Abstract
A new method using a confocal scanning laser microscope for melting oxide at 1673 K (1400 °C) to produce a diffusion couple, which consists of oxide and an iron-based alloy, has been developed for the investigation of a solid-state reaction between them. It is important to obtain good contact between the Fe-Mn-Si alloy and MnO-SiO2-FeO oxide before the diffusion experiment to observe diffusion of elements between them at 1473 K (1200 °C). In this system, the melting point of the oxides is higher than the temperature where the reaction would be observed. The pretreatment involving melting oxide at 1673 K (1400 °C) to obtain good contact with the alloy was carried out using a new method. The effect of the pretreatment at 1673 K (1400 °C) on the diffusion experiment at 1473 K (1200 °C) was minimized by this new method. The effect of diffusion of oxygen on the solid-state reaction between the Fe-Mn-Si alloy and MnO-SiO2-FeO oxide at 1473 K (1200 °C) was observed clearly and showed good agreement with Wagner’s model of internal oxidation of metal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precise control of inclusion composition is important for the production of high-quality steel. Generally, inclusion composition can be adjusted by a secondary refining process. However, the inclusion composition in the final steel product is occasionally different from that in the molten steel.
The process of changing inclusion composition by reaction with solid steel during heat treatment has already been clarified. Shibata et al.[1,2] investigated the solid-state reaction at the interface between Fe-Cr steel and MnO-SiO2 oxide created by heat treatment at 1473 K (1200 °C) using the diffusion couple method. It was shown that MnO-SiO2-type inclusions changed to MnO-Cr2O3-type inclusions in the case of low Si content in the steel. On the other hand, in the case of high Si content, MnO-SiO2-type inclusion was stable after heat treatment. Kim et al.[3–5] have also carried out an experiment using the diffusion couple method to investigate the solid-state reaction between Fe-Mn-Si alloy and MnO-SiO2-FeO oxide under heat treatment at 1473 K (1200 °C). It was found that the Mn-depleted zone (MDZ) and particle precipitation zone (PPZ) were formed near the interface in the alloy. Because of the formation of fine metal particles near the interface in the oxide, they considered that the diffusion of oxygen from the oxide to the alloy occurred during heat treatment and caused the formation of fine oxides and the consumption of Mn. However, in this study, the sample was first heated at 1673 K (1400 °C) for 10 minutes in order to ensure good contact of the oxide with the solid alloy. Therefore, the results of subsequent heat treatment experiments were influenced by the diffusion between the alloy and oxide at this temperature.
As a result of this research, the experimental method to ensure good contact between the oxide and the alloy was improved and the influence of diffusion at 1673 K (1400 °C) was suppressed. The solid-state reaction between Fe-Mn-Si alloy and MnO-SiO2-FeO oxide under heating at 1473 K (1200 °C) is now better understood.
Experimental Method
Compositions of Fe-Mn-Si alloy and MnO-SiO2-FeO oxide used for making diffusion couples are shown in Table I. The compositions of alloy and oxide in diffusion couples AM0, AM1, and AM2 were the same as those with the previous A0, A1, and A2 in References 4 and 5, respectively. According to the previous experimental results in References 4 and 5, the compositions of the alloy and oxide in the case of AM1 had an equilibrium relation at 1823 K (1550 °C) including sulfur. In the cases of diffusion couples AM0 and AM2, the sulfur content in the oxide was lower (0 mass pct) and higher (0.28 mass pct) than the equilibrium, respectively.
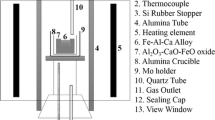
The experimental method was almost the same as that described in Reference 5 except for the heating method at 1673 K. To produce the diffusion couple, pre-melting of the oxide at 1673 K (1400 °C) is necessary to ensure good contact. In this study, to avoid the intense diffusion during pre-melting of oxide, a new technique using confocal scanning laser microscopy (CSLM) was developed. The alloy with the appropriate composition was prepared using reagent-grade electrolytic iron, ferromanganese, and silicon by an arc furnace and machined into a hexahedral shape (4 mm × 4 mm × 3 mm, 2 g). The oxide with the target composition was melted using reagent powders of MnO, SiO2, FeO, and MnS in an electrical resistance furnace. Details for making oxide containing sulfur were described in Reference 4. The contents of Mn, Si, and Fe were analyzed by an electron probe microanalyzer (EPMA), and the initial content of sulfur was analyzed by combustion-infrared spectrometer. A small piece of the oxide about 0.005 g was placed in the hole that was manufactured in the alloy. The oxide placed on the alloy was inserted into an Al2O3 crucible. A schematic diagram of the experimental set-up is shown in Figure 1. After the evacuation of the CSLM chamber up to 5.0 × 10−3 Pa, pure Argon gas was introduced. For protection against the oxidation of alloy and oxide, Ti foil was inserted around the Al2O3 crucible. After that, the temperature was increased from room temperature to 1673 K (1400 °C) by 100 K/min. During the heating, the surface of the oxide was observed, and when the oxide was melted, the sample was quenched immediately. Cooling rate was about 1000 K/min. By this method, the holding time at 1673 K (1400 °C) was minimized and the reaction at this temperature was suppressed. The diffusion couple produced by the above method was sealed into a quartz tube filled with pure Ar gas, together with a piece of Ti foil and a block of alloy with the same composition. The quartz tube was heated at 1473 K (1200 °C) for 10 and 50 hours. After the heat treatment, the quartz tube was quenched by water. A vertical cross section of each quenched sample was mirror-polished. Then, the morphology and the chemical composition of the alloy and oxide at the interface were observed and analyzed using an electron probe micro analyzer (EPMA). In order to reduce analysis error, each value was obtained from the average of three or more analyses.
Results
Influence of Heating at 1673 K (1400 °C) to Produce Diffusion Couple
Figure 2 shows the interface between the alloy and the oxide in AM0, AM1, and AM2 after the production of a diffusion couple. Good contact between oxide and alloy was confirmed, and the intense reaction observed by the previous method shown in Reference 5 was mostly suppressed, although a narrow particle precipitation zone (PPZ) was observed. In these figures, several phases observed in the oxide are also shown, plus the compositions analyzed by EPMA. This result indicated that the composition of the gray phases was close to 2MnO·SiO2 and that of the dark gray phases was close to MnO·SiO2. White particles were recognized as a metallic phase. In addition, in the case of AM0 shown in Figure 2(a), the oxide was almost homogeneous 2MnO·SiO2 with a small amount of MnO·SiO2. The content of FeO in the MnO·SiO2 phase near the alloy was about 4 pct. This value, measured by EPMA, must be affected by the alloy, since the thickness of the MnO·SiO2 phase was about 1 μm. In the cases of AM1 and AM2, shown in Figures 2(b) and (c), respectively, the main phase was also 2MnO·SiO2, but the amount of MnO·SiO2 increased. Moreover, some metallic iron particles were observed.
Mn and Si contents in the alloy near the interfaces of AM0, AM1, and AM2 are shown in Figure 3. In these figures, the results obtained by the previous method[4,5] are also shown for comparison. It is clear that the decrease in Mn and Si content near the interface was suppressed by the new method. The width of the Mn-depleted zone (MDZ) was only 15 μm or less.
In this study, the particle precipitation zone (PPZ) and Mn-depleted zone (MDZ) in the alloy near the interface were measured by the same method as described in Reference 5. PPZ was defined as the zone with more than two particles counted in a rectangular area 50 μm parallel to the interface and 5 μm perpendicular to the interface. MDZ was defined as the area with Mn content lower than that of the bulk. Figure 4 shows the PPZ and MDZ of diffusion couples AM0, AM1, and AM2 produced by the new method compared with those produced by the previous method shown in Reference 5. In this study, the widths of the PPZ were 17, 12, and 15 μm, and the widths of the MDZ were 10, 10, and 13 μm in the case of AM0, AM1, and AM2, respectively. It can be noted that the formation of PPZ and MDZ was suppressed in our study, as those widths decreased considerably.
Figure 5 shows the size distributions of particles in AM0, AM1, and AM2. The particles were manually measured in the obtained image. Compared with the experiment results in Reference 5, the size and number of particles became small, though the number of the particles smaller than 0.5 μm in diameter per 1000 μm2 increased a little with the decrease of PPZ width. The numbers of particles of larger size per 1000 μm2 were close to the previous result.
Solid-State Reaction by Heat Treatment at 1473 K (1200 °C)
Figure 6 shows the interface of the alloy and the oxide in AM0 after heat treatment at 1473 K (1200 °C) for 10 hours (AM0-10) and 50 hours (AM0-50) in this study. After the heat treatment, 2MnO·SiO2 and MnO·SiO2 phases were observed and the ratio of the MnO·SiO2 phase increased; the number and size of iron particles formed in the oxide also increased. In the alloy phase, with the increase of time for heat treatment, the width of the PPZ and the size of each oxide particle precipitated in the alloy near the interface increased.
Mn and Si contents in the alloy near the interface of AM0 before and after the heat treatment at 1473 K (1200 °C) in this study are shown in Figure 7. After the heat treatment for 10 hours, Mn and Si contents gradually decreased toward the interface from 3.1 and 0.1 pct to about 1.3 and 0.01 pct, respectively. However, they slightly increased within about 15 μm of the interface. As the heat treatment time increased to 50 hours, the width of the MDZ became larger, the minimum value of Mn content slightly increased to 1.5 pct, and the distance from the interface to the position where Mn content showed minimum value slightly increased to 30 μm. As for Si content in the alloy, as the heat treatment time increased to 50 hours from 10 hours, the region where the Si content was lower than that in the bulk alloy also increased. The minimum value of Si content and the distance from the interface to the position where Si content showed minimum value were 0.01 pct and 70 μm, respectively.
Figure 8 displays the PPZ and MDZ of AM0-10 and AM0-50 in this study compared with those in Reference 5. In this figure, AM0-0 indicates the result of the diffusion couple before heat treatment at 1473 K. As shown here, the MDZ was always larger than the PPZ after heat treatment. In addition, the widths of the PPZ and MDZ of AM0-10 in this study were almost the same as those in Reference 5. However, in the case of AM0-50, the widths of the PPZ and MDZ, which were 138 and 155 μm, respectively, in this study, were larger than those in Reference 5.
Figure 9 presents the composition of oxide particles precipitating in the PPZ of the alloy near the interface before and after the heat treatment in this study and in Reference 5. It is clear from Figure 9(a) that in this study, before and after the heat treatment for 10 hours, the composition of the particles was located in the range between pure MnO and 2MnO·SiO2 without containing MnS. On the other hand, after heat treatment for 50 hours, most of the oxide particles were close to 2MnO·SiO2, containing 0~7 mass pct MnS. As shown in Figure 9(b), the compositions of the particles in A0-0 and A0-50 reported in Reference 5 were similar to those in this study. However, the MnS content of some particles shown in AM0-10 of this study was lower than that reported in Reference 5, although the particle composition was close to 2MnO·SiO2 in both cases.
Composition of the particles in the PPZ before and after the heat treatment in this study and the previous work. (a) Composition of particles in this study and (b) composition of particles in Ref. [5]
Figure 10 presents the size distribution of the oxide particles precipitating in the PPZ of the alloy near the interface before and after the heat treatment in this study and the previous work. From the experiment results in this study shown in Figure 10(a), it can be concluded that, after heat treatment for 10 hours, the number of the particles smaller than 0.5 μm per 1000 μm2 decreased and continued decreasing after heat treatment for 50 hours. The number of the particles of larger size per 1000 μm2 increased a little after heat treatment for 50 hours. In agreement with the results in Reference 5 shown in Figure 10(b), the number of the particles smaller than 0.5 μm per 1000 μm2 increased with a decrease in PPZ width. After heat treatment for 10 hours, the number of particles smaller than 0.5 μm per 1000 μm2 in this study was close to the previous result. The number of particles of each size per 1000 μm2 in this study was smaller after heat treatment for 50 hours than that in the previous work.
Size distribution of the particles in the PPZ before and after heat treatment in this study and the previous work. (a) This study and (b) previous work in Ref. [5]
Effect of Sulfur Content in the Oxide
Figure 11 shows the effect of S content in the oxide on the concentration profiles of Mn and Si in the alloy near the interface after the heat treatment for 10 hours. When S content increased from trace (AM0) to 0.09 pct (AM1) and 0.28 pct (AM2), the minimum concentration of Mn and Si near the interface increased and the width of the MDZ became smaller. Figure 12 shows the effect of S content in the oxide on the concentration profiles of Mn and Si in the alloy near the interface after the heat treatment for 50 hours. The effects of S on the minimum concentrations of Mn and Si near the interface and on the width of the MDZ were similar to those after heat treatment for 10 hours. With the increase in the heating time, the width of the MDZ increased and the Mn content close to the interface increased in every case.
The PPZ and MDZ after the heat treatment at 1473 K (1200 °C) for 10 and 50 hours are shown in Figure 13. In this study, as shown in Figure 13(a), the MDZ and PPZ showed a linear relation, but the MDZ was larger than the PPZ. In addition, with an increase in the heating time and a decrease in the oxide’s S content, the widths of the PPZ and MDZ increased. For example, given a heat treatment lasting 10 hours, the width of the MDZ decreased as S was added, from 120 μm (AM0-10) to 62 μm (AM1-10) and 52 μm (AM2-10). The width of the PPZ decreased from 79 μm (AM0-10) to 37 μm (AM1-10) and 33 μm (AM2-10). Compared with the previous work of Reference 5 shown in Figure 13(b), the widths of the MDZ and PPZ before heating at 1473 K (1200 °C) were much smaller. After 10 hours of heating, the widths of the MDZ and PPZ of A1 and A2 were similar, but the MDZ of A0 was larger. In addition, after 50-h heating at 1473 K (1200 °C), the widths of the MDZ and PPZ of A0 became much larger.
PPZ and MDZ before and after heat treatment in this study and the previous work. (a) This study and (b) previous work in Ref. [5]
Discussion
In this study, the method to produce diffusion couples was modified using CSLM, and the chemical reaction at 1673 K (1400 °C) between the alloy and the oxide was significantly suppressed. It was found that the width of the PPZ in this experiment is little different from the previous experiment.[4,5] The diffusion length of oxygen was estimated by the same procedure carried out by Kim et al.[5] For this calculation, Wagner’s equation,[6] which estimates the thickness of the internal oxidation zone, was used to estimate the width of the PPZ.
Wagner’s equation is shown in Eq. [1]:
where ξ indicates the depth of the internal oxidation zone, N (s)O indicates the mole fraction of oxygen at the interface, D O means the diffusivity of oxygen in the alloy, ν is the number of oxygen atoms per A atom in AO x oxide, N (O)B is the mole fraction of the solute element in the alloy, and t indicates time.
Kim et al.[5] have assumed that N (s)O was determined by the solubility of oxygen in the alloy. In this study, to evaluate the influence of FeO content in the oxide, N (s)O was determined by the equilibrium relation with the alloy and the oxide. The partial pressure of oxygen equilibrated with the oxide was calculated by Eqs. [2][8] and [3][8] at 1673 K and 1473 K (1400 °C and 1200 °C). The regular solution parameters given in Reference 7 were for liquid oxides and were assumed to describe behavior at the temperatures in this study. The activity coefficient of FeO at each temperature was calculated by regular solution model using each parameter.
Using the calculated partial pressure of oxygen, the oxygen content at the interface was estimated by Eq. [4].[9] In this calculation, the activity coefficient of oxygen was assumed to be unity. Accordingly, N (s)O was obtained by changing the unit from mass percent to mol fraction.
In addition, the diffusivity of oxygen in γ-iron at each temperature was calculated by Eq. [5].[9] ν was calculated by assuming the formation of pure 2MnO·SiO2, and N (O)B was calculated from the composition of the alloy used in this experiment.
Firstly, the calculation was carried out at 1673 K (1400 °C) using the initial FeO content (3 mass pct) in the oxide. In the previous work, the heating time was 10 minutes. In this study, after the temperature reached 1673 K (1400 °C), the power to the CSLM was cut off and the sample was quenched immediately. The heating time at 1673 K (1400 °C) was then assumed to be 0 minute. By the first step of the calculation, the width of the PPZ in the diffusion couple before heating at 1473 K (1200 °C) was determined. The calculation results for the initial condition of this study and the previous study were 0 and 29 μm, respectively. After this calculation, the widths of the PPZ after heat treatment at 1473 K (1200 °C) for varying lengths of time were calculated for several values of FeO content in the oxide. Calculation results and experimental results for diffusion couple AM0 of this study and A0 of Reference 5 are shown in Figure 14. In these figures, the calculation results of various initial FeO contents in the oxide before heating at 1473 K (1200 °C) were drawn for comparison. The observed width of the PPZ in this experiment corresponded with the line of 3 pctFeO, which was the initial content of FeO in the oxide, as shown in Figure 14. A small quantity of manganese and silicon also diffused from bulk oxide to the alloy during heat treatment, as shown in Figure 7. However, the effect of the diffusion of manganese and silicone on the formation of PPZ did not predominate in comparison with the diffusion of oxygen as discussed. In the case of the previous experiment, experimental results did not correspond with the 3 mass pct FeO line but with the 1 mass pct FeO line. This result indicates that the FeO in the oxide gradually decreased with the heat treatment time at 1673 K (1400 °C). In other words, significant diffusion of oxygen due to reducing FeO in oxide was verified to have occurred after heating at 1673 K (1400 °C) for 10 minutes in the previous experiment. This indicates that this modified method of making a diffusion couple is helpful in understanding the diffusion of elements between oxide and alloy at 1473 K (1200 °C) although the observed width of the PPZ roughly corresponded with a calculated line due to the reduction of FeO content in the oxide during heat treatment.
Conclusions
A new method to produce diffusion couples was developed, and the solid-state reaction between Fe-Mn-Si alloy and MnO-SiO2-FeO oxide at 1473 K (1200 °C) was clarified. Oxide was melted in the CSLM before heat treatment at 1473 K (1200 °C). By this method, the holding time at 1673 K (1400 °C) was minimized and the reaction at this temperature was suppressed. The diffusion couple was heated at 1473 K (1200 °C) for 10 and 50 hours, and the interface of the oxide and alloy was observed to be the same as in the previous experiment. Similar to the results found in the previous method, the particle precipitation zone (PPZ) and the manganese-depleted zone (MDZ) were observed to be in the alloy near the interface. The widths of the PPZ and MDZ increased with heat treatment time and decreased with the increasing sulfur content of oxide. The distance of oxygen diffusion was estimated by Wagner’s equation, with FeO content in the oxide. The results show that the diffusion of the oxygen governs the reaction between the oxide and the alloy. It means that our modified method has an advantage in investigating the solid-state reaction between the oxide and the alloy.
References
H. Shibata, K. Kimura, T. Tanaka and S. Kitamura: ISIJ Int., 2011, vol. 51, no. 12, pp. 1944-1950.
H. Shibata, T. Tanaka, K. Kimura and S. Kitamura: Ironmak. Steelmak., 2010, vol. 37, no. 7, pp. 522-528.
K.-H. Kim, H. Shibata, and S. Kitamura: Proceedings of the 5th International Congress On ‘Science and Technology of Steelmaking’, Dresden, October 2012, CD-ROM.
K. -H. Kim, H. Shibata and S. Kitamura: ISIJ Int., 2014, vol. 54, no. 12, pp. 2678-2686.
K. -H. Kim, S. -J. Kim, H. Shibata and S. Kitamura: ISIJ Int., 2014, vol. 54, no. 10, pp. 2144-2153.
C. Wagner: Z. Elektrochem., 1959, vol. 63, no. 7, pp. 772-783.
S. Ban-ya: ISIJ Int., 1993, vol. 33, no. 1, pp. 2-11.
The Japan Institute of Metals: Physical Chemistry of Metals, Maruzen Press, Tokyo, 1996, pp. 198–208.
J. H. Swisher and E. T. Turkdogan: Trans. Met. Soc. of AIME, 1967, vol. 239, no. 4, pp. 426-431.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 18, 2014.
Rights and permissions
About this article
Cite this article
Liu, Cs., Kim, KH., Kim, SJ. et al. Reaction Between MnO-SiO2-FeO Solid Oxide and Solid Steel Deoxidized by Si and Mn During Heat Treatment at 1473 K (1200 °C). Metall Mater Trans B 46, 1875–1884 (2015). https://doi.org/10.1007/s11663-015-0356-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-015-0356-4