Abstract
The aim of this study was to evaluate the long-term effects of a combined periodontal treatment of scaling and root planing (SRP) and Nd:YAG laser (NDL) in chronic periodontitis (CP) patients. This was accomplished by determining the periodontal indices and the interleukin-1beta (IL-1β) and matrix metalloproteinase-8 (MMP-8) levels of the gingival crevicular fluid (GCF). This study was performed according to a random split-mouth-design, controlled clinical trial for sulcular debridement on 40 teeth from 20 patients with generalized moderate chronic periodontitis. The periodontal healing outcomes were compared after periodontal treatment with either SRP + NDL at 1 W (test side) or SRP (control side). Plaque index (PI), gingival index (GI), probing pocket depth (PPD), and clinical attachment level (CAL) were recorded, and samples of gingival crevicular fluid (GCF) were taken at baseline and post-therapy (3 and 9 months after treatment). The GCF samples were analyzed for IL-1β and MMP-8. There was postoperative improvement of all clinical parameters in both groups, but test side GI, PPD, and CAL recovery was higher than that of the control side (p < 0.05). Although levels of IL-1β and MMP-8 in GCF after treatment were lower in the test side than the control side, there was not a statistically significant difference (p > 0.05). In the long term, we found that SRP + NDL treatment of periodontal pockets was more effective than SRP alone in reducing PPD, CAL, GI, and GCF values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic inflammatory periodontal disease is initiated by Gram-negative microflora [1], and this infection elicits a host inflammatory response resulting in destruction of the periodontal attachment apparatus, including non-mineralized connective tissue and bone [2]. The breakdown of collagen and resorption of bone associated with tissue breakdown, remodeling and tissue repair or wound healing occurs during the course and treatment of periodontal disease [3].
Previous studies have demonstrated higher levels of collagenase activity in the GCF of progressive periodontitis patients compared to stable and gingivitis patients [4, 5], and successful periodontal treatment is followed by reduction of raised GCF collagenase activities [4, 6–8]. The major collagenase species detected in inflamed human periodontium is MMP-8 [4, 7].
The cytokine IL-1 is a mediator of the host inflammatory response to infections and other inflammatory stimuli [8]. IL-1β has been found to be significantly increased in the periodontal tissues and gingival fluid from diseased sites compared to healthy sites [9]. IL-1β up-regulates matrix metalloproteinases and down-regulates tissue inhibitors of metalloproteinase production [10]. It is also a powerful and potent bone-resorbing cytokine [11, 12], which suggests that it has a role in degrading the extracellular matrix in periodontitis [11].
The aim of periodontal treatment is the removal of bacterial deposits to stop disease progression [13]. Bacterial endotoxines and other antigenic components often stimulate the host response, causing inflammation and periodontal tissue destruction [10]. Therefore, debridement of the subgingival plaque must be the main goal in the treatment of inflammatory periodontal disease [14]. However, complete removal of bacterial deposits and their toxins from the root surface and within the periodontal pockets is not necessarily achieved with conventional mechanical therapy. In particular, access to areas such as furcations, concavities, grooves, and distal sites of molars is limited [15]. While different treatment methods are available for subgingival debridement, hand instrumentation with curettes is still regarded as the gold standard. In addition to machine-driven instruments (e.g., sonic and ultrasonic scalers), rotating, reciprocating, and laser instruments are also available [16].
As lasers can achieve excellent tissue ablation with strong bactericidal and detoxification effects, they are one of the most promising new technical modalities for nonsurgical periodontal treatment. The adjunctive use of lasers with conventional tools may facilitate treatment and has the potential to improve healing [17]. The laser-treated root surface may therefore provide favorable conditions for the attachment of periodontal tissue. This procedure might be more effective for the treatment of residual pockets after initial therapy and during maintenance [17]. Although there is no clear evidence to date that laser applications improve clinical outcomes due to the action of curettage [18], laser treatment has a potential advantage of accomplishing soft tissue wall treatment effectively along with root surface debridement, and therefore should be further investigated. The NDL has been used in dentistry in recent decades. Several potential roles for lasers in periodontal treatment have been proposed, such as the removal of calculus, the epithelial lining of periodontal pockets, and granulomatous tissue [19–22]. It is easy to deliver the NDL by a flexible optical fiber with a contact tip of 200 μm suitable for pocket insertion [23]. Basic research and clinical trials have been performed on periodontal pocket curettage and root surface debridement [24].
Therefore, the aim of this study is to compare the long-term effects of SRP and SRP + NDL in CP patients by determining the periodontal indices and IL-1β and MMP-8 levels of the GCF prior to and following treatment.
Materials and methods
Study population
The study population consisted of 20 patients with generalized moderate CP (ten females, ten males) with a median age of 46.1 ± 8.3 years. The patients were recruited from the patient pool of the Department of Periodontology at the University of Ataturk. Inclusion criteria for this study were: three or more teeth having at least two quadrants with probing depth between 4 and 6 mm and radiographic signs of bone loss. Criteria for exclusion from the study were: (1) no diagnosis of generalized moderate CP; (2) received periodontal therapy within the last 12 months; (3) systemic diseases that could affect periodontal treatment outcomes; (4) having taken systemic antibiotics within the last 6 months; (5) pregnancy or breast-feeding for women; (6) teeth with restoration such as crowns, bridges, or fillings.
Study design and treatment protocols
The present study was designed as a single-blinded, randomized-controlled, split-mouth clinical trial of 9-month duration. The study protocol was approved by the ethics committee of the University of Ataturk in accordance with the Declaration of Helsinki, and all participants signed informed consent forms.
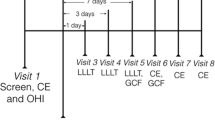
This study was performed on 40 teeth (probing depth between 4 and 6 mm) from 20 patients with chronic periodontitis. Patients underwent two different treatment modalities. The tooth on the test side (n = 20) received SRP + NDL treatment, whereas those on the control side (n = 20) were treated with SRP alone (Fig. 1). Both treatment modalities were equally distributed between quadrants. All patients received initial periodontal therapy consisting of thorough oral hygiene instructions and full-mouth supragingival and subgingival SRP with a combined use of hand (Hu-Friedy, Chicago, IL, USA) and ultrasonic instruments under local anesthesia. Subsequently, the periodontal pockets of the teeth in the test side were operated on with 1.0 W NDL (wavelength 1,064 nm, 100 mJ, 10 Hz). The laser beam was applied at these settings in accordance with the instructions given by the manufacturer (Smarty A10, DEKA, Florence, Italy). A clinical NDL delivered by optic fiber (diameter 200 μm) inside the periodontal pocket was selected for the treatment. Laser application was performed by the contact fiber tip inserted at the bottom of the periodontal pocket and slowly moved from apical to coronal in a sweeping motion during laser light emission. This was done mesially, distally, buccally, and lingually during an exposition time of 120 s. During the laser application, protective eyeglasses were worn by the patient, the operator, and the assistants.
Clinical examination
The following clinical periodontal parameters were collected immediately before SRP (baseline) and 3 and 9 months after treatment for each test and control tooth. Plaque index (PI) [25] and gingival index (GI) [26] were recorded according to Silness & Löe and Löe & Silness, respectively. Probing pocket depth (PPD) was measured as the distance between the gingival margin and the deepest aspect of the pocket. Clinical attachment level (CAL) was measured as the distance between the cementoenamel junction of the tooth and the deepest aspect of the pocket.
All clinical parameter measurements were conducted using a manual periodontal probe (PCP- 12, Hu-Friedy, Chicago, IL, USA). PI and GI measurements were assessed at four surfaces per tooth (mesial, distal, buccal, lingual, or palatal surface), while PPD and CAL measurements were taken at six surfaces per tooth (mesio-buccal, mid-buccal, distobuccal and mesio-lingual, mid-lingual, disto-lingual or -palatal surface). Measurements at all visits for a given subject were made by one calibrated examiner who was not involved in providing treatment during the study. Before the start of the study, the examiner was trained to adequate levels of accuracy and reproducibility in recording the clinical parameters and indices.
GCF collection
In each patient, four GCF samples were taken from the control and test tooth with PD between 4 and 6 mm. GCF samples were used to determine their levels of IL-1β and MMP-8. These were taken just before treatment and 3 and 9 months after treatment. The GCF samplings were carried out in the same manner.
Each clinical evaluation was preceded by collection of GCF as previously described [27] from the mesiobuccal surfaces of all teeth included in the study. Briefly, the teeth were air dried and isolated with cotton rolls, supragingival plaque was gently removed, and GCF was collected with prefabricated paper strips (Periopaper, Oraflow Inc., Plainview, NY, USA), which were inserted into the pockets until resistance was felt and kept there for 30 s. Strips were measured for fluid volume with a calibrated Periotron 8000 (Oraflow Inc.) and then removed to separate microcentrifuge tubes containing 200 μl of phosphate buffered saline (PBS). After extraction on a vortex mixer for 30 s, each sample was centrifuged at 3,000 × g for 5 min. The tubes were stored at –80°C until the day of the analysis. In case of visible contamination with blood, the strips were discarded.
Analysis of IL-1β
The level of IL-1β was determined by enzyme-linked immunoadsorbent assays (ELISA). The assays were carried out in accordance with the manufacturer’s instructions. Briefly, IL-1β present in the GCF sample was first captured by the antibody. A biotinylated second monoclonal antibody to each cytokine was added, which bound to the complex already present in the well of the 96-well plate. Then, a polymer of horseradish peroxidase conjugate to streptavidin was added, and finally a substrate solution was added, which gave a colored product in proportion to the levels of IL-1β present in each GCF sample. After the reaction was terminated, the absorbency at 450 nm was read within 20 min in a spectrophotometer.
Analysis of MMP-8
The MMP-8 protein levels in GCF was determined using human MMP-8 ELISA systems (Amersham Pharmacia Biotech UK Limited, Amersham Life Science Ltd, Buckinghamshire, England), and the assays were carried out in accordance with the manufacturer’s instructions. Briefly, monoclonal antibody specific for MMP-8 had been precoated onto a microplate. Samples diluted 20 times and a standard were pipetted into the wells and incubated at room temperature for 2 h. The plates were then washed, and a monoclonal antibody against MMP-8 conjugated to horseradish peroxidase was added and incubated again as before. After a new washing procedure, the substrate solution was added and the reaction was stopped after 15 min with a stop solution. After the reaction was terminated, the absorbency at 450 nm was read within 20 min in a spectrophotometer.
Statistical analysis
The mean of clinical and gingival crevicular fluid data was calculated for one tooth which had PD between 4 and 6 mm at baseline, in the test and control sides in each subject. All statistical analysis was performed with the SPSS for Windows version 15.0 software. Kruskal–Wallis and Mann–Whitney U tests were used to determine the significance of the differences between groups, while Friedman and Wilcoxon tests were used to determine the significance of the differences within groups. Probability values higher than 0.05 were considered as not significant.
Results
Clinical outcomes
The results of the present study demonstrated that SRP with or without laser applications led to significant improvements in all investigated clinical and laboratory parameters at 3 and 9 months after treatment. Table 1 presents the full-mouth mean values for the clinical parameters at baseline and 3 and 9 months after treatment for the 20 subjects. There was a statistically significant decrease in the PI, GI, PPD, and CAL for all patients after treatment (p < 0.05).
The mean changes in clinical indices in both groups are shown in Table 2. There was postoperative improvement of all clinical parameters in the two sides, but in the test side, GI, PPD, and CAL recovery were higher than in the control side (p < 0.05 ). The change in the PI was similar between groups after treatment (p > 0.05). There was no statistically significant PI difference between groups at 3 and 9 months. GI was decreased after treatment in both groups. In addition, the reductions in the GI were significantly greater in the test side than in the control side (p < 0.05). The differences in the GI values from baseline were obvious at 3 and 9 months (p < 0.05). However, the average change in the GI values for both sides was not statistically significant between 3 and 9 months (p > 0.05). Statistically significant differences between the laser and control sides were demonstrated in PPD and CAL values at all treatment times (p < 0.05). In contrast to the GI in both sides, there was a statistically significant average change in the PPD and CAL values between 3 and 9 months (p < 0.05).
Laboratory variables
The evaluation of the laboratory results is summarized in Table 3. In both groups, there were diminished, statistically significant GCF values, GCF Il-1β, and MMP-8 levels at 3 and 9 months after treatment (p < 0.05). Despite the fact that Il-1β and MMP-8 values were lower in the test side than in the control side, the difference was not statistically significant (p > 0.05). Although it was observed that there was diminished numeric from 3 months to 9 months, there was no statistical significance (p > 0.05).
As another marker of inflammation, the change in the GCF value was also similar to that of the GI. The GCF value was decreased after treatment in both sides (p < 0.05), but the reductions in GCF value were significantly greater in the test side than in the control side (p < 0.05). The mean of the changes in the GCF value within the group was similar for both groups at 3 and 9 months after treatment (p > 0.05).
Discussion
In this study, SRP and SRP + NDL groups are shown to result in major changes both clinical and laboratory, however, GI, PPD, and CAL recovery in the test side were higher than in the control side. Changes in Il-1β and MMP-8 levels were similar in both groups. In addition, the gingival inflammation significantly decreased in test sides compared to control sides. Our findings suggest that NDL may have a beneficial effect on periodontal healing, especially PPD and CAL improvement.
Antimicrobial properties of NDL were further investigated in clinical studies, and few studies have reported significant effects on antimicrobial that favored NDL [24, 28–30]. Due to deep penetration of the laser light, the bacterial level was reduced to 1 mm below the root surface. On the other hand, Radvar et al. reported that NDL failed to improve the clinical and microbiological parameters of periodontal disease as compared to SRP [20].
In addition, several studies evaluating gingival inflammation have reported that SRP plus NDL irradiation showed significantly greater improvements in gingival index and GCF values [30, 31]. Liu et al. [24] reported that laser therapy alone was less effective than traditional SRP for the improvement of clinical parameters. They reported that no additional benefit was found when laser treatment was used secondary to traditional SRP therapy. Apparently, recent studies have been gaining support for the antibacterial and inflammation-reducing effect of the NDL beam. The GI and GCF values may reflect the presence of inflammation at the periphery of the periodontium. Our results showed that the reduction in GI and GCF values in the test group was significantly greater than in the control group. Our results supported that the NDL as an adjunct to SRP was capable of reducing the level of inflammation within the periodontium via its antibacterial property.
Recently, two studies investigated the effects of NDL on collagen formation in the dermis on skin. The first study demonstrated that dermal remodeling after the 1,320-nm laser was mainly through the synthesis and deposition of collagen type I. Inflammatory reactions were in favor of the formation of type III collagen [32]. Similarly, the second study showed that the long-pulse 1,064-nm NDL induces collagen formation in the reticular dermis in porcine skin [33]. Because CAL was significantly reduced in the NDL application side rather than in the control side, the present study’s results are consistent with those of the previous report on induced collagen formation by the NDL beam.
Several in vitro studies reported significant effects on PPD and CAL that favored NDL. Radvar et al. [20] reported a greater mean decrease in PD in SRP-treated sites than in those treated by laser. Horton et al. compared subgingival application of the NDL with conventional SRP [29]. The subgingival application of the NDL was at least equally effective on measures of probe depths and attachment loss. Neil and Mellonig [30] reported that the reduction of probing depth for 6 months after treatment was similar between the SRP + laser and SRP alone. The mean attachment level of laser-treated pockets also showed a tendency to improve steadily for 6 months, whereas the SRP group showed a reduction in attachment level. The use of a laser beam in periodontal therapy is based on the purported benefits of subgingival curettage, laser-induced new attachment through regeneration of cementum, periodontal ligament, and supporting alveolar bone, and significant decreases in subgingival pathogenic bacteria [24]. When measuring outcomes of non-surgical periodontal therapy, gain in CAL represents the gold standard. PD and levels of subgingival microbes are important primarily because, in cases of traditional non-surgical mechanical therapy, they have been shown to be associated with changes in CAL. Our study showed that the NDL application combined with SRP significantly reduced the PPD and CAL at 3 and 9 months, compared to sites treated by SRP alone.
In the present study, IL-1β and MMP-8 were detected in almost all GCF samples. Our findings are consistent with previous studies; IL-1β and MMP-8 levels were detected in a higher number of GCF samples from CP patients [34, 35], and were decreased in GCF following periodontal treatment [36]. Previously, only four studies evaluated the effect on GCF contents of NDL in periodontal treatment. Liu et al. [24] showed that laser therapy alone was less effective than traditional SRP for the reduction of crevicular IL-1β. Laser treatment followed by SRP after 6 weeks showed greater reduction of IL-1β. In another study, Miyazaki et al. [31] compared the effectiveness of the Nd:YAG and CO2 laser and ultrasonic scaling in periodontal pockets of CP patients. The Nd:YAG group also tended to show a decrease in IL-1 level, but no significant difference was observed among the three groups. Qadri et al. [37] compared the short-term outcome of combined SRP and NDL irradiation with SRP alone. At the 1 week follow-up, PPD, PI, GCF volume, the IL-1β and MMP-8 levels showed significant improvement on the test side compared to the control side. At the 3-month follow-up, PPD, PI, GI, and GCF volume also showed significant improvement on the test side compared to the control side. The 3-month follow-up IL-1β and MMP-8 levels confirmed that the improvements on the test side had been sustained compared to the control side. In a recent study, Gomes et al. [23] reported that levels of IL-1β in GCF were significantly lower with SRP + NDL treatment compared to SRP alone after 4 weeks of treatment. Levels of TNF-alpha were significantly lower after the SRP + NDL treatment. The total antioxidative status of GCF increased after the SRP + NDL treatment.
Few studies have focused on the association between GCF content and NDL. Therefore, our study examined the effects of NDL on IL-1β and MMP-8 levels of the GCF content. Our findings showed that the IL-1β and MMP-8 also decreased in both groups, but the difference was not statistically significant. These results conflicted with the results for GI and GCF value as inflammation markers, because the laser group showed a greater statistically significant reduction of GI and GCF value than did the control group. We suggest that this situation can be attributed to the effect of the NDL beam on biological tissue. Previously, several studies have identified that the osseous healing response was severely delayed by NDL irradiation of bone. In addition, cellular damage, inhibitions of the DNA metabolism rate and the cell division rate, and degeneratively changed cytomorphology up to cell pyknosis of cultured gingival fibroblasts were induced by NDL irradiation [38–40], but there is no in vitro research in this field. In this respect, our study had two important limitations; our study did not investigate effects antimicrobial and in biological tissue of NDL beam.
Conclusions
We found that SRP + NDL treatment of periodontal pockets was more effective than SRP alone in reducing PPD, CAL, GI, and GCF values. Further studies are needed to investigate long-term biological effects and various properties such as power, frequency, energy, and time in the application of the Nd:YAG laser to periodontal tissue.
References
Haffajee AD, Socransky SS (1994) Microbial etiological agents of destructive periodontal diseases. Periodontol 2000 5:78–111
Engebretson SP, Grbic JT, Singer R, Lamster IB (2002) GCF IL-1beta profiles in periodontal disease. J Clin Periodontol 29(1):48–53
Kinane DF, Darby IB, Said S, Luoto H, Sorsa T, Tikanoja S, Mäntylä P (2003) Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J Periodontal Res 38(4):400–404
Romanelli R, Mancini S, Laschinger C, Overall CM, Sodek J, McCulloch CA (1999) Activation of neutrophil collagenase in periodontitis. Infect Immun 67:2319–2326
Mancini S, Romanelli R, Laschinger CA, Overall CM, Sodek J, McCulloch CA (1999) Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J Periodontol 70:1292–1302
Chen HY, Cox SW, Eley BM, Mäntylä P, Rönkä H, Sorsa T (2000) Matrix metalloproteinase-8 levels an elastase activities in gingival crevicular fluid from chronic adult periodontitis patients. J Clin Periodontol 27:366–369
Sorsa T, Mäntylä P, Rönkä H, Kallio P, Kallis GB, Lundqvist C, Kinane DF, Salo T, Golub LM, Teronen O, Tikanoja S (1999) Scientific basis of a matrix metalloproteinase-8 specific chair-side test for monitoring periodontal and periimplant health and disease. Ann NY Acad Sci 878:130–140
Delaleu N, Bickel M (2004) Interleukin-1 beta and interleukin-18: regulation and activity in local inflammation. Periodontol 2000 35:42–52
Stashenko P, Fujiyoshi P, Obernesser MS, Prostak L, Haffajee AD, Socransky SS (1991) Levels of interleukin 1 beta in tissue from sites of active periodontal disease. J Clin Periodontol 18:548–554
Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS (1997) Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000 14:216–248
Schwartz Z, Goultschin J, Dean DD, Boyan BD (1997) Mechanisms of alveolar bone destruction in periodontitis. Periodontol 2000 14:158–172
Shirodaria S, Smith J, McKay IJ, Kennett CN, Hughes FJ (2000) Polymorphisms in the IL-1A gene are correlated with levels of interleukin-1alpha protein in gingival crevicular fluid of teeth with severe periodontal disease. J Dent Res 79:1864–1869
Kepic TJ, O'Leary TJ, Kafrawy AH (1990) Total calculus removal: an attainable objective? J Periodontol 61(1):16–20
Wennstrom JL, Tomasi C, Bertelle A, Dellasega E (2005) Full-mouth ultrasonic debridement versus quadrant scaling and root planing as an initial approach in the treatment of chronic periodontitis. J Clin Periodontol 32:851–859
Adriaens PA, Edwards CA, De Boever JA, Loesche WJ (1988) Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol 59:493–503
AAP Position Paper (2000) Sonic and ultrasonic scalers in periodontics. J Periodontol 71:1792–1801
Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 2000 36:59–97
AAP (The American Academy of Periodontology) (1999) The Research, Science and Therapy Committee of the American Academy of Periodontology. Statement regarding use of dental lasers for Excisional New Attachment Procedure (ENAP). AAP Website in August 1999: http://www.perio.org/resources-products/enap_laser.htm.
Wang QQ, Zhang CF, Yin XZ (2007) Evaluation of the bactericidal effect of Er,Cr:YSGG, and Nd:YAG lasers in experimentally infected root canals. J Endod 33:830–832
Radvar M, MacFarlane TW, MacKenzie D, Whitters CJ, Payne AP, Kinane DF (1996) An evaluation of the Nd:YAG laser in periodontal pocket therapy. Br Dent J 180:57–62
Ishikawa I, Sculean A (2007) Laser dentistry in periodontics. In: Gutknecht N (ed) Proceedings of the 1st International Workshop of Evidence Based Dentistry on Lasers in Dentistry. Quintessence Publishing, New Malden, Surrey, UK, pp 115–129
Gomez C, Costela A, Garcı´a-Moreno I, Garcı´a JA (2006) In vitro evaluation of Nd:YAG laser radiation at three different wavelengths (1064, 532, and 355 nm) on calculus removal in comparison with ultrasonic scaling. Photomed Laser Surg 24:366–376
Gómez C, Domínguez A, García-Kass AI, García-Nuñez JA (2010) Adjunctive Nd:YAG laser application in chronic periodontitis: clinical, immunological, and microbiological aspects. Lasers Med Sci. Jun 10. doi:10.1007/s10103-010-0795-8
Liu CM, Hou LT, Wong MY, Lan WH (1999) Comparison of Nd:YAG laser versus scaling and root planing in periodontal therapy. J Periodontol 70:1276–1282
Silness J, Loe H (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand 22:121–135
Loe H, Silness J (1963) Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand 21:533–551
Lamster IB, Oshrain RL, Celenti R, Levine K, Fine JB (1991) Correlation analysis for clinical and gingival crevicular fluid parameters at anatomically related gingival sites. J Clin Periodontol 18(4):272–277
Coluzzi DJ (2002) Lasers and soft tissue curettage: an update. Compendium 23:1104–1111
Horton JE, Lin PY (1992) A comparison of the Nd:YAG laser used subgingivally with root planing. The 3rd International Congress on Lasers in Dentistry, Salt Lake City, University of Utah. Abstract handbook, 23, abstract 46
Neil ME, Mellonig JT (1997) Clinical efficacy of the Nd:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent 9:1–5
Miyazaki A, Yamaguchi T, Nishikata J, Okuda K, Suda S, Orima K, Kobayashi T, Yamazaki K, Yoshikawa E, Yoshie H (2003) Effects of Nd:YAG and CO2 laser treatment and ultrasonic scaling on periodontal pockets of chronic periodontitis patients. J Periodontol 74:175–180
Dang Y, Ren Q, Liu H, Ma J, Zhang J (2006) Effects of the 1,320-nm Nd:YAG laser on transepidermal water loss, histological changes, and collagen remodeling in skin. Lasers Med Sci 21:147–152
Dayan S, Damrose JF, Bhattacharyya TK, Mobley SR, Patel MK, O'Grady K, Mandrea S (2003) Histological evaluations following 1,064-nm Nd:YAG laser resurfacing. Lasers Surg Med 33(2):126–131
Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A (2000) Levels of interleukin-1b, -8 and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71:1535–1545
Boström L, Linder LE, Bergström J (2000) Smoking and GCF levels of IL-1b and IL-1ra in periodontal disease. J Clin Periodontol 27:250–255
Gonzales JR, Herrmann JM, Boedeker RH, Francz PI, Biesalski H, Meyle J (2001) Concentration of interleukin-1b and neutrophil elastase activity in gingival crevicular fluid during experimental gingivitis. J Clin Periodontol 28:544–549
Qadri T, Poddani P, Javed F, Tunér J, Gustafsson A (2010) A short-term evaluation of Nd:YAG laser as an adjunct to scaling and root planing in the treatment of periodontal inflammation. J Periodontol 81(8):1161–1166
Chen YJ, Jeng JH, Lee BS, Chang HF, Chen KC, Lan WH (2000) Effects of Nd:YAG laser irradiation on cultured human gingival fibroblasts. Lasers Surg Med 27(5):471–478
Chen YJ, Jeng JH, Jane YC, Chen MH, Hou LT, Lan WH (2005) Long-term effect of pulsed Nd:YAG laser irradiation on cultured human periodontal fibroblasts. Lasers Surg Med 36(3):225–233
Gutknecht N, Kanehl S, Moritz A, Mittermayer C, Lampert F (1998) Effects of Nd:YAG-laser irradiation on monolayer cell cultures. Lasers Surg Med 22(1):30–36
Source of funding
The study was self-funded by the authors and their institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eltas, A., Orbak, R. Effect of 1,064-nm Nd:YAG laser therapy on GCF IL-1β and MMP-8 levels in patients with chronic periodontitis. Lasers Med Sci 27, 543–550 (2012). https://doi.org/10.1007/s10103-011-0939-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0939-5