Abstract
The aim of the present study was to compare the effectiveness of four different laser wavelengths (660, 810, 980, and 1,064 nm) used for low-level laser therapy (LLLT) on the healing of mucositis in an animal model of wound healing by investigating the expression of platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and blood-derived fibroblast growth factor (bFGF). Thirty-five male Wistar albino rats with a weight of 250–300 g body mass and 5 months old were used in the study. All animals were intraperitoneally injected with 100 mg/kg of 5-fluorouracil (5-FU) on the first day and 65 mg/kg of 5-FU on the third day. The tip of an 18-gauge needle was used in order to develop a superficial scratching on the left cheek pouch mucosa by dragging twice in a linear movement on third and fifth days. After ulcerative mucositis were clinically detected on the animals' left cheek pouch mucosa, the laser therapy was started. Four different laser wavelengths (660 nm, HELBO, Bredent; 810 nm, Fotona XD, Fotona; 980 nm, ARC Fox; and 1,064 nm, Fidelis Plus 3, Fotona) used for LLLT at ED 8 J/cm2 daily from the first to the fourth days. Oval excisional biopsy was taken from the site of the wound, and the expression of PDGF, TGF-β, and bFGF was evaluated. The obtained data were analyzed by one2-way ANOVA, and then Tukey HSD tests were used for pairwise comparisons among groups (α = 0.05). The one-way ANOVA test indicated that expression values of the growth factors, PDGF and bFGF, were significantly affected by irradiation of different wavelengths of lasers (p < 0.001). However, expression value of the TGF-β was not affected by irradiation of different wavelengths of lasers (p > 0.05). The highest PDGF expression was detected in neodymium-doped yttrium aluminum garnet (Nd:YAG) laser group (p < 0.05), and there were no statistically significant differences among the other groups (p > 0.05). The highest bFGF expression was detected in 980-nm diode and Nd:YAG laser groups (p < 0.05), and there were no statistically significant differences among the other groups (p > 0.05). These findings suggest that low-level Nd:YAG and 980-nm diode laser therapy accelerate the wound healing process by changing the expression of PDGF and bFGF genes responsible for the stimulation of the cell proliferation and fibroblast growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oropharyngeal mucositis is an acute and distressing toxic effect of chemotherapy and head and neck irradiation [1]. Varying degrees of mucositis severity are seen in patients receiving cytotoxic therapy, ranging from mild sensation changes to multiple, ulcerative, bleeding lesions. These lesions may be present throughout the oral cavity and the gastrointestinal tract. Common complaints of patients related to oral mucositis include changes in sensation, difficulty talking and swallowing, the presence of mouth sores, and sometimes dryness and pain. The symptoms including bleeding, infection, ulceration, and xerostomia alter the nutritional status of the patient [2,3].
Wound healing process is divided into three phases: an inflammatory phase, a proliferative phase, and a remodeling phase. During the inflammatory phase, platelets, neutrophils, macrophages, and lymphocytes migrate to a wound. The proliferative phase shows an increase in fibroblasts. During the remodeling phase, fibroblasts help recreate the extracellular matrix and deposit collagen [4]. Platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and blood-derived fibroblast growth factor (bFGF) are known to have anti-inflammatory and wound healing effects [5]. PDGF increases the secretion of other growth factors by stimulating macrophages. TGF-β is an important signal in stopping immune and inflammatory responses and has potent effects on wound healing processes. TGF-β also antagonizes some lymphocytic responses. bFGF stimulates the growth of fibroblasts, which have an important role in healing processes [6]. PDGF, TGF-β, and bFGF are among the most important growth factors of tissues, and therefore, healing processes may be promoted by laser irradiation via increased production of these growth factors.
Low-level laser therapy (LLLT) is a form of phototherapy that involves the application of low-power, monochromatic, coherent light to injuries and lesions. It has been used to successfully induce wound healing in cases of healing defects by inducing increases in mitotic activity, numbers of fibroblasts, collagen synthesis, and neovascularization. Laser light with a longer wavelength, such as the (infrared) diode laser, produced using the gallium arsenide (GaAs) or gallium aluminum arsenide (GaAlAs) laser penetrates deeper, whereas laser light with a shorter wavelength, such as red light, produced using the He–Ne laser penetrates less deeply [7]. Different biological effects have been observed after diode laser application, namely, a significantly higher fibroblast count than controls in vitro [8], modulation of angiogenic factor production by T lymphocytes [9], and upregulation of the expression of TGF-β1 after stroke in rats [10]; increase of collagen deposition; and greater proliferation of myofibroblasts in experimental cutaneous wounds [11].
The mechanisms of laser radiation interaction at the molecular level are described by Karu, who suggests different action mechanisms for wavelengths emitted in the visible and infrared range [12]. Photobiological reactions involve the absorption of a specific wavelength of light by the functioning photoacceptor molecule. The photobiological nature of the LLLT effects means that a molecule (photoacceptor) must first absorb the light used for irradiation. After the promotion of electronically excited states, primary molecular processes from these states may lead to a measurable biological effect at the cell level [12]. The biological effects include the alteration of cellular metabolism as a result of its being absorbed by cytochrome c oxidase [12]. Cytochrome c oxidase is the enzyme that catalyzes the final step in mitochondrial respiratory chain—the transfer of electrons from cytochrome c to molecular oxygen [12]. The biological effects also includes the increased mitochondrial respiration and adenosine triphosphate (ATP) synthesis, cellular proliferation, enhancement and promotion of skeletal muscle regeneration following injury, enhanced collagen synthesis in the wound area, and increased wound tensile strength [13–15]. Stimulation of cell proliferation results from an increase in mitochondrial respiration and ATP synthesis, among other proposed mechanisms including nitric oxide, and reactive oxygen species-mediated pathways [16].
The benefits of diode lasers in wound healing are still controversial, and many other investigators found no improvement in the wound healing process [17–21]. The majority of negative wound healing studies have been performed on healthy individuals, and the positive results are found in compromised wounds, such as diabetic wounds [22]. Because of these contradictory results, there is still no consensus on the effects of LLLT in the wound healing process. Recent wound healing studies have used various diode lasers with different wavelengths, laser power, and doses [23–25].
With regard to the type of laser and sufficiency of wavelength, generally, little is known about the use of the neodymium-doped yttrium aluminum garnet (Nd:YAG) as a biostimulator, and there has been little attention overall of this aspect of Nd:YAG. The recent investigations contain no comparison in mucosal wound healing effectiveness of diode laser (660, 810, and 980 nm) and Nd:YAG lasers investigating the expression of PDGF, TGF-β, and bFGF. Therefore, the aim of the present study was to compare the effectiveness of four different laser wavelengths (660, 810, 980, and 1,064 nm) used for LLLT on the healing of mucositis in an animal model of wound healing by investigating the expression of PDGF, TGF-β, and bFGF.
Materials and methods
The animals
Thirty-five male Wistar albino rats with a weight of 250–300 g body mass and 5 months old were used. All animals were kept in the laboratory in order to orientate them to the laboratory conditions for at least 5 days prior to the study. All studies were performed in accordance with the National Institutes of Health guidelines on animal care and with the approval of the Ethics Committee of the Gaziantep University, Gaziantep, Turkey.
Mucosa wounding
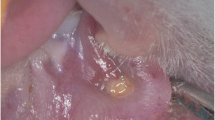
All animals were intraperitoneally injected with 100 mg/kg of 5-fluorouracil (5-FU) on the first day and 65 mg/kg of 5-FU on the third day. The tip of an 18-gauge needle was used in order to develop a superficial scratching on the left cheek pouch mucosa by dragging twice in a linear movement on third and fifth days. This technique has been repeatedly used to develop an ulcerative mucositis, which is similar to the human oral mucositis. The animals were anesthetized with xylazine hydrochloride (XylazineBio) 3 mg/kg and ketamine hydrochloride (Ketasol) 90 mg/kg prior to performing these procedures. After ulcerative mucositis was clinically detected on the animals' left cheek pouch mucosa, the laser therapy was started.
Irradiation protocol
The animals were randomly divided into four groups (n = 10). Laser irradiation was performed with the laser handpiece being kept perpendicular to the mucositis at a distance of 1 cm per point. The laser devices with four different wavelengths were summarized in Table 1.
Group 1
Diode laser (810 nm; Fotona XD-2 diode laser, Fotona, Slovenia) was used for the daily treatment of mucositis from the first to fourth days (continuous mode, beam area 0.28 cm2, application time 9 s, total energy per session 2.24 J, energy density 8 J/cm2).
Group 2
Diode laser (980 nm; ARC Fox, Germany) was used for the daily treatment of mucositis from the first to fourth days (continuous mode, beam area 0.12 cm2, application time 10 s, total energy per session 0.99 J, energy density 8.3 J/cm2).
Group 3
Nd:YAG laser (1,064 nm; Fidelis Plus 3, Fotona, Slovenia) was used for the daily treatment of mucositis from the first to fourth days (pulsed mode, average power 0.25 W, beam area 0.28 cm2, application time 9 s, total energy per session 2.24 J, energy density 8 J/cm2).
Group 4
Diode laser (660 nm; HELBO, Bredent, Germany) was used for the daily treatment of mucositis from the first to fourth days (average power 100 mW, beam area 0.75 cm2, application time 60 s, total energy per session 6 J, energy density 8 J/cm2).
Group 5
In the control group, no laser irradiation was applied to the mucositis. Thirty minutes after completion of the laser therapy, the rats were scarified.
Oval excisional biopsy was taken from the site of the wound, and frozen samples were used for future laboratory procedures, and the specimens were kept in nitrogen tank.
Ribonucleic acid extraction
Ribonucleic acid (RNA) extraction was conducted using binding buffer OBB (20 mM Tris–Cl (pH 7.5), 1 M NaCl, 2 mM EDTA, 0.2 % sodium dodecyl sulfate (SDS)). In brief, 100 μl of frozen sample was mixed with 200 μl Oligotex Suspension 10 % (w/v) in 10 mM Tris–Cl (pH 7.5), 500 mM NaCl, 1 mM EDTA, 0.1 % SDS, and 0.1 % NaN3 (Qiagen cat. no. 79000) and incubated for 24 h at room temperature. Chloroform (50 μl) was added and centrifuged at 12,000 rpm for 15 min at 4 °C. Total RNA was ethanol precipitated and dissolved in 10 μl diethyl pyrocarbonate-treated water.
cDNA preparation for mRNA expression
The kit enabled the transcription of 25 mg RNA into cDNA. In a standard assay of 20 ml with 0.2–2 mg RNA, the synthesis rates were directly proportional to the amount of RNA. The reagents were allowed to thaw, mixed well, and centrifuged briefly to collect the solutions at the bottom of the vials. The reagents were kept on ice while performing the assay and then stored at 215 to 225 °C after the experiment. For checking the synthesis rates of the first and second strand, labeling an aliquot of the assay with dCTP was performed. The incorporation rate was in the standard assay with 2 mg RNA and 20 mCi dCTP, 3,000 Ci/mmol, after 60-min incubation at 42 °C amounts to 2 × 105 cpm. For labeling of the second strand, an aliquot of the nonradioactive first-strand synthesis was used correspondingly (20 mCi per 2 mg input RNA in 20 ml standard assay). The incorporation rates of the second strand were comparable with those of the first strand. For separation of the incorporated and free nucleotides, the application of spin columns was used. For analysis of the reaction products denaturing, alkaline agarose gels were used.
Reverse transcription PCR
To perform PCR using RNA as a starting template, the RNA was first reversely transcribed into cDNA in a reverse transcription (RT) reaction. RT and PCR were carried out either sequentially in the same tube (one-step RT-PCR) or separately (two-step RT-PCR).
RNA samples were reversely transcribed using reverse transcription kit, and DNase treatment was performed according to the manufacturer's protocol (Qiagen Sciences, San Diego, CA). Pre-amplification of cDNA was done using the TaqMan® PreAmp Master Mix kit. Real-time PCR was performed using the TaqMan Universal Master Mix and optimized TaqMan probe sets. Samples were amplified using the ABI 7000 thermocycler. The comparison of normal and laser-treated tissue was used to analyze gene expression. For internal control, GAPDH or beta-actin gene was used.
Statistical analysis
All the values were calculated as mean ± standard deviation by the software for all the evaluated growth factors using the SPSS software version 10.0 (SPSS, Inc., Chicago, IL, USA). The obtained data were analyzed by one-way ANOVA, and then Tukey HSD tests were used for pairwise comparisons among groups (α = 0.05).
Results
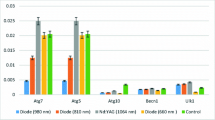
The results of mean TGF-β, PDGF, and bFGF expressions after laser irradiations with different wavelengths are showed in Fig. 1.
The one-way ANOVA test indicated that expression values of the growth factors, PDGF and bFGF, were significantly affected by irradiation of different wavelength types of lasers (p < 0.001); however, expression value of the TGF-β was not affected by irradiation of different wavelength types of lasers (p > 0.05) (Table 2).
When the PDGF values were evaluated, there were statistically significant differences among Nd:YAG laser- and 660-nm laser-irradiated groups and control group (p < 0.05); however, there were no statistically significant differences among Nd:YAG laser-, 980-nm laser-, and 810-nm laser-irradiated groups (p > 0.05).
When bFGF values were evaluated, there were statistically significant differences between 980-nm laser-irradiated group and control group and 660-nm laser-irradiated group (p < 0.05) and also between Nd:YAG laser-irradiated group and control group (p < 0.05); however, there were no statistically significant differences among other groups (p > 0.05) (Table 3).
Discussion
The art and science of photomedicine or phototherapy involving LLLT have become promising and effective tools in prophylactic and therapeutic interventions for oral mucositis and associated orofacial pain [26]. Although in vitro cell culture studies can help determine the potential mechanism of action of LLLT, they cannot duplicate the complex wound healing processes that occur in vivo. Studies examining the effect of LLLT on wound healing in different animal models can provide a more realistic stimulation of the effects on the human skin. Because of the relative ease of working with rodents, most studies have used a rodent model. The relevant investigations have, with mixed results, addressed surgical wound healing, skin flap survival, burn wound healing, and wound tensile strength. In addition to studies of LLLT's effects on skin wounds, studies on bone fractures and nerve regeneration have been conducted [27]. In the current study, an animal wound healing of oral mucositis was seen after chemotherapeutic agent injection was evaluated.
Several possible pathways may lead to the clinical findings reported on trials conducted with LLLT to prevent or treat mucositis; most likely several mechanisms may take place at the same time. Even though control clinical studies are scarce in the literature, the evidence suggests that LLLT may be useful to control and/or treat mucositis [28]. In this context, besides control clinical studies, a clear explanation for this positive effect must be identified. Thus, basic research has to be performed in this field to clarify the exact scope of the LLLT in this clinical condition. The experimental design proposed in the current study seems to be suitable for this end, and the obtained data demonstrated that the results achieved in clinical trials can be simulated with this model. The current study used the model of creating mucositis-inducing 5-FU as described by França et al. [29].
The fibroblast plays an important role in wound healing. Most of the studies published in the LLLT literature have examined the effect of LLLT on fibroblast cell growth. The growth factors which were evaluated in the current study have anti-inflammatory effects. The bFGF stimulates cell proliferation and differentiation of many cell types, stimulates fibroblast growth, has an important role in healing processes, and regulates melanocyte growth and melanogenesis [30]. The TGF-β regulates multiple cellular functions including inhibition and stimulation of cell growth, apoptosis, cellular differentiation, and extracellular matrix synthesis, is an important signal in stopping immune and inflammatory responses, has potent effects on wound healing processes, and inhibits melanocyte proliferation [31]. The PDGF increases the secretion of other growth factors by stimulating macrophages and accelerates the wound healing process [32]. In the results of the current study, LLLT therapy increased the expression of PDGF and bFGF; however, it was not effective in the expression of TGF-β.
Various wavelengths can be used to cause photobiological effects in cytochrome c oxidase. These wavelengths can be in blue-to-green [33] or red-to-near IR [34] regions. Various wavelengths in the red-to-near IR region (most frequently 632.8, 670–680, 780, and 820–830 nm) are used in laser phototherapy, as well as in cellular studies of the mechanisms of action of this modality. More recent studies have shown an increase in proliferation of human fibroblasts exposed to 904-nm GaAs lasers with no change in procollagen synthesis [35], an increase in rat myofibroblasts and collagen deposition with exposure to a 670-nm GaAlAs laser [11], and an increase in gingival fibroblasts after exposure to diode lasers (670, 692, 780, and 786 nm) [36]. Hawkins and Abrahamse performed cell proliferation studies and exposed the cells with the various wavelengths with and without light and compared the results. They concluded that wounded cells responded optimally to 5 J/cm2 using 632.8 nm in the dark, 830 nm in the light, and 1,064 nm in the dark by maintaining cell viability, reducing cytotoxicity, and stimulating the release of cytokines and growth factors, thus ultimately stimulating cell proliferation and cell growth [37]. Jahangiri et al. used two intervention (laser) groups which underwent LLLT using 670-nm diode laser in the wound context and 810-nm diode laser to the wound margins. However, the results of measured wound healing parameters were not significantly different in the LLLT group compared with the control group [23]. In the current study, the effectiveness of four different laser systems (660, 810, 980, and 1,064 nm) was compared, and results of the current study have shown that in the increased expression of PDGF, the most effective laser was 1,064-nm Nd:YAG laser, and in that of bFGF, 1,064-nm Nd:YAG and 980-nm diode lasers were the most effective.
Al-Watban et al. [38] compared the effects of laser therapy on wound healing using different wavelengths (He–Ne 632.8 nm; He–Cd 442 nm; Ar 514, 670, 780, and 830 nm; and CO2) and found that the optimum effect belongs to He–Ne laser with 632.8 nm. Also, Enwemeka et al. [39] reported the positive effects of various wavelengths of laser light on tissue repair, with 632.8 nm, have the highest treatment effect. Al-Watban et al. compared the effects of continuous wave (CW) and pulsed lasers on wound healing and found that the effects of treatment using CW laser was higher than those of pulse frequency [40]. According to the results of the current study, the 660- and 810-nm diode lasers induced an increase in expression in all growth factors, but the difference between the control groups was not statistically significant.
Karu observed that high fluences cause destruction of photoreceptors which is accompanied by growth inhibition and cell lethality [41]. Other researchers have also demonstrated that irradiation with fluences higher than 10 J/cm2 damages DNA [37,42]. Finally, Bensadoun suggested the optimal dose in the range of 2–3 J/cm2 for prophylaxis and not less than 4 J/cm2 for therapeutic effect and the application on single spot on a lesion rather than a scanning motion over the entire lesion [26]. In the current study, the energy density used was 8 J/cm2 because of the positive findings of this density on fibroblasts [5,25].
Conclusion
Within the limitations of this study, the following conclusions were drawn:
-
1.
Expression values of the growth factors, PDGF and bFGF, were significantly affected by irradiation of different lasers wavelengths; however, expression value of the TGF-β was not affected by irradiation of different laser wavelengths tested.
-
2.
The highest PDGF expression was detected in Nd:YAG laser-applied group, and the highest bFGF expression was detected in 980-nm diode and Nd:YAG laser-applied group.
-
3.
The PDGF and bFGF expressions were lower in 660-nm diode laser group than those in previously reported studies which have used red light.
References
Kwong KK (2004) Prevention and treatment of oropharyngeal mucositis following cancer therapy: are there new approaches? Cancer Nurs 27:183–205
Eilers J (2004) Nursing interventions and supportive care for the prevention and treatment of oral mucositis associated with cancer treatment. Oncol Nurs Forum 31(4 Suppl):13–23
Fulton JS, Middleton GJ, McPhail JT (2002) Management of oral complications. Semin Oncol Nurs 18:28–35
Kirsner R (2003) Wound healing. In: Bolognia J, Jorizzo J, Rapini R (eds) Dermatology. Mosby, Bolognia, pp 2207–2218
Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M (2008) Effects of low-level He–Ne laser irradiation on the gene expression of IL-1beta, TNF-alpha, IFN-gamma, TGF-beta, bFGF, and PDGF in rat's gingiva. Lasers Med Sci 23:331–335
Abbas AK, Lichtman AH, Pober JS (1999) Cellular and molecular immunology, chapter 3. Saunders, Philadelphia
Cameron MH, Perez D, Otaho-Lata S (1999) Electromagnetic radiation. In: Cameron MH (ed) Physical agents in rehabilitation: from research to practice. Saunders, Philadelphia, pp 303–344
Webb C, Dyson M, Lewis WHP (1998) Stimulatory effect of 660 nm level laser energy on hypertrophic scar derived fibroblasts: possible mechanisms for increase in cell counts. Lasers Surg Med 22:294–301
Agaiby AD, Ghali LR, Wilson R, Dyson M (2000) Laser modulation of angiogenic factor production by T Lymphocytes. Lasers Surg Med 26:357–363
Leung MC, Lo SC, Siu FK, So KF (2002) Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and upregulates the expression of transforming growth factor-Beta. Lasers Surg Med 31:283–288
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low-level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Carnevalli CM, Soares CP, Zângaro RA, Pinheiro AL, Silva NS (2003) Laser light prevents apoptosis in Cho K-1 cell line. J Clin Laser Med Surg 21:193–196
Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66:866–871
Stadler I, Evans R, Narayan V, Buehner N, Naim JO, Lanzafame RJ (2001) 830 nm irradiation increases wound tensile strength in a diabetic murine model. Lasers Surg Med 28:220–226
Karu T (2010) Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg 28:159–160
Anneroth G, Hall G, Ryden H, Zetterqvist L (1988) The effect of low-energy infra-red laser radiation on wound healing in rats. Br J Oral Maxillofac Surg 26:12–17
Cambier DC, Vanderstraeten GG, Mussen MJ, van der Spank JT (1996) Low-power laser and healing of burns: a preliminary assay. Plast Reconstr Surg 97:555–558
Walker MD, Rumpf S, Baxter GD, Hirst DG, Lowe AS (2000) Effect of low-intensity laser irradiation (660 nm) on a radiation-impaired wound- healing model in murine skin. Lasers Surg Med 26:41–47
Schlager A, Kronberger P, Petschke F, Ulmer H (2000) Low-power laser light in the healing of burns: a comparison between two different wavelengths (635 nm and 690 nm) and a placebo group. Lasers Surg Med 27:39–42
Al-Watban FA, Delgado GD (2005) Burn healing with a diode laser: 670 nm at different doses as compared to a placebo group. Photomed Laser Surg 23:245–250
Al-Watban FA (2009) Laser therapy converts diabetic wound healing to normal healing. Photomed Laser Surg 27:127–135
Jahangiri Noudeh Y, Shabani M, Vatankhah N, Hashemian SJ, Akbari K (2010) A combination of 670 nm and 810 nm diode lasers for wound healing acceleration in diabetic rats. Photomed Laser Surg 28:621–627
Cury V, Bossini PS, Fangel R, Crusca Jde S, Renno AC, Parizotto NA (2009) The effects of 660 nm and 780 nm laser irradiation on viability of random skin flap in rats. Photomed Laser Surg 27:721–724
de Oliveira Guirro EC, de Lima Montebelo MI, de Almeida BB, da Costa Betito Torres MA, Polacow ML (2010) Effect of laser (670 nm) on healing of wounds covered with occlusive dressing: a histologic and biomechanical analysis. Photomed Laser Surg 28:629–634
Bensadoun RJ, Nair RG (2012) Efficacy of low-level laser therapy (LLLT) in oral mucositis: what have we learned from randomized studies and meta-analyses? Photomed Laser Surg 30:191–192
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Genot MT, Klastersky J (2005) Low-level laser for prevention and therapy of oral mucositis induced by chemotherapy or radiotherapy. Curr Opin Oncol 17:236–240
França CM, França CM, Núñez SC, Prates RA, Noborikawa E, Faria MR, Ribeiro MS (2009) Low-intensity red laser on the prevention and treatment of induced-oral mucositis in hamsters. J Photochem Photobiol B 94:25–31
Takamiya M, Saigusa K, Nakayashiki N, Aoki Y (2003) Studies on mRNA expression of basic fibroblast growth factor in wound healing for wound age determination. Int J Legal Med 117:46–50
Ihn H (2002) Pathogenesis of fibrosis: role of TGF-b and CTGF. Curr Opin Rheumatol 14:681–685
Heldin CV, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316
Hallén S, Brzezinski P (1994) Light-induced structural changes in cytochrome c oxidase: implication for the mechanism of electron and proton gating. Biochim Biophys Acta 1184:207–218
Kato M, Shinzawa K, Yoshikawa S (1981) Cytochrome oxidase is a possible photoacceptor in mitochondria. Photochem Photobiophys 2:263–269
Pereira AN, Eduardo Cde P, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Almeida-Lopes L, Rigau J, Zangaro RA et al (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184
Hawkins D, Abrahamse H (2007) Influence of broad-spectrum and infrared light in combination with laser irradiation on the proliferation of wounded skin fibroblasts. Photomed Laser Surg 25:159–169
Al-Watban FA, Zhang XYA (1996) Comparison of the effects of laser therapy on wound healing using different wavelengths. J Laser Ther 8:127–135. https://www.jstage.jst.go.jp/article/islsm/8/2/8_2_127/_article.
Enwemeka CS, Parker JC, Dowdy DS, Harkness EE, Sanford LE, Woodruff LD (2004) The efficacy of low-power lasers in tissue repair and pain control: a meta-analysis study. Photomed Laser Surg 22:323–329
Al-Watban FA (2004) The comparison of effects between pulsed and CW lasers on wound healing. J Clin Laser Med Surg 22:15–18
Karu T (2002) Low-power laser effects. In: Waynant RW (ed) Lasers in medicine. CRC, Boca Raton, pp 171–210
Houreld N, Abrahamse H (2007) In vitro exposure of wounded diabetic fibroblast cells to a Helium–Neon laser at 5 and 16 J/cm2. Photomed Laser Surg 25:78–84
Acknowledgments
The author wishes to express their sincere appreciation to ARC Fox and HELBO companies for supplying the laser devices for this study and to Jan Tunér and René-Jean Bensadoun for sharing their knowledge and experiences about LLLT.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is based on the Master Thesis in “Lasers in Dentistry” in RWTH Aachen University.
Rights and permissions
About this article
Cite this article
Usumez, A., Cengiz, B., Oztuzcu, S. et al. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med Sci 29, 1807–1813 (2014). https://doi.org/10.1007/s10103-013-1336-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1336-z