Abstract
Sn-, Zn-, and As-bearing iron ores are typical complex ores and are abundantly reserved in China. This kind of ore is difficult to use effectively due to the complicated relationships between iron and the other valuable metal minerals. Excessive Sn, Zn, and As contents would adversely affect ferrous metallurgy operation as well as the quality of the products. In this study, thermodynamic calculations revealed that it was feasible to synchronously volatilize Sn, Zn, and As via CO reduction. Experimental results showed that preoxidation was necessary for the subsequent reductive volatilization of Zn from the pellets, and the proper preoxidation temperature was 700–725°C under air atmosphere. Synchronous volatilization of Sn, Zn, and As was realized by roasting under weak reductive atmosphere after the pellets were preoxidized. The volatilization ratios of 75.88% Sn, 78.88% Zn, and 84.43% As were obtained, respectively, under the conditions by reduction at 1000°C for 100 min with mixed gas of 50% CO + 50% CO2 (in vol.). A metallic pellet (direct reduction iron) with total iron grade of 87.36%, Fe metallization ratio of 89.27%, and residual Sn, Zn, and As contents of 0.071%, 0.009%, and 0.047%, respectively, was prepared. Sn and As were mainly volatilized during weak reductive atmosphere roasting, and those volatilized in the metallization reduction process were negligible. Most of Zn (78.88%) was volatilized during weak reductive atmosphere roasting, while the metallization reduction process only contributed to 16.10% of total Zn volatilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the global iron and steel industry has experienced rapid development and China is in urgent need of massive amounts of iron ore due to its tremendous growth of crude steel production. To relieve the heavy dependence on the imported iron resources and to diminish the cost of iron and steel production in China, using domestic iron resources with relatively inferior quality is an important avenue toward the sustainable development of the steel industry. As reported, complex iron ores have taken an overwhelmingly dominant position.1
Currently, iron ores containing nonferrous metals have not been efficiently used on an industrial scale because these nonferrous metals would exert an adverse effect on the quality of steel products if they are not effectively separated and recovered. Sn-, Zn-, and As-bearing iron ore is a typical intractable ore abundant in China. This kind of iron ore has great potential value in use because of its large reserves and high contents of valuable metals.2 However, they cannot be directly used in ironmaking or a steelmaking because Sn, Zn, and As have unfavorable effects on the ferrous metallurgical processes and the properties of steel products.3 For example, if Zn is not effectively removed from iron ores, then the Zn compounds that existed in finished sinter or pellets will be reduced to Zn(g) and volatilized during the blast furnace (BF) metallurgy process. Then, Zn(g) vapor would diffuse into the refractory brick, resulting in the accretion on the inner wall or throat of BFs.4 , 5 In addition, because Sn and As are difficult to remove during steelmaking, they will reduce the quality of steel products. Excessive Sn and As in the steel products will substantially deteriorate their mechanical properties, such as plasticity, ductility, and tenacity. Furthermore, As and Zn are harmful to human health and the environment if they escape to the atmosphere.
Up to now, many efforts have been made to develop more economical and effective ways to recover iron and other valuable elements from this kind of iron ore. However, the available technologies are infeasible to thoroughly separate or recover all valuable metals. Combined physical separation processes are efficient to increase iron grade, while unfavorable Sn, Zn, and As remain because they are complexly embedded in magnetite.6 Selective sulfurization or chlorination roasting processes are effective to separate Sn, Zn components from iron ores, but issues of environmental pollution and equipment corrosion are difficult to avoid.7 , 8 It is also reported that As is readily removed by the sintering process, whereas the majority of Sn and Zn are left in finished sinter.9 Sn and Zn are capable of being removed by conventional coal-based rotary kiln process, and a wustite pellet with good strength is prepared.10 However, the reducibility is worsened because the wustite pellet is difficult to reduce further in BF.11 , 12
Currently, ministeelmaking is one of the cutting-edge technologies for steel production, which is characterized by low energy consumption, high efficiency, and environmental friendliness. Direct reduction iron (DRI) is a superior burden for ministeelmaking because of its stable composition, low content of harmful elements, and uniform particle size.
To prepare DRI from Sn-, Zn-, and As-bearing magnetite concentrate, redox thermodynamic behaviors of Sn, Zn, and As compounds were initially discussed, and technological parameters including roasting atmosphere, roasting temperature, roasting time, and CO concentration were investigated to synchronously volatilize Sn, Zn, and As. Finally, a metallic pellet (DRI), a burden for electric arc furnace (EAF) production, with a high iron metallization ratio and low residual Sn, Zn, and As contents, was prepared.
Experimental
Materials
Iron Concentrate
The Sn-, Zn-, and As-bearing iron concentrate used in this study was taken from the Inner Mongolia Autonomous Region, China. The specific surface area of the sample was 1837.5 cm2/g. The main chemical composition is given in Table I. The total iron grade (TFe) of the concentrate was 65.70%, but the excessive contents of 0.26% Sn, 0.14% Zn, and 0.29% As exceed the limitation (Sn < 0.08%, Zn, As < 0.1%) when used as a feed for ironmaking.
A previous study on the mineralogy of a similar sample from the same mine demonstrated that the main minerals of Sn, Zn, and As in the iron concentrate were cassiterite (SnO2), sphalerite (ZnS), and arsenopyrite (FeAsS), respectively, and most of the Sn-, Zn-, and As-bearing minerals were embedded in magnetite, which resulted in difficult separation of Sn-, Zn-, and As-bearing minerals from magnetite by physical separation processes.13 It is worthy to mention that the tested sample has higher TFe, and lower Zn and As contents than the sample used in the literature 13 by improving magnetic separation of the run-of mine.
Bentonite
The main chemical composition of the bentonite, the binder for pelletizing, is shown in Table II.
Gases
The purity of gases (CO, CO2, and N2) used in this study was greater than 99.99%.
Methods
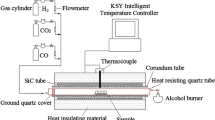
The experimental flowsheet is shown in Fig. 1, which includes balling, drying, preoxidation roasting, reduction roasting under weak reductive atmosphere, metallization reduction roasting, and cooling. The iron concentrate was blended with 1% bentonite and balled into green pellets with 12–15 mm in diameter by using a Φ1000-mm disk pelletizer. The green ball was dried in an oven at 105°C for 4 h. After that, the dried pellets were put in a corundum crucible and then roasted in the electrically heated horizontal furnace, whose schematic diagram was described in Fig. 2.
The corundum crucible loaded with pellets was pushed rapidly from the pipe orifice to the roasting zone after the furnace was heated to a given temperature. The pellets were first roasted at the given temperature for 10 min under air atmosphere, then consecutively reduced under weak reductive atmosphere and by metallization reduction with different CO concentrations [CO/(CO + CO2)]. The CO content of the reduction gas was adjusted by varying the proportion of CO and CO2. The flow rate of inlet gas was fixed at 4.0 L/min, equivalent to a flow velocity of 9 cm/s (in standard state). After the pellets were roasted at given temperature for a period of time, the crucible containing the roasted pellets was pulled to the entrance of the furnace and cooled to room temperature under N2 atmosphere. Finally, the composition of the roasted pellets, including their residual Sn, Zn, and As contents, TFe and metallic iron (MFe) contents were analyzed. The contents of MFe and TFe were measured by chemical titration method, and the contents of Sn, Zn, and As were assayed by the inductively coupled plasma optical emission spectrometer (ICP-OES; T-900 F3.5, American Baird Co., Ltd).
The phase composition of roasted pellets was determined by XRD analysis using a diffractometer (Rigaku D/Max 2500; Rigaku Corporation, Tokyo, Japan) under the conditions of radiation: Cu Kα, tube current and voltage: 250 mA, 40 kV, scanning range: 10° to 80° (2θ), step size: 0.02° (2θ), and scanning speed: 8°/min. Optical microscopy (LEICA DM RXP; Leica Microsystems, Wetzlar, Germany) was used to observe the microstructure of the roasted pellet. The microstructure and elemental composition of the reduced pellet were analyzed by using environmental scanning electron microscopy (ESEM; FEI QUANTA 200; FEI, Eindhoven, The Netherlands) equipped with an EDAX energy dispersive x-ray spectroscopy (EDS) detector (EDAX Inc., Mahwah, NJ). ESEM images were recorded by backscattering in the low-pressure environment (0.5 Torr and 20 keV).
Evaluation Indexes
The volatilization ratios of Sn, Zn, and As were calculated according to the following equation:
where γ is the volatilization ratio (%); ε is the mass loss ratio of the pellets (%); α is the residual Sn, Zn, or As mass contents of the roasted pellets (%); and β is the original Sn, Zn, or As mass contents of the dried pellets (%).
The iron metallization ratio was calculated according to the following equation:
where η is the iron metallization ratio (%), MFe is the mass content of metallic iron of the reduced pellets (%), and TFe is the total iron grade of the reduced pellets (%).
Thermodynamics of Sn, Zn, and As Volatilization
It is well known that ZnO is more readily reduced to Zn(g) for volatilization than ZnS.14 Therefore, the dried pellets need to be preoxidized so that sphalerite (ZnS) is transformed into ZnO for the subsequent separation of Zn via reduction.
The main reactions of Sn, Zn, and As compounds and the corresponding \( \varDelta G_{T}^{\theta } - T \) equations during oxidation and reduction processes are shown in Table III. The gas-phase equilibrium diagram of reductive volatilization of Sn, Zn, and As is shown in Fig. 3. According to Table III, ZnS is capable of being oxidized to ZnO (Eq. 1, T < 5712 K), and the temperature required for ZnO reduction is above 1584 K (Eq. 2) under the standard condition [P Zn(g) = 1 atm.]. However, because the partial pressure of gaseous Zn(g) is very low during reduction roasting, the temperature required for ZnO reduction decreases obviously compared with the standard condition. The temperature required for ZnO reduction is only 1074 K when P Zn(g) is 0.001 atm. and CO concentration is 50%. As seen from Fig. 3, the temperature and CO equilibrium concentrations for reducing ZnO(s) to Zn(g) increase gradually as the partial pressure of gaseous Zn(g) increases.
Arsenopyrite (FeAsS), the main As-bearing phase of the iron concentrate, is capable of being oxidized and forming As2O3(g) under oxidative atmosphere of 6–12% O2 concentration [O2/(O2 + N2)]. However, arsenopyrite will also be oxidized to arsenate (such as FeAsO4) under higher O2 concentration,15 and the formation of FeAsO4 is easier than As2O3(g) as the \( \varDelta {\text{G}}_{T}^{\theta } \) of Eq. 3 is lower than those of Eqs. 4 and 5. As a result, solid FeAsO4 must be reduced to gaseous As4O6 or As2O3 for As volatilization from the pellets.16 At a low CO content, FeAsO4 is also capable of being reduced to As2O3(g) (Eq. 6); however, it will also be reduced to solid-state FeAs under higher CO concentration (Eq. 7), which restrains the volatilization of As greatly. Therefore, the CO concentration should be controlled between the CO equilibrium concentrations of Eqs. 6 and 7 to generate gaseous As2O3.
It is reported that cassiterite remains relatively stable under an oxidative atmosphere. SnO2 will be reduced stepwise in the order of SnO2 → SnO(s) → Sn via CO reduction.17 SnO(s) has high saturated vapor pressure (e.g., 2.07 kPa at 1100°C),11 which facilitates the volatilization of Sn. However, the generated metallic Sn will be combined with metallic Fe and form Fe-Sn alloy, leading to an adverse effect on the Sn volatilization under the strong reductive atmosphere. Therefore, the reduction roasting conditions for Sn volatilization should be strictly controlled at the stage of SnO(s).18 , 19 Unfavorably, the predominant area of SnO(s) is relatively small, as shown in Fig. 3.
From the above discussion, it can be concluded that ZnS must be first oxidized to ZnO for the subsequent reduction volatilization. Sn, Zn, and As are capable of synchronously volatilizing via a reduction roasting process. The CO content should be controlled under weak reductive atmosphere for their synchronous volatilization from a thermodynamic point of view. The thermodynamic area with oblique lines is the phase-stability section where SnO(s), Zn(g), and As2O3(g) coexist. The reduction conditions are mainly restrained by the volatilization of Sn.
Experimental Results and Discussion
Effect of Preoxidation on the Reductive Volatilization of Sn, Zn, and As
Thermodynamic analysis indicated that Sn, Zn, and As were feasible to be synchronously volatilized under weak reductive atmosphere. It was also concluded that ZnS must be oxidized to ZnO in advance for the subsequent reduction volatilization. Thus, the effect of preoxidation temperature on the downstream reduction volatilization of Sn, Zn, and As was investigated emphatically. The dried pellets were preoxidized at different temperatures for 10 min under air atmosphere, and then the preoxidized pellets were reduced at 1000°C for 60 min with the mixed gas of 50% CO + 50% CO2.
It was detected that the volatilization of Sn, Zn, and As is negligible under the experimental conditions of preoxidization. As a result, their volatilization during preoxidization was ignored. The volatilization of Sn, Zn, and As during reduction roasting and their residual contents of the reduced pellets are shown in Fig. 4.
Compared with the pellets not preoxidized, the Zn volatilization ratio of the preoxidized pellets increases obviously. The Zn volatilization ratio is only 18.57% without preoxidization, while it goes up to 32.75% after being preoxidized at 600°C and then increases to 65.63% at 725°C. Thus, preoxidation plays an important role in the subsequent reduction volatilization of Zn. The results agree well with the thermodynamic analysis in which ZnS must be oxidized before Zn is volatilized.
However, it is observed that preoxidation exerts obviously an adverse effect on the volatilization of Sn. The volatilization of Sn is as high as 79.85% for pellet without preoxidization, but it markedly decreases to about 50% after being preoxidized.
Simultaneously, preoxidation is slightly advantageous for the subsequent volatilization of As during reduction. Therefore, the preoxidation temperature of 700–725°C was recommended for the subsequent reduction process.
Synchronous Volatilization of Sn, Zn, and As via Weak Reductive Atmosphere Roasting
In this section, preoxidation process was performed at 725°C for 10 min under air atmosphere, and the preoxidized pellets were subsequently reduced in weak reductive atmosphere without discharging from the furnace. The residual Sn, Zn, and As contents of the reduced pellets and their volatilization ratios were determined.
Effect of Reduction Temperature
The effect of reduction temperature on the volatilization of Sn, Zn, and As is shown in Fig. 5. The preoxidized pellets were reduced at temperatures varying from 925°C to 1050°C for 60 min with the mixed gas of 50% CO + 50% CO2. It can be observed that the reduction temperature has a significant impact on the volatilization of Sn, Zn, and As. The volatilization of Sn increases with increasing reduction temperature up to 1000°C and then decreases slightly at higher temperatures. The maximum Sn volatilization ratio of 46.62% is obtained at 1000°C. In the meantime, the residual Zn content of the reduced pellets decreases gradually from 0.075% to 0.043% with the increase of reduction temperature, and the volatilization ratio of Zn increases from 50.06% to 71.68% correspondingly. Similarly, the residual As content decreases as the reduction temperature increases from 925°C to 1000°C, and then keeps stable. A minimum As content of 0.049% and a maximum As volatilization of 84.37% are obtained at 1000°C. Thus, the proper reduction temperature of 1000°C was recommended.
Effect of Reduction Time
The effect of reduction time on the volatilization of Sn, Zn, and As is shown in Fig. 6. The pellets were reduced at 1000°C in the mixed gas of 50% CO + 50% CO2 for different time periods from 60 min to 110 min.
The results indicate that the residual Sn content of the reduced pellets decreases sharply with the reduction time increasing from 60 min to 100 min and then varies slightly. The residual Sn content and the volatilization ratio of Sn are 0.068% and 75.88%, respectively, when reduced at 1000°C for 100 min. The Zn content decreases obviously from 60 min to 80 min and then decreases gradually. A residual Zn content of 0.038% and 74.90% of Zn volatilization are acquired after being reduced for 80 min. With respect to As, the effect of reduction time on its volatilization is weak. Both the residual As content and the volatilization ratio of As remain nearly stable throughout the reduction process.
Considering on the lower residual Sn content, a reduction time of 100 min is recommended at 1000°C.
Effect of CO Content of the Mixed Gas
Fixing the reduction temperature of 1000°C and reduction time of 100 min, the effect of CO content of the mixed gas on the volatilization of Sn, Zn, and As was investigated. The results presented in Fig. 7 indicate that the volatilization of Sn increases first and then decreases with increasing CO content. This is mainly attributed to the formation of metallic Sn under a stronger reduction atmosphere, which restrains the volatilization of Sn. The maximum Sn volatilization ratio of 75.88% is obtained at 50% CO + 50% CO2.
Adversely, the volatilization ratio of Zn obviously increases from 56.74% to 91.81% with the increase of CO content up to 70%. The thermodynamic analysis also reveals that a higher reduction potential is advantageous for the volatilization of Zn.
The As volatilization changes slightly when the CO content is lower than 50%, but it decreases sharply when the CO content is further increased. This is mainly attributed to the formation of FeAs under a stronger reduction atmosphere, and the experimental result is in accordance with the thermodynamic results.
Considering the synchronous volatilization of Sn, Zn, and As, 50% CO + 50% CO2 content of the mixed gas is recommended. This optimal CO content is higher than that obtained via a thermodynamics analysis. The difference may be partly attributed to the preoxidation processing, which causes a more compact pellet, and to the slower diffusion of CO into pellet restrained by lower porosity. In addition, it can be observed that the optimal CO content of the mixed gas is mainly determined by the volatilization of Sn, which is in line with the predominant region restrained by Sn volatilization in the thermodynamic analysis (in Fig. 1).
The micrographs of the pellets reduced under weak CO atmosphere obtained by scanning electron microscopy (SEM) and optical microscope analysis are shown in Fig. 8a and b, respectively. The energy dispersive x-ray (EDX) detector results of point A and B are presented in Fig. 8 simultaneously. It can be observed that the pellet is mainly composed of wustite (FeO, exhibited at point A) together with a small amount of gangues (exhibited at point B).
From the above results, the proper conditions for synchronous volatilization of Sn, Zn, and As via weak reductive atmosphere roasting are obtained as follows: reducing at 1000°C for 100 min with the mixed gas of 50% CO + 50% CO2. The volatilization ratio of 75.88% Sn, 78.88% Zn, 84.43% As, the residual content of 0.068% Sn, 0.032% Zn, 0.046% As, 83.03% FeO, and 0.57% MFe of the reduced pellets are attained, respectively. The results indicate that it is necessary for the pellet to be further reduced under a stronger reduction atmosphere for DRI preparation.
Metallization Reduction Roasting for DRI Preparation
The results described above reveal that most of Sn, Zn, and As is capable of being synchronously volatilized from pellets under a weak reductive atmosphere, but iron oxide merely transforms to wustite. For DRI preparation, the effects of CO content, reduction temperature, and time on the metallization ratio of Fe were studied in detail.
In this section, preoxidation process was performed at 725°C for 10 min under air atmosphere, and the weak reduction was conducted at 1000°C for 100 min with the mixed gas of 50% CO + 50% CO2.
Effect of CO Content
For optimizing the CO content required for the metallization reduction, the roasting temperature and time were fixed at 1050°C and 60 min, respectively, and the CO content of gas varied in the range 70–100%. The results shown in Fig. 9 reveal that the metallization ratio of Fe increases rapidly with increasing CO content from 70% to 100%. A maximum Fe metallization ratio of 89.27% and total iron grade of 87.36% were attained for 100% CO content. Therefore, 100% CO gas is considered to be the most favorable.
Effect of Reduction Temperature
The effect of reduction temperature on the metallization reduction of pellets is shown in Fig. 10 when the reduction time is 60 min and CO concentration of the gas is 100%. The metallization ratio of Fe increases from 85.39% to 89.27% with the reduction temperature going up to 1050°C, and then it changes slightly. Hence, 1050°C is recommended for the metallization reduction process.
Effect of Reduction Time
The results plotted in Fig. 11 reveal the effect of reduction time on the metallization reduction of pellets. It was observed that the metallization of Fe increases gradually with duration up to 60 min, and there is no appreciable gain in Fe metallization as the reduction time is further extended to 70 min. Thus, 60 min is considered to be optimum for metallization reduction of pellets at 1050°C with 100% CO gas.
Therefore, the proper metallization reduction conditions are recommended at the reduction temperature of 1050°C for 60 min with 100% CO gas.
Under the proper roasting conditions, the reduction of iron and the volatilization of Sn, Zn, and As at different stages are shown in Table IV. It can be seen that the metallization ratio of Fe reaches 89.27%; Sn and As are mainly deprived under weak reductive atmosphere, while their volatilization in metallization reduction process are very slight. Most of Zn (78.88%) is volatilized at a weak reduction stage, and metallization reduction plays a part in deep volatilization of Zn.
Characterization of DRI Product
The XRD pattern of the metallic pellets (DRI) obtained under the optimal conditions is demonstrated in Fig. 12. For a comparison, the XRD patterns of the iron concentrate and the weakly reduced pellet are simultaneously presented. As seen from Fig. 12, the diffractions of magnetite in the iron concentrate disappear and wustite is formed after weak reduction. Then, the diffractions of wustite in the weakly reduced pellets disappear whereas metallic iron is newly generated during the metallization reduction.
The main chemical composition presented in Table V shows that the prepared DRI product contains 87.36% TFe as well as 0.071% Sn, 0.009% Zn, and 0.047% As.
Conclusions
Sn-, Zn-, and As-bearing iron concentrate was efficiently used for synchronous volatilization of nonferrous metals and preparation of DRI by the successive processes including preoxidation, reduction under a weak CO atmosphere, and metallization reduction.
Preoxidation was necessary for the reductive volatilization of Zn, and the preoxidation temperature of 700–725°C under air atmosphere for 10 min was sufficient for the subsequent reductive volatilization of Zn.
Sn, Zn, and As were synchronously volatilized under the weak reductive atmosphere. The Sn, Zn, and As volatilization ratios of 75.88%, 78.88%, and 84.43% were attained, respectively, via reduction at 1000°C for 100 min with the mixed gas of 50% CO + 50% CO2.
A metallic pellet (DRI) with 89.27% Fe metallization ratio, 87.36% TFe, and the residual content of 0.071% Sn, 0.009% Zn, and 0.047% As was prepared for EAF steelmaking.
References
J. Zou, Miner. Eng. 2, 1 (2003).
Y.B. Zhang, L.Y. Chen, G.H. Li, T. Jiang, and Z.C. Huang, J. Cent. South Univ. Sci. Technol. 42, 1501 (2010).
T. Jiang, Y.B. Zhang, G.Q. He, G.H. Li, and Z.C. Huang (Paper presented at the Proceedings of 24th IMPC, Beijing, 2008), pp. 3952–3958.
C.D. Xu, R. Lin, and D.C. Wang, Physical and Chemical of Zinc Metallurgy (Shanghai: Science and Technology Press, 1979), pp. 829–836.
Y.B. Zhang, Y.F. Guo, G.H. Li, Y.B. Yang, Y.S. Jin, Z.C. Huang, X.H. Fan, and T. Jiang, Proceedings of Sessions and Symposia Sponsored by the Extraction and Processing Division (Warrendale, PA: TMS, 2005), pp. 417–426.
L. Lin and S.Q. Wang, Process. Metall. 7, 26 (1998).
A.W. Fletcher, D.V. Jackson, and A.G. Valentine, Trans. Nonferr. Met. Soc. 76, 145 (1967).
T. Jiang, Y.B. Zhang, Z.C. Huang, G.H. Li, Y.F. Guo, Y.B. Yang, and Y.S. Jin, Trans. Nonferr. Met. Soc. 15, 902 (2005).
Q. Lu, S.H. Zhang, and X. Hu, Adv. Mater. Res. 284, 238 (2011).
S. Cetinkaya and S. Eroglu, Int. J. Miner. Process. 110, 71 (2012).
Y.B. Zhang, T. Jiang, G.H. Li, Z.C. Huang, and Y.F. Guo, Ironmak. Steelmak. 38, 612 (2011).
D. Huang, Y.B. Zhang, G.H. Han, G.H. Li, and T. Jiang, Supplemental Proceedings: Materials Processing and Properties (Warrendale, PA: TMS, 2010), Vol. 1, pp. 393–401.
Y.B. Zhang, G.H. Li, T. Jiang, Y.F. Guo, and Z.C. Huang, Int. J. Miner. Process. 114, 109 (2012).
L. Bentell, L. Norrman, and M. Sundgren, Scand. J. Metall. 13, 308 (1984).
S.H. Zhang, Q. Lu, and X. Hu, Chin. J. Nonferr. Met. 21, 1075 (2011).
N. Chakraborti and D.C. Lynch, Metall. Mater. Trans. B 14, 239 (1983).
J. Joffre, Foreign Tin Ind. 4, 38 (1984).
H. Giefers, F. Porsch, and G. Wortmann, Solid State Ion. 176, 199 (2005).
M.S. Moreno, G. Punte, G. Rigotti, R.C. Mercader, A.D. Weisz, and M.A. Blesa, Solid State Ion. 144, 81 (2001).
D.L. Ye, Practical Thermodynamics Data Handbook of Inorganic Substances (Beijing: Metallurgy Industry Press, 2002), pp. 875–878.
Acknowledgement
The authors wish to express their thanks to the National Science Fund for Distinguished Young Scholars (No. 50725416) and the Program for New Century Excellent Talents in University (NCET-11-0515) for financial support of this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yuanbo Zhang and Tao Jiang have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, G., You, Z., Zhang, Y. et al. Synchronous Volatilization of Sn, Zn, and As, and Preparation of Direct Reduction Iron (DRI) from a Complex Iron Concentrate via CO Reduction. JOM 66, 1701–1710 (2014). https://doi.org/10.1007/s11837-013-0852-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-013-0852-4