [4, 5], and more alveolar bone loss [6, 7]. Smokers are more likely to lose their teeth [8, 9] and have more furcation involvement than non-smokers [10, 11]. Smoking also affects the progression of periodontal disease. Heitz-Mayfield indicated that smoking is a powerful dose-related predictor for periodontitis progression [12]. Kibayashi et al. investigated the relationship between smoking and periodontal disease progression. They found that 38.5% of disease progression was associated with current smoking status [13]. It has been also demonstrated that some sites in smokers presented high gingival crevicular fluid (GCF) MMP-8 levels. These sites were also more likely to have progressive disease [14]. Longitudinal studies reported that smokers developed more sites with increased probing depth and alveolar bone loss [15,16,17]. Considering the wound healing after periodontal therapy in smokers, smokers exhibit decreased healing response compared to non-smokers after periodontal treatment [18,19,20].
Plaque control, scaling, and root planing (SRP) are main procedures for reducing or eliminating bacteria and provide clinical improvement for periodontal health [21]. Although the success of non-surgical periodontal treatment is well proven, there are some limitations decreasing the success rate such as inaccessible areas including furcation lesions, concavities and grooves on root surfaces, and distal sites of molars [21]. Until this time, dental lasers have been used for improving the treatment outcomes as an adjunct or alternative treatment procedure in the treatment of periodontitis with a great interest [22, 23]. One commonly used dental laser in periodontal therapy is Neodymium-doped yttrium aluminum garnet (Nd:YAG) laser with 1064 nm wavelength. It has low absorption in water. It penetrates deeply into soft tissues and has an affinity for pigmented tissues [24, 25]. Excellent soft tissue ablation, potential bactericidal, and detoxification effects are important features of this laser [26,27,28]. Nd:YAG laser can be easily inserted into periodontal pockets with a flexible optical fiber and has been used for periodontal pocket curettage and root debridement [22]. It can remove contaminated gingival epithelium completely without damaging the connective tissue and micro vessels [29]. In the literature, clinical researches demonstrated beneficial effects of Nd:YAG laser applications as an adjunct to non-surgical periodontal therapy on improving clinical parameters such as probing depth (PD), clinical attachment level (CAL), gingival index (GI), plaque index (PI), and bleeding on probing (BOP) [29,30,31,32,33], reducing interleukin-1β (IL-1β) and matrix metalloproteinase-8 (MMP-8) levels [30] and periodontal bacteria counts such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Treponema denticola [29, 33]. Considering the application of Nd:YAG laser with SRP in smokers with periodontitis, there is only one study in the literature. In that study, Eltas et al. reported that SRP+Nd:YAG laser did not provide any considerable improvements for clinical parameters and GCF volume compared to SRP alone in smokers with chronic periodontitis [34].
It has been demonstrated that LLLT in periodontology has various advantages, such as reducing pain or discomfort, promoting wound healing and regeneration of alveolar bone, and suppressing inflammation [23]. Considering in vitro studies investigating the bio stimulatory effects of Nd:YAG laser, it has been reported that LLLT with this laser may stimulate gingival [35], periodontal ligament fibroblast [36], and osteoblast proliferation [37]. In a mucositis animal model, LLLT with Nd:YAG laser stimulated wound healing by modulating basic fibroblast growth factor and platelet-derived growth factor gene expressions related to fibroblast growth and cell proliferation [38]. In the literature, no clinical research investigating the effects of LLLT with Nd:YAG laser in addition to SRP for periodontitis treatment has been found.
To the best of our knowledge, there is no information about LLLT with Nd:YAG laser or combination of pocket debridement and LLLT with Nd:YAG laser in periodontal treatment of smokers with periodontitis. Our hypothesis is that different Nd:YAG laser applications as pocket debridement, LLLT, and combination of pocket debridement and LLLT with SRP may enhance the healing response in smokers with periodontitis. The purpose of the present clinical trial was to explore the short-term effects of different application methods of Nd:YAG laser (pocket debridement, LLLT, and pocket debridement+LLLT) in addition to SRP in treatment of smokers with stage III grade C periodontitis.
Material and methods
This study was a single-blind, randomized controlled trial and carried out using parallel group design. The protocol for this clinical trial was reviewed and approved by the Ethics Committee of Izmir Katip Celebi University for human subjects (2017/106) and also the Republic of Turkey Ministry of Health, Turkey Pharmaceuticals and Medical Devices Agency (No: 71146310-511.06-E.213417 Subject: 2017-102). The registration number for clinical trials is TCTR20190926001. A written informed consent form following the Helsinki Declaration (1975; revised, 2013) was obtained from all participants.
Sample size calculation was performed using G*Power software, version 3.1.5 (Franz Faul, Christian-Albrechts-Universität Kiel, Kiel, Germany). PD parameter was used for sample size calculation, and effect size was 0.37 considering the previous research similar to the present study [34]. A minimum of 20 participants for each group were needed in this study with a power of 80 % and α = 0.05 according to power analysis software.
For the present study, of a total 250 individual screened, systemically healthy 80 smokers with chronic periodontitis (CP) (44 females, 36 males aged 23 to 64 years) were enrolled from October 2017 to May 2019 at the Department of Periodontology, Faculty of Dentistry, Izmir Katip Celebi University, Izmir, Turkey. According to the consensus report of the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions [39], the individuals who participated in the present clinical trial match with the definition of generalized stage III periodontitis in terms of the extent and severity and grade C due to radiographic bone loss (%)/age being > 1.0 and smoking ≥ 10 cigarettes a day. Radiographic bone loss %/age was calculated according to previous reports [40, 41]. For determination of radiographic bone loss/age, the tooth showing the most severe bone loss is used. Radiographic bone loss %/age measured as radiographicbone loss in percentage of root length divided by the age of the subject.
Periodontitis patients had PD ≥ 6 mm, CAL ≥ 5 mm, alveolar bone loss at radiographs reaching to the middle or apical third of the root, BOP ≥ 30%, and 30% or more of the teeth with CAL ≥ 5 mm (generalized type) in terms of disease extent and distribution
Exclusion criteria were as follows: (i) having periodontal therapy in the last 1 year, (ii) using any drugs (anti-inflammatory/antibiotics/steroids) and in last 6 months, (iii) pregnancy or lactation, and (iv) alcohol consumption.
Clinical assessments
The following measurements were performed at 1–2 days before GCF sampling visit and 1 and 3 months after periodontal treatment by the same blinded calibrated periodontist (İ.T):
-
Six sites (mesiobuccal, mid-buccal, disto-buccal, mesiolingual/mesio-palatal, mid-lingual/mid-palatal, and disto-lingual/disto-palatal) in each tooth for PD and CAL
-
Four sites (mesial, buccal, lingual/palatinal and distal) in each tooth for GI [42], PI [43], and BOP [44]. A periodontal probe (Hu-Friedy, Chicago, IL, USA) was used for measurements.
Calibration of examiner
For calibration of examiner, four patients, each having two pairs of contralateral teeth with PD > 5 mm, were chosen. The examiner (İ.T) evaluates these patients on 2 visits, 48 h apart. Calibration was accepted if the baseline and 48 h later data were similar to the millimeter at a > 90% level.
Treatment procedure
Supragingival debridement was performed using hand instruments (Hu- Friedy, Chicago, IL, USA) and ultrasonic scalers (EMS Mini-Piezon, Nyon, Switzerland), and detailed oral hygiene instructions were given for all participants after the initial GCF sampling by an experienced periodontologist (AE). The randomization for therapy allocation was done at this visit. A computer-generated set of random numbers obtained from a website (http://www.random.org) were used for randomization process. A computer-generated random list was used for randomizing the participants to one of the four study groups. Opaque sealed envelopes were used for allocation concealment. Blinding was provided by the study coordinator (MS) who registered the treatment assignment and was not associated with the treatments and examinations of the study. Study treatment groups were as follows:
-
Group 1: SRP alone (control group)
-
Group 2: SRP + LLLT with Nd:YAG laser
-
Group 3: SRP + pocket debridement with Nd:YAG laser
-
Group 4: SRP + combined pocket debridement and LLLT with Nd:YAG laser
In group 1:
Subgingival SRP procedures were performed using hand instruments (H6/7 scaler, Gracey Curets, Hu- Friedy, Chicago, IL, USA) and ultrasonic scaler with subgingival tips under local anesthesia in full mouth manner. Saline irrigation was done subgingivally in addition to SRP procedure.
In group 2:
First, the same procedure in group 1 was performed. Then LLLT was applied to the pocket site for 2 min (1 min buccally + 1 min lingually/palatinally) from 1 cm distance using Nd:YAG laser (Fotona Fidelis AT, Ljubljana, Slovenia) (0.50-W, 10 Hz, long-pulse mode) with a 950-µm fiber handpiece (R24).
In group 3:
First, the same procedure in group 1 was performed. Then pocket debridement with Nd:YAG laser (Fotona Fidelis AT, Ljubljana, Slovenia) (2 W, 200 mj, 10 Hz, short pulse) was performed for removing pocket epithelium and detoxification purpose. A 320-µm fiber tip was used for this procedure. The laser fiber tip was placed in the periodontal pocket parallel to the root surface, and a sweeping motion was used in apical to coronal direction during the laser irradiation. Laser irradiation was applied to the pocket site for 30 s.
In group 4:
First, the same procedure in group 1 was performed. Then pocket debridement with Nd:YAG laser (2 W, 200 mj, 10 Hz, short pulse) was performed as in group 3, and finally LLLT with Nd:YAG laser (0.50 W, 10 Hz, long-pulse) was performed as in group 2.
All these treatment procedures were performed by an experienced periodontologist (AE).
GCF sampling and analysis
GCF sampling was performed at baseline, 1 and 3 months after treatment. The paper strip method was chosen for GCF sampling. Sampling was done 1–2 days after baseline clinical recordings. An interproximal site (deepest pocket with PD ≥ 6 mm, CAL ≥5 mm, BOP (+) and GI = 2) of one selected tooth from each quadrant was chosen for GCF sampling in each participant.
After isolation of the sampling area was provided, a sterile curette was used for supragingival biofilm removal. Sampling sites were gently dried before sampling, and then paper strips (Periopaper, Oraflow Inc.) were gently placed into the periodontal pocket (1–2 mm subgingivally) and removed after 30 s. Paper strips contaminated with blood and saliva were discarded. GCF volume was measured by a calibrated device (Periotron 8000, Oraflow). Totally four strips were obtained from each participant and pooled in the same sterile tube. Samples were stored at –80 °C before biochemical analysis.
A commercial enzyme-linked immunosorbent assay kit (Elabscience, Houston, USA) was used for analyzes of GCF MMP-8 level in accordance with the manufacturer’s instructions.
Outcome variables
In this study, PD was the primary outcome and used for calculating the study sample size. CAL, GI, PI, BOP (%), and GCF levels of MMP-8 were secondary outcome variables.
Statistical analyses
A software program (SPSS v. 20.0; IBM, Chicago, IL) was used for statistical analyses. The normality of the data was evaluated by the Shapiro-Wilk test. One-way analysis of variance (ANOVA) followed by Tukey’s test was used for intergroup analysis, and repeated measures ANOVA test was used for intragroup analysis in full-mouth PD, CAL, GI, PI, and BOP (%) parameters and MMP-8 levels. The post-hoc test was used after repeated measures ANOVA for intragroup comparison between two different time points (baseline, 1 month, and 3 months after treatment). Kruskal–Wallis test followed by Dunn’s test were used for intergroup analysis in PD and CAL changes. A p value of < 0.05 was considered to be statistically significant.
Results
A study flowchart is presented in Fig. 1. All participants completed the study, and no adverse events were observed in treatment groups. Table 1 presents baseline demographic data of all participants in study groups.
Clinical findings
Full-mouth PD, CAL, PI, GI, BOP (%), and GCF volume values are presented in Table 2. These parameters significantly reduced at 1 and 3 months postoperatively compared to baseline in all study groups (p ˂ 0.01). However, there were no significant differences between study groups at any time point in terms of these parameters (p ˃ 0.05).
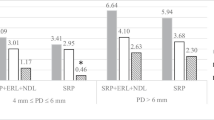
PD and CAL changes for moderately deep pockets (4 mm ≤ PD ≤ 6 mm) are presented in Table 3. The number of sites for moderately deep pockets for groups was as follows: group 1: 914, group 2: 901, group 3: 926, and group 4: 906. All test groups provided more PD and CAL reductions compared to group 1 between baseline and 1 month and between baseline and 3 months (p < 0.001). Test groups showed similar effects in PD and CAL reductions between baseline and 1 month and between baseline and 3 months (p > 0.05).
PD and CAL changes for deep pockets (PD ≥ 7 mm) are presented in Table 3. The number of sites for deep pockets for groups was as follows: group 1: 76, group 2: 75, group 3: 72, and group 4: 81. There were greater PD and CAL reductions in group 2 and group 4 compared to group 1 between baseline and 1 month (p = 0.001). These reductions were similar between test groups and also between group 1 and group 3 (p > 0.05). There was more significant reduction for PD in group 2 compared to other groups between baseline and 3 months (p ˂ 0.05). PD reduction was higher in group 4 compared to group 1 (p ˂ 0.05) but similar to group 3 between baseline and 3 months (p > 0.05). PD reduction was also similar between group 1 and group 3 between baseline and 3 months (p > 0.05). There were more significant reductions in CAL in group 2 and group 4 compared to group 1 between baseline and 3 months (p < 0.001). These reductions were similar between test groups (p > 0.05) and also between group 1 and group 3 (p > 0.05).
Biochemical findings
GCF total amounts of MMP-8 for study groups are presented in Table 4. All study groups provided significant reductions in GCF MMP-8 levels at 1 and 3 months after therapy compared to baseline (p ˂ 0.05). There were no significant differences between all study groups at 1 and 3 months after treatment in terms of MMP-8 levels (p > 0.05).
Discussion
In this study, we examined whether different Nd:YAG laser applications in addition to non-surgical periodontal therapy improve the healing response to periodontal therapy in smokers.
Based on our clinical findings, full-mouth PI, GI, and BOP values were significantly reduced in all treatment groups compared to baseline. No statistically significant differences were found between the test groups and group 1 at 1 and 3 months postoperatively in terms of these parameters. There were also no significant differences between test groups. This finding confirmed the importance of conventional SRP procedure and performing daily optimal individual oral hygiene instructions in the healing of periodontal disease independently of the treatment procedure. Thus, in this study, it has been shown once again that SRP with oral hygiene instructions is the main procedure (gold standard) in the resolution of periodontal inflammation.
The most striking findings in this study were significant reductions in PD and CAL parameters in moderately deep pockets and deep pockets in laser-treated groups compared to the control group. Considering group 2, it has been shown in the literature that LLLT with Nd:YAG laser has beneficial effects on wound healing and suppressing inflammation such as inducing proliferation of gingival fibroblasts [35] and periodontal ligament fibroblasts [36], stimulation of epidermal growth factor [35], type I collagen, platelet-derived growth factor and basic fibroblast growth factor expressions [45], and inhibiting TNF-α expression for bone marrow mesenchymal stem cells in an inflammatory environment [46]. In our study, the favorable results for LLLT with Nd:YAG laser in PD and CAL parameters may be related to these beneficial effects of Nd:YAG laser mentioned above. As it was mentioned before, we could not find any study investigating the effects of LLLT with Nd:YAG laser as an adjunct to non-surgical periodontal therapy in the treatment of periodontitis. In most of clinical studies investigating the efficacy of LLLT, diode lasers have been used. Considering some of these studies, it has been demonstrated that LLLT with diode laser provide significant improvements in PD and CAL parameters compared to SRP alone [47,48,49]. Our results were in agreement with these studies.
Considering group 3, the literature suggests that the pocket therapy with Nd:YAG laser is beneficial for periodontal healing such as complete removal of pocket epithelium [50], formation of new cementum and new connective tissue attachment on root surface [51], and eliminating endotoxins and smear layer from root surface [52]. These favorable effects may provide significant PD and CAL reductions in group 3 compared to group 1 in our study. Based on the clinical studies, there are contrary results in the literature. While some investigators have reported additional benefits of Nd:YAG laser as an adjunct to conventional therapy in periodontal pocket healing [30, 32, 53], others have not [54,55,56]. These controversial findings might be due to the different application power densities, type of laser fiber tip, application time, single or repeated application of laser, and severity of the periodontal disease. Our clinical results are in agreement with the studies that demonstrated beneficial effects of Nd:YAG laser [30, 32, 53]. Considering the smoker population in our study, we could find two clinical studies investigating the efficiency of additional Nd:YAG laser in smokers with periodontitis patients [34, 57].
Maden reported that SRP+Nd:YAG laser (2 W, 100 mj, 20 Hz) provided significant improvements in PD and CAL parameters compared to SRP alone between baseline and first month and between baseline and 3 months in moderately deep pockets (4 mm ≤ PD ≤ 6 mm). They also found significant improvements in PD compared to SRP alone between baseline and first month in deep pockets (PD ≥ 7 mm) [57]. Our clinical results were in agreement with this study in terms of PD and CAL changes in moderately deep pockets but not for deep pockets. These different findings may be related to the application times of laser and application methods. While laser irradiation was performed at buccal and lingual/palatinal root surfaces for total 50 s and laser treatment was performed three times, 1 week apart in their study, laser irradiation was performed to the only pocket site for 30 s, and laser treatment was performed only once in our study. In another study, Eltas et al. demonstrated that SRP+Nd:YAG laser and SRP alone have similar effects in PD and CAL parameters in smokers with chronic periodontitis patients [34]. Their energy setting was 1 W, 100 mj, and 10 Hz, and laser irradiation was conducted mesially, distally, buccally, and lingually for a total of 120 s. The different findings between their study and ours may be due to the energy setting. In our study, energy setting was 2 W, 200 mj, and 10 Hz, and it was demonstrated that this energy setting could completely remove diseased pocket epithelium without damaging the healthy epithelium [50].
While we found that additional pocket debridement with Nd:YAG laser provides significant improvements in PD and CAL parameters compared to group1 in moderately deep pockets, we did not find these improvements in deep pockets. This situation may be associated with the same laser application time (30 s) for both moderately deep pockets and deep pockets. This application time may be beneficial for moderately deep pockets but insufficient for deep pockets. Another reason is that, since mechanical debridement may be insufficient in pockets of 7 mm and greater, dark colored subgingival calculus at the apical region of the pocket may absorb the Nd:YAG laser beam, causing thermal damage on the cementum surface and adversely affect the healing.
In group 4, a combined laser procedure was applied for the first time in this study to the best of our knowledge. Considering the beneficial effects of pocket debridement with Nd:YAG laser and LLLT with Nd:YAG laser as we mentioned above, these findings for PD and CAL parameters were expected.
It is known that the Nd:YAG laser is well absorbed by dark pigments [58] and gingival melanin pigmentation is common in smokers. Nd:YAG laser may have been well-absorbed by these pigmented tissues and become more effective in the treatment of smokers with periodontitis.
Considering the effects of lasers on microbiological parameters in the literature, Petrovic et al. reported that additional LLLT could decrease the levels of T. forsythia, T. denticola, P. gingivalis, P. intermedia, and A. actinomycetemcomitans in chronic periodontitis patients [59]. Hatit et al. also indicated that additional Nd:YAG could decrease the levels of T. forsythia, T. denticola, P. gingivalis, and A. actinomycetemcomitans in periodontitis patients [28]. In our study, we did not examine the microbiologic parameters, but the probable anti-microbial effect of Nd:YAG laser biostimulation and pocket debridement with Nd:YAG may have improved the PD and CAL parameters in laser groups compared to group 1.
Comparing the test groups, we generally observed similar effects in PD and CAL reductions except for PD reduction between baseline and 3 months in deep pockets. PD reduction for deep pockets was significantly greater in group 2 compared to group 3 and group 4 between baseline and 3 months. This situation may be explained by possible recolonization facilitating effect of pocket debridement with Nd:YAG laser in group 3 and group 4. It has been reported that Nd:YAG laser may cause pits and crater formation (porous structures) on the surface with charring, carbonization, and melting, even if the laser application is made parallel to the root surface [60]. This root surface may accelerate the recolonization rate of bacteria.
Considering the MMP-8 levels in GCF, significant reductions were found in all treatment groups at 1 and 3 months postoperatively compared to baseline. We did not observe any significant differences between the control and test groups at any time point in terms of MMP-8 levels. Considering the studies investigating the MMP-8 levels after Nd:YAG laser applications, Qadri et al. demonstrated that there was no significant difference in MMP-8 levels after treatment between SRP+LLLT with diode laser and SRP alone [47]. In another clinical study, Eltas et al. reported that SRP+Nd:YAG laser and SRP alone have similar effects on reducing MMP-8 levels [31]. Our findings for MMP-8 levels were in agreement with these studies. Reductions in MMP-8 levels and full-mouth PI, GI, and BOP were consistent. Three different laser procedures were effective in the resolution of inflammation, but they did not provide any additional advantage.
Considering the limitations of this study, microbiological parameters could be investigated. If we could examine the levels of periodontopathogens before and after treatment in our study, we could better explain the significant improvements in PD and CAL parameters in the test groups. Smoking status could be managed as light, moderate, and heavy smokers. Thus, we would have observed how much smoking status affects the response to treatment. At last the study could be conducted with a greater sample size.
Conclusion
The findings of this study demonstrated that additional Nd:YAG laser applications provided more significant reductions in PD and CAL parameters in moderately deep and deep pockets compared to SRP alone in smokers with stage III grade C periodontitis. Therefore, in this study, we showed that the Nd:YAG laser may be beneficial for the PD and CAL parameters of the healing response of smokers to non-surgical periodontal therapy.
Considering the application methods, LLLT with Nd:YAG laser is more suitable for clinicians and patients regarding treatment time and ease of application. Well-designed, greater sample-sized studies searching the long-term results of these procedures are needed to understand the efficacy of Nd:YAG laser on the healing response of smokers more clearly.
References
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS (2018) Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89(Suppl 1):S173–S182. https://doi.org/10.1002/JPER.17-0721
Bergstrom J, Preber H (1994) Tobacco use as a risk factor. J Periodontol 65(5):545–550. https://doi.org/10.1902/jop.1994.65.5.545
Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, Norderyd OM, Genco RJ (1994) Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol 65(3):260–267. https://doi.org/10.1902/jop.1994.65.3.260
Preber H, Bergstrom J (1986) Cigarette smoking in patients referred for periodontal treatment. Scand J Dent Res 94(2):102–108. https://doi.org/10.1111/j.1600-0722.1986.tb01372.x
Bergstrom J (1989) Cigarette smoking as risk factor in chronic periodontal disease. Community Dent Oral Epidemiol 17(5):245–247. https://doi.org/10.1111/j.1600-0528.1989.tb00626.x
Bergstrom J, Eliasson S (1987) Cigarette smoking and alveolar bone height in subjects with a high standard of oral hygiene. J Clin Periodontol 14(8):466–469. https://doi.org/10.1111/j.1600-051x.1987.tb02253.x
Grossi SG, Genco RJ, Machtei EE, Ho AW, Koch G, Dunford R, Zambon JJ, Hausmann E (1995) Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol 66(1):23–29. https://doi.org/10.1902/jop.1995.66.1.23
Holm G (1994) Smoking as an additional risk for tooth loss. J Periodontol 65(11):996–1001. https://doi.org/10.1902/jop.1994.65.11.996
Albandar JM, Streckfus CF, Adesanya MR, Winn DM (2000) Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol 71(12):1874–1881. https://doi.org/10.1902/jop.2000.71.12.1874
Mullally BH, Linden GJ (1996) Molar furcation involvement associated with cigarette smoking in periodontal referrals. J Clin Periodontol 23(7):658–661. https://doi.org/10.1111/j.1600-051x.1996.tb00590.x
Axelsson P, Paulander J, Lindhe J (1998) Relationship between smoking and dental status in 35-, 50-, 65-, and 75-year-old individuals. J Clin Periodontol 25(4):297–305. https://doi.org/10.1111/j.1600-051x.1998.tb02444.x
Heitz-Mayfield LJ (2005) Disease progression: identification of high-risk groups and individuals for periodontitis. J Clin Periodontol 32(Suppl 6):196–209. https://doi.org/10.1111/j.1600-051X.2005.00803.x
Kibayashi M, Tanaka M, Nishida N, Kuboniwa M, Kataoka K, Nagata H, Nakayama K, Morimoto K, Shizukuishi S (2007) Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J Periodontol 78(5):859–867. https://doi.org/10.1902/jop.2007.060292
Mantyla P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, Sorsa T (2006) Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res 41(6):503–512. https://doi.org/10.1111/j.1600-0765.2006.00897.x
Ogawa H, Yoshihara A, Hirotomi T, Ando Y, Miyazaki H (2002) Risk factors for periodontal disease progression among elderly people. J Clin Periodontol 29(7):592–597. https://doi.org/10.1034/j.1600-051x.2002.290702.x
Bergstrom J, Eliasson S, Dock J (2000) A 10-year prospective study of tobacco smoking and periodontal health. J Periodontol 71(8):1338–1347. https://doi.org/10.1902/jop.2000.71.8.1338
Norderyd O, Hugoson A, Grusovin G (1999) Risk of severe periodontal disease in a Swedish adult population. A longitudinal study. J Clin Periodontol 26(9):608–615. https://doi.org/10.1034/j.1600-051x.1999.260908.x
Preber H, Bergstrom J (1986) The effect of non-surgical treatment on periodontal pockets in smokers and non-smokers. J Clin Periodontol 13(4):319–323. https://doi.org/10.1111/j.1600-051x.1986.tb02229.x
Preber H, Linder L, Bergstrom J (1995) Periodontal healing and periopathogenic microflora in smokers and non-smokers. J Clin Periodontol 22(12):946–952. https://doi.org/10.1111/j.1600-051x.1995.tb01800.x
Preber H, Bergstrom J (1986) Effect of non-surgical treatment on gingival bleeding in smokers and non-smokers. Acta Odontol Scand 44(2):85–89. https://doi.org/10.3109/00016358609041312
Drisko CH (2001) Nonsurgical periodontal therapy. Periodontology 2000(25):77–88
Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontology 2000(36):59–97. https://doi.org/10.1111/j.1600-0757.2004.03679.x
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM (2000) Izumi Y (2009) Application of lasers in periodontics: true innovation or myth?. Periodontology 50:90–126. https://doi.org/10.1111/j.1600-0757.2008.00283.x
Cobb CM, Low SB, Coluzzi DJ (2010) Lasers and the treatment of chronic periodontitis. Dent Clin N Am 54(1):35–53. https://doi.org/10.1016/j.cden.2009.08.007
Parker S (2007) Lasers and soft tissue: periodontal therapy. Br Dent J 202(6):309–315. https://doi.org/10.1038/bdj.2007.224
White JM, Goodis HE, Rose CL (1991) Use of the pulsed Nd:YAG laser for intraoral soft tissue surgery. Lasers Surg Med 11(5):455–461. https://doi.org/10.1002/lsm.1900110511
White JM, Goodis HE, Cohen JN (1991) Bacterial reduction of contaminated dentin by Nd:YAG laser. J Dent Res 70:412
Ben Hatit Y, Blum R, Severin C, Maquin M, Jabro MH (1996) The effects of a pulsed Nd:YAG laser on subgingival bacterial flora and on cementum: an in vivo study. J Clin Laser Med Surg 14(3):137–143. https://doi.org/10.1089/clm.1996.14.137
Giannelli M, Bani D, Viti C, Tani A, Lorenzini L, Zecchi-Orlandini S, Formigli L (2012) Comparative evaluation of the effects of different photoablative laser irradiation protocols on the gingiva of periodontopathic patients. Photomed Laser Surg 30(4):222–230. https://doi.org/10.1089/pho.2011.3172
Qadri T, Poddani P, Javed F, Tuner J, Gustafsson A (2010) A short-term evaluation of Nd:YAG laser as an adjunct to scaling and root planing in the treatment of periodontal inflammation. J Periodontol 81(8):1161–1166. https://doi.org/10.1902/jop.2010.090700
Eltas A, Orbak R (2012) Effect of 1,064-nm Nd:YAG laser therapy on GCF IL-1beta and MMP-8 levels in patients with chronic periodontitis. Lasers Med Sci 27(3):543–550. https://doi.org/10.1007/s10103-011-0939-5
Qadri T, Tuner J, Gustafsson A (2015) Significance of scaling and root planing with and without adjunctive use of a water-cooled pulsed Nd:YAG laser for the treatment of periodontal inflammation. Lasers Med Sci 30(2):797–800. https://doi.org/10.1007/s10103-013-1432-0
Martelli FS, Fanti E, Rosati C, Martelli M, Bacci G, Martelli ML, Medico E (2016) Long-term efficacy of microbiology-driven periodontal laser-assisted therapy. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 35(3):423–431. https://doi.org/10.1007/s10096-015-2555-y
Eltas A, Orbak R (2012) Clinical effects of Nd:YAG laser applications during nonsurgical periodontal treatment in smoking and nonsmoking patients with chronic periodontitis. Photomed Laser Surg 30(7):360–366. https://doi.org/10.1089/pho.2011.3184
Gkogkos AS, Karoussis IK, Prevezanos ID, Marcopoulou KE, Kyriakidou K, Vrotsos IA (2015) Effect of Nd:YAG low level laser therapy on human gingival fibroblasts. Int J Dent 2015:258941. https://doi.org/10.1155/2015/258941
Prevezanos ID, Karoussis IK, Gkogkos AS, Marcopoulou KE, Kyriakidou K, Chernysheva A, Vrotsos IA (2018) Effect of Nd: YAG Low Level Laser Therapy on human periodontal ligament cells: A preliminary in-vitro study. Biomed J Sci Tech Res 2(4):2797–2802
Arisu HD, Turkoz E, Bala O (2006) Effects of Nd: Yag laser irradiation on osteoblast cell cultures. Lasers Med Sci 21(3):175–180. https://doi.org/10.1007/s10103-006-0398-6
Usumez A, Cengiz B, Oztuzcu S, Demir T, Aras MH, Gutknecht N (2014) Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci 29(6):1807–1813. https://doi.org/10.1007/s10103-013-1336-z
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, Greenwell H, Herrera D, Kao RT, Kebschull M, Kinane DF, Kirkwood KL, Kocher T, Kornman KS, Kumar PS, Loos BG, Machtei E, Meng H, Mombelli A, Needleman I, Offenbacher S, Seymour GJ, Teles R, Tonetti MS (2018) Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal Clin Periodontol 45(Suppl 20):S162–S170. https://doi.org/10.1111/jcpe.12946
Tonetti MS, Greenwell H, Kornman KS (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol 89(Suppl 1):S159–S172. https://doi.org/10.1002/JPER.18-0006
Kornman KS, Papapanou PN (2020) Clinical application of the new classification of periodontal diseases: Ground rules, clarifications and “gray zones.” J Periodontol 91(3):352–360. https://doi.org/10.1002/JPER.19-0557
Loe H (1967) The gingival index, the plaque index and the retention index systems. J Periodontol 38(6):610–616. https://doi.org/10.1902/jop.1967.38.6.610
Silness J, Loe H (1964) Periodontal disease in pregnancy. Ii. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand 22:121–135. https://doi.org/10.3109/00016356408993968
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25(4):229–235
Chellini F, Sassoli C, Nosi D, Deledda C, Tonelli P, Zecchi-Orlandini S, Formigli L, Giannelli M (2010) Low pulse energy Nd:YAG laser irradiation exerts a biostimulative effect on different cells of the oral microenvironment: an in vitro study. Lasers Surg Med 42(6):527–539. https://doi.org/10.1002/lsm.20861
Wang L, Wu F, Liu C, Song Y, Guo J, Yang Y, Qiu Y (2019) Low-level laser irradiation modulates the proliferation and the osteogenic differentiation of bone marrow mesenchymal stem cells under healthy and inflammatory condition. Lasers Med Sci 34(1):169–178. https://doi.org/10.1007/s10103-018-2673-8
Qadri T, Miranda L, Tuner J, Gustafsson A (2005) The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol 32(7):714–719. https://doi.org/10.1111/j.1600-051X.2005.00749.x
Aykol G, Baser U, Maden I, Kazak Z, Onan U, Tanrikulu-Kucuk S, Ademoglu E, Issever H, Yalcin F (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82(3):481–488. https://doi.org/10.1902/jop.2010.100195
Saafan A, El-Nahass H, Nasr AS, Radwan R (2013) Effect of low power diode laser 810 nm on TGF-β1 level in GCF in aggressive periodontitis. J Dent Lasers 7(2):59–65
Ting CC, Fukuda M, Watanabe T, Sanaoka A, Mitani A, Noguchi T (2014) Morphological alterations of periodontal pocket epithelium following Nd:YAG laser irradiation. Photomed Laser Surg 32(12):649–657. https://doi.org/10.1089/pho.2014.3793
Yukna RA, Carr RL, Evans GH (2007) Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent 27(6):577–587
Fukuda M, Minoura S, Ishikawa K, Ogura N, Ueda N, Murase M, Sugihara N, Kato K, Nakagaki H, Noguchi T (1994) Effects of Nd:YAG laser irradiation on endotoxin in exposed cementum [in Japanese, English abstract]. Jpn J Conserv Dent 37:711–716
Abduljabbar T, Vohra F, Kellesarian SV, Javed F (2017) Efficacy of scaling and root planning with and without adjunct Nd:YAG laser therapy on clinical periodontal parameters and gingival crevicular fluid interleukin 1-beta and tumor necrosis factor-alpha levels among patients with periodontal disease: a prospective randomized split-mouth clinical study. J Photochem Photobiol B Biol 169:70–74. https://doi.org/10.1016/j.jphotobiol.2017.03.001
Gomez C, Dominguez A, Garcia-Kass AI, Garcia-Nunez JA (2011) Adjunctive Nd:YAG laser application in chronic periodontitis: clinical, immunological, and microbiological aspects. Lasers Med Sci 26(4):453–463. https://doi.org/10.1007/s10103-010-0795-8
Slot DE, Kranendonk AA, Van der Reijden WA, Van Winkelhoff AJ, Rosema NA, Schulein WH, Van der Velden U, Van der Weijden FA (2011) Adjunctive effect of a water-cooled Nd:YAG laser in the treatment of chronic periodontitis. J Clin Periodontol 38(5):470–478. https://doi.org/10.1111/j.1600-051X.2010.01695.x
Radvar M, MacFarlane TW, MacKenzie D, Whitters CJ, Payne AP, Kinane DF (1996) An evaluation of the Nd:YAG laser in periodontal pocket therapy. Br Dent J 180(2):57–62. https://doi.org/10.1038/sj.bdj.4808976
Maden I (2009) Effects of Nd:YAG laser treatment as an adjunct to conventional periodontal therapy [PhD Thesis]. Istanbul: Istanbul University;:113p
Cobb CM (2006) Lasers in periodontics: a review of the literature. J Periodontol 77(4):545–564. https://doi.org/10.1902/jop.2006.050417
Petrovic MS, Kannosh IY, Milasin JM, Mihailovic DS, Obradovic RR, Bubanj SR, Kesic LG (2018) Clinical, microbiological and cytomorphometric evaluation of low-level laser therapy as an adjunct to periodontal therapy in patients with chronic periodontitis. Int J Dent Hyg 16(2):e120–e127. https://doi.org/10.1111/idh.12328
Morlock BJ, Pippin DJ, Cobb CM, Killoy WJ, Rapley JW (1992) The effect of Nd:YAG laser exposure on root surfaces when used as an adjunct to root planing: an in vitro study. J Periodontol 63(7):637–641. https://doi.org/10.1902/jop.1992.63.7.637
Acknowledgements
We would like to thank Assoc. Prof. Ferhan Elmalı for his statistical assistance.
Funding
This study was funded by The Scientific Research Coordination Office of Izmir Katip Çelebi University (2017-TDU-DİŞF-0034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures in this study were approved by the Ethics Committee of Izmir Katip Celebi University for human subjects (2017/106) and also the Republic of Turkey Ministry of Health, Turkey Pharmaceuticals and Medical Devices Agency (No: 71146310-511.06-E.213417 Subject: 2017-102). Clinical trial serial number: TCTR20190926001.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ezber, A., Taşdemir, İ., Yılmaz, H.E. et al. Different application procedures of Nd:YAG laser as an adjunct to scaling and root planning in smokers with stage III grade C periodontitis: a single-blind, randomized controlled trial. Ir J Med Sci 192, 457–466 (2023). https://doi.org/10.1007/s11845-022-02940-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-022-02940-z