Abstract
The effect of heat-treatment temperature on the interfacial reaction between MnO-SiO2-FeO oxide and Fe-Mn-Si alloy was investigated by the diffusion couple method in the temperature range of 1173–1573 K. The reaction at the interface between the alloy and oxide was not obvious during treatment at 1173 K, but, with increasing heat-treatment temperature, the interfacial reaction was strengthened and the proportion of the MnO·SiO2 phase in the oxide increased. The width of the particle-precipitation zone in the alloy increased with increasing temperature from 1173 K to 1473 K but decreased at 1573 K owing to coarsening of the precipitated particles. In addition, Mn2+ and Si4+ in the oxide significantly diffused into the alloy at 1573 K, resulting in an obvious increase of the Mn and Si contents in the alloy near the interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With increasingly stringent quality requirements for steel, clean steel production has become an important direction of development in this industry. The key technique of clean steel production is inclusion control, including the removal and modification of inclusions.1,2,3,4,5,6 The development of secondary refining has enabled the total oxygen content in steel to be controlled at a very low level, so further removal of inclusions in molten steel has become increasingly difficult. Exploring new inclusion control techniques is therefore necessary for further improvement of product quality.
An attempt to modify the characteristics of inclusions in solid steel by heat treatment was recently conducted and has so far proved to be practicable for resulfurized free-cutting steel, Fe-Al-Ti steel, and austenitic stainless steel.7,8,9,10,11,12,13 Compared with the detrimental inclusions such as large-sized MnS, Al2O3, and MgO·Al2O3, the finer and uniformly distributed MnS, titanium oxide, and MnO-Cr2O3 particles are preferred in these steels. The elongated MnS inclusions in resulfurized steel split into small particles and became obviously spheroidized after heat treatment at 1273 K and 1473 K.7 The composition of inclusions in Fe-Al-Ti steel changed from Al2O3 and TiO x to finer Al-Ti-Fe-O and Fe-Ti-O, respectively, during heat treatment at 1473 K.8 The MnO-SiO2 inclusions in an austenitic stainless steel (Fe-18%Cr-8%Ni) changed to MnO-Cr2O3 after heat treatment.11,12,13
The changes in characteristics of inclusions in solid steel during heat treatment are related to diffusion of elements at the interface between the solid steel and the inclusions.14 Kim et al.15,16 investigated the interfacial reaction between Fe-Mn-Si alloy and MnO-SiO2-FeO oxide under heat treatment at 1473 K using the diffusion couple method. They found that FeO in the oxide was reduced during heat treatment and the excess oxygen diffused into the alloy, forming a particle-precipitation zone (PPZ) and an Mn-depleted zone (MDZ) in the alloy. Liu et al.17,18 also studied the reaction between MnO-SiO2-FeO oxide and Si-Mn killed steel during heat treatment at 1473 K using an improved diffusion couple method. In their study, a confocal scanning microscope with rapid heating and cooling functions was used to prepare diffusion couples at 1673 K. The impact of the pre-treatment process on the experimental results at 1473 K was reduced using this technique, enabling the interfacial reaction between the solid steel and oxide inclusion to be better clarified.
Changes to inclusions in solid steel during heat treatment are significantly affected by the heat-treatment conditions.7,11 The effect of heat-treatment temperature on the reaction between the oxide inclusions and Si-Mn killed steel is still unclear. In this study, the interfacial reaction between MnO-SiO2-FeO oxide and Si-Mn killed steel during heat treatment at temperatures of 1173 K to 1573 K was therefore investigated.
Experimental
The compositions of the Fe-Mn-Si alloy and MnO-SiO2-FeO oxide are listed in Table I. These two components are in equilibrium at 1823 K.15
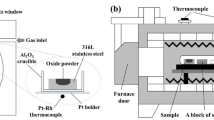
Electrolytic iron, Fe-Mn alloy, and Si metal were melted in a vacuum induction furnace to produce the Fe-Mn-Si alloy. The oxide was prepared by heating reagent-grade MnO, SiO2, and FeO powders under an Ar atmosphere in an electrical resistance furnace. An electron probe microanalyzer (EPMA) was used to determine the composition of the alloy. The alloy was then machined into a cylindrical shape (5 mm in diameter × 3 mm height, about 0.350 g) and a hole (diameter: 2 mm; height: 1.5 mm) was manufactured within the alloy in which the oxide was placed.
A confocal scanning laser microscope (CSLM) was employed to melt the oxide to produce the diffusion couples. Specific details are provided in Ref. 17. Alloy containing about 0.005 g oxide powder (~ 100 mesh) in the hole was placed in the CSLM. The CSLM chamber was evacuated to 5 × 10−3 Pa and high-purity Ar gas (> 99.999%, flow rate: 50 mm3/min) introduced to prevent oxidation of the sample. The pre-treatment temperature was then increased to 1673 K, which is 50 K higher than the melting point of the oxide, at 100 K/min. When the oxide was observed to be completely melted, the sample was immediately quenched by a helium stream. The cooling rate was approximately 1000 K/min.
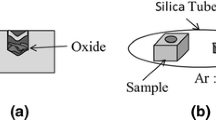
The diffusion couple sample, an alloy block with the same composition, and a Ti foil were sealed into a quartz tube, into which pure Ar gas was introduced. The quartz tube was placed in a heating furnace when temperature of the furnace was increased to target values. Five quartz-tube samples were then heat treated at a temperature between 1173 K and 1573 K for 30 h, respectively. After heat treatment, the experimental sample was quenched by water, and a vertical cross-section of each diffusion couple was analyzed using EPMA. The widths of the MDZ and PPZ in the alloy were measured by the same method as described in Ref. 15.
Results
Figure 1 shows the interface of the oxide and alloy before and after heat treatment at temperatures ranging from 1173 K to 1573 K. Some fine particles formed in the alloy before heat treatment, which was mainly caused by the reaction between the liquid oxide and solid alloy during pretreatment at 1673 K. The oxide was a uniform 2MnO·SiO2 phase; some dark striped patterns caused by the phase separation during cooling in the CSLM were also observed.
After heat treatment at 1173 K, the number and size of the precipitated oxide particles in the alloy did not obviously change. A few iron particles were observed in the oxide, but the oxide was still homogeneous 2MnO·SiO2.
On increasing the heat-treatment temperature from 1273 K to 1473 K, both the number and size of the precipitated particles increased; however, at 1573 K, the number of the precipitated particles in the alloy was considerably reduced, but their size became larger. The oxide consisted of both 2MnO·SiO2 and MnO·SiO2 phases after heat treatment at temperatures from 1273 K to 1573 K, and many iron particles were observed in the oxide. With increasing heat-treatment temperature, the proportion of the MnO·SiO2 phase in the oxide and the size of the iron particles gradually increased.
Figure 2 shows the changes in Mn and Si contents in the alloy near the interface before and after heat treatment at temperatures from 1273 K to 1573 K. The Mn and Si contents in the alloy slightly decreased within 10 μm of the interface before heat treatment. Compared with the experimental results before heat treatment, there was no obvious change in the Mn and Si contents after heat treatment at 1173 K; however, after heat treatment at temperatures of 1273 K to 1573 K, the Mn content near the interface in the alloy of all diffusion couples was reduced, but it slightly increased within 30 μm or less of the interface. With increasing heat-treatment temperature, both the range of the regions in which the Mn contents were lower than the initial value and the minimum Mn and Si contents in the alloy gradually increased. In particular, the Mn and Si contents in the alloy after heat treatment at 1573 K were higher.
Figure 3 shows the influence of heat-treatment temperature on the width of the MDZ and PPZ. There were no obvious changes before and after heat treatment at 1173 K. With increasing heat-treatment temperature, the width of the MDZ increased; however, the influence of temperature on change in the width of the PPZ was different: the width of the PPZ increased with increasing heat-treatment temperature in the range of 1173–1473 K, but decreased at 1573 K.
Discussion
It has been proposed that the FeO in the oxide becomes unstable and decomposes during heat treatment and that the excess oxygen in the oxide diffuses into the solid alloy and reacts with Mn and Si, causing the formation of a MDZ and PPZ in the alloy.15 This phenomenon is similar to that of internal oxidation.
In this study, the oxygen partial pressure that equilibrated with the MnO-SiO2-FeO oxide was defined as the experimental oxygen partial pressure (\( {\text{P}}_{{{\text{O}}_{2} }} \)), which could be calculated by Eq. 1.19 The activity of FeO in the oxide was determined by the regular solution model, assuming regular solution parameters for liquid inclusions to describe the behavior at each heat-treatment temperature:20
In addition, the minimum oxygen partial pressure at which the alloy elements in the matrix started to oxidize was defined as the critical oxygen partial pressure. The critical partial pressures of Mn (\( {\text{P}}_{{{\text{O}}_{2} }}^{\text{Mn}} \)) and Si (\( {\text{P}}_{{{\text{O}}_{2} }}^{\text{Si}} \)) in the Fe-3.1mass%Mn-0.1 mass%Si alloy at each heat-treatment temperature were calculated by Eqs. 2 and 3,19,21 respectively, assuming the activity coefficients of Mn and Si were unity:
The diffusion coefficient of oxygen in the austenite iron-based alloy was determined by Eq. 4:22
Figure 4 shows the change of the oxygen partial pressure and diffusivity of oxygen (DO) with temperature. The experimental oxygen partial pressures were larger than the critical oxygen partial pressures of Mn and Si in the temperature range of 1173–1573 K. Mn and Si in the alloy could therefore be oxidized during heat treatment at all temperatures in this study. With increasing heat-treatment temperature, the values of \( P_{{{\text{O}}_{2} }} \) and DO increased, strengthening the reaction between the oxide and alloy at higher temperatures. In addition, the \( P_{{{\text{O}}_{2} }} \) and DO values were very small at 1173 K, which meant that the interfacial reaction during heat treatment was not obvious. It was concluded that any reaction between the oxide inclusions and Si-Mn killed steel during heat treatment below 1173 K can be neglected.
With increasing heat-treatment temperature, the interfacial reaction became more intense, and more oxygen in the oxide diffused into the alloy. Meanwhile, more cations (Mn2+ and Si4+) would also diffuse into the alloy to ensure electrical neutrality of the oxide, causing an increase of Mn and Si contents in the alloy. As shown in Fig. 2, the Mn and Si contents in the alloy after heat treatment at 1573 K were obviously higher.
The equation proposed by Wagner,23 shown in Eq. 5, was used to predict the range of the internal oxidation zone. The width of the PPZ at different heat-treatment temperatures was estimated using this equation:
where \( \xi \) is the thickness of the internal oxidation zone; \( N_{\text{O}}^{{ ( {\text{s)}}}} \) is the mole fraction of oxygen at the interface; DO indicates the diffusivity of oxygen in the alloy; \( \nu \) indicates the number of oxygen atoms per A atom in an AO v oxide; \( N_{\text{B}}^{{ ( {\text{O)}}}} \) indicates the mole fraction of the solute elements in the alloy; t is the heat-treatment time.
The parameters in Eq. 5 were determined at each heat-treatment temperature using the method described in Ref. 17. Figure 5 shows a comparison between the theoretical calculations and experimental results. In the temperature range of 1173–1473 K, the experimental results were in good agreement with the theoretical calculations; however, the experimental results for the width of the PPZ were obviously lower than the theoretical calculations at 1573 K. With increasing heating temperature, the diffusivities of solute elements (Mn, Si, and O) in the alloy increased, making the coarsening of particles easier.24,25 Due to the significant coarsening of precipitated particles at 1573 K, the number of particles was reduced, causing the decrease in the width of PPZ.
Conclusion
In this study, the interfacial reaction between oxide inclusions and Si-Mn killed steel during heat treatment at temperatures of 1173 K to 1573 K was investigated using the diffusion couple method. The following conclusions were drawn:
-
1.
The interfacial reaction between oxide inclusions and Si-Mn killed steel during heat treatment below 1173 K can be neglected.
-
2.
With increasing heat-treatment temperature, the interfacial reaction between the oxide and alloy was strengthened, and the proportion of the MnO·SiO2 phase in the oxide increased.
-
3.
Large amounts of Mn2+ and Si4+ in the oxide diffused into the solid alloy during heat treatment at 1573 K, resulting in an obvious increase of the Mn and Si contents of the alloy.
-
4.
The width of the PPZ can be well estimated using the dynamic model established based on Wagner’s equation in the temperature range of 1173–1473 K; however, the width of the PPZ was obviously lower than the theoretical calculations at 1573 K owing to significant coarsening of the precipitated particles.
References
S.F. Yang, Q.Q. Wang, L.F. Zhang, J.S. Li, and K. Peaslee, Metall. Trans. B 43B, 731 (2012).
S.F. Yang, W. Liu, and J.S. Li, JOM 67, 2993 (2015).
C. Liu, S.F. Yang, J.S. Li, L.B. Zhu, and X.G. Li, Metall. Trans. B 47B, 1882 (2016).
S.F. Yang, J.S. Li, C. Liu, L.Y. Sun, and H.B. Yang, Metall. Trans. B 45B, 2453 (2014).
H.T. Ling and L.F. Zhang, JOM 65, 1155 (2013).
S.P. He, G.J. Chen, Y.T. Guo, B.Y. Shen, and Q. Wang, Metall. Trans. B 46B, 585 (2015).
X.J. Shao, X.H. Wang, M. Jiang, W.J. Wang, and F.X. Huang, ISIJ Int. 51, 1995 (2011).
W. Choi, H. Matsuura, and F. Tsukihashi, ISIJ Int. 51, 1951 (2011).
W. Choi, H. Matsuura, and F. Tsukihashi, Metall. Trans. B 47B, 1851 (2016).
M.G. Li, H. Matsuura, and F. Tsukihashi, Metall. Trans. B 48B, 1915 (2017).
I. Takahashi, T. Sakae, and T. Yoshida, Tetsu-to-Hagane 53, 168 (1967).
K. Takano, R. Nakao, S. Fukumoto, T. Tsuchiyama, and S. Takaki, Tetsu-to-Hagane 89, 616 (2003).
H. Shibata, T. Tanaka, K. Kimura, and S.-Y. Kitamura, Ironmak. Steelmak. 37, 522 (2010).
H. Shibata, K. Kimura, T. Tanaka, and S.-Y. Kitamura, ISIJ Int. 51, 1944 (2011).
K.-H. Kim, S.-J. Kim, H. Shibata, and S.-Y. Kitamura, ISIJ Int. 51, 2144 (2014).
K.-H. Kim, H. Shibata, and S.-Y. Kitamura, ISIJ Int. 54, 2678 (2014).
C.S. Liu, K.-H. Kim, S.-J. Kim, J.S. Li, S. Ueda, X. Gao, H. Shibata, and S.-Y. Kitamura, Metall. Trans. B 46B, 1875 (2015).
C.S. Liu, S.F. Yang, K. Kim, J.S. Li, H. Shibata, and S.-Y. Kitamura, Int. J. Miner. Metall. Mater. 22, 811 (2015).
S. Ban-Ya and E. Tasuhiko, Physical Chemistry of Metals, 1st ed. (Tokyo: Maruzen Press, 1996), pp. 198–208.
S. Ban-Ya, ISIJ Int. 33, 2 (1993).
M. Hino and K. Ito, Thermodynamic Data for Steelmaking, 1st ed. (Sendai: Tohoku University Press, 2010), pp. 167–169.
J.H. Swisher and E.T. Turkdogan, Trans. Metall. Soc. AIME 239, 426 (1967).
C. Wagner, Z. Elektrochem. 63, 772 (1959).
C.S. Jayanth and P. Nash, J. Mater. Sci. 24, 3041 (1989).
Y. Ren, L.F. Zhang, and P.C. Pistorius, Metall. Trans. B 48B, 2281 (2017).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 51574020, 51674023 and 51604201).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, S., Liu, C. et al. Effect of Heat-Treatment Temperature on the Interfacial Reaction Between Oxide Inclusions and Si-Mn Killed Steel. JOM 70, 958–962 (2018). https://doi.org/10.1007/s11837-018-2738-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2738-y