Abstract
In the selective reduction process of lateritic nickel ore, the formation of the liquidus phase could be obtained by increasing the temperature and additive, resulting in an uneconomic process. Nevertheless, it could also be obtained by modifying the ore basicity by adding low-cost fluxes, such as CaO, SiO2, MgO, and Al2O3. In this work, the effect of three types of basicity (binary, ternary, and quaternary) in selective reduction of saprolitic nickel ore on metallic grade and recovery, phase transformation, and microstructure of ferronickel was investigated clearly. The nickel ore, sodium sulfate, coal, and flux mixture was reduced to 1150 °C for 60 min. Then, the wet magnetic separation process was continued to separate ferronickel from impurities. The result showed that the optimum basicity for the selective reduction of saprolitic nickel ore was 0.6 of ternary basicity (CaO + MgO/SiO2), modified by CaO addition. It produced 16.11% and 50.57% for nickel grade and recovery in concentrate, respectively. Modified basicity with CaO addition is more effective than MgO, Al2O3, and SiO2 addition due to its ability to break the forsterite structure and release iron and nickel. It also could be reacted with magnesium silicate structure to form calcium–magnesium silicate structure such as melilite and monticellite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nickel's global demand has grown rapidly. Almost 65% of nickel consumption is used for stainless steel, while others go to batteries and chemicals [16]. There are two kinds of nickel ore resources on land, i.e., lateritic and sulfidic. Nowadays, nickel laterite production increases due to the depletion of nickel sulfide [3]. Nickel laterite has lower nickel content than nickel sulfide. Nickel is found in complex structure and has high water content, which causes difficulties in improving the nickel content by physical separation and flotation method [6, 23]. Therefore, it has become challenging in lateritic nickel ore processing.
Selective reduction continued with magnetic separation process is a new method to generate high nickel concentrate from nickel laterite by using lower temperature than conventional pyrometallurgy technology, such as blast furnace and rotary kiln electric arc furnace [12, 28]. The main role of selective reduction is to inhibit the reduction of iron oxide to metallic iron for generating high-nickel-grade ferronickel [1].
Nickel laterite is classified into saprolite and limonite. Saprolite has higher nickel content than limonite. Nevertheless, it also has higher impurities, such as silicon dioxide (SiO2) and magnesium oxide (MgO). Iron and nickel are mainly associated with magnesium silicon hydroxide or lizardite ((Fe,Ni,Mg)3Si2O5(OH)4). From the previous study, lizardite is more difficult to reduce than goethite. Low recovery of iron and nickel has been resulted from the selective reduction process of saprolitic nickel ore. However, it produces a higher nickel grade in concentrate than limonite [17]. The reduction mechanism of saprolite is expressed in Reaction (1–5) [8].
There are some important parameters in the selective reduction process of nickel laterite to generate ferronickel with high nickel grade and recovery; they are (1) temperature, (2) duration time, (3) reductant, and (4) additives. The initial temperature reduction of nickel oxide is lower than iron oxide [20, 24]. However, reducing nickel laterite below the reduction temperature of iron oxide will result in a low recovery of nickel [29]. The reduction rate of iron and nickel oxide increases with the increase in the temperature and duration time of the reduction process. Zhu et al. [32] reported that iron and nickel grades increase significantly with increasing temperature from 1100 to 1250 °C and duration time from 20 to 60 min. According to Jiang et al. [9], the particle size of ferronickel increases with the increase in the temperature. The large particle size of ferronickel will promote the liberation degree of the metallic phase from impurities in this selective reduction process.
Coal is a carbon-bearing material commonly used as a reductant in the nickel ore reduction process [7, 13]. From the Bouduard reaction, as expressed in Reaction (1), the CO gas will be generated and used to reduce the metal oxide. The increasing reductants will increase the reducing atmosphere, thus increasing the reduction rate of metallic oxide. However, more metallic iron in ferronickel will be harmful to nickel grade [19, 25]. Thus, the optimum coal addition should be selected.
Additives are very important in the selective reduction of nickel laterite to produce high nickel grade in ferronickel [4]. Additives, such as carbonate [30], chloride [31, 14], and sulfur or sulfate [20, 8, 26] are already used. From the previous result, sodium sulfate showed its superiority for enhancing ferronickel's nickel content over sodium chloride and sodium carbonate [22]. The increase of nickel grade in concentrate is resulted from the addition of sulfate or sulfur additive in the reduction process to suppress the metallization of iron by sulfidation mechanism [11, 5, 21], as expressed in Reaction (6–8). It promotes non-magnetic iron sulfide (FeS); thus, some iron will go to the tailing after the magnetic separation [15]. Iron sulfide also has a low melting point temperature, 985 °C; thus, the liquidus phase could lower the diffusions rate of reductant gas, which could inhibit the reduction of iron oxide [32]. Using sodium sulfate as additives could promote nepheline (Na2Si2O5) which also has a low melting point. Iron sulfide and nepheline play an important role in the agglomeration of ferronickel particles. The large ferronickel size improves the liberation degree of metallic particles from impurities, increasing the metal recovery [8].
The previous result required 10 wt% of sodium sulfate in selective reduction of saprolitic nickel ore with 1.78% Ni at temperature 1250 °C for 60 min to obtain 12.35% and 34.4% for nickel grade and recovery, respectively [17]. More iron and nickel from magnesium silicate structure will be released with the increase of additives and temperatures, which could affect the increase in the cost production. Therefore, it becomes a challenge in the selective reduction of saprolitic nickel ore to conduct the reduction process at a temperature below 1200 °C. Nevertheless, the more sulfur or sulfate addition, the more sulfur content in ferronickel will be found. Sulfur content should be minimized in stainless steel products due to lowering its mechanical properties. Therefore, the use of more sulfur additives in selective reduction should be considered.
The liquidus phase plays an important role in the pyrometallurgy process. Diffusion rate, chemical reaction, and agglomeration of a metallic particle are performed faster in the liquidus phase than in the solidus phase. In the selective reduction process of lateritic nickel ore, the formation of the liquidus phase could be obtained by increasing the temperature and sodium sulfate, resulting in an uneconomical process. The low melting point phase can be obtained by modifying the basicity of ore in the smelting process of metallic ore [27]. Generally, there are three types of basicities, i.e., binary (BB), ternary (BT), and quaternary (BQ) basicity, which is expressed in Eq. (9–11). Modification of the basicity is carried out with the addition of calcium oxide (CaO), magnesium oxide (MgO), aluminum oxide (Al2O3), and silicon dioxide (SiO2).
Chuang et al. [2] have investigated the effect of binary basicity (CaO/SiO2) in the reduction process of iron-bearing minerals on low melting point phase formation, which promoted the strength of direct reduced iron pellet. Nevertheless, there is no information about its effect on metal and recovery grade. Pan et al. [19] have studied the effect of basicity (CaO/SiO2) on selective reduction of nickel slag containing iron–nickel–copper oxide with 37.8 Fe-0.9 Ni-0.34Cu. Reducing the material to 1200 °C for 20 min with basicity of 0.15 resulted in 3.25% Ni and 1.2% Cu with 82.2% and 80.0% for nickel and copper recovery, respectively. However, there is still less information about the effect of basicity on selective reduction of lateritic nickel ore.
From a previous study, the effect of basicity in the selective reduction of limonitic nickel ore has been investigated. Binary basicity is more appropriate than ternary and quaternary basicity for limonitic ore due to the fact that it contains high SiO2, while it has low MgO, Al2O3, and CaO. The optimum condition has resulted from 0.1 of binary basicity (CaO/SiO2) [18]. Saprolite has a different composition than limonite; thus, the proper basicity type could be different. However, the effect of modified basicity in the selective reduction of saprolitic nickel ore is still less studied. Tian et al. [25] reported that the addition of CaCO3 in the reduction of saprolitic ore has increased the nickel recovery due to the destruction of iron and nickel in magnesium silicate compounds. Unfortunately, this study did not reveal the effect of basicity in the reduction of saprolite.
2 Materials and Methods
2.1 Materials
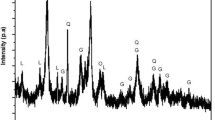
The saprolitic nickel ore was from Halmahera Island, Indonesia. The chemical composition of saprolite was analyzed by using XRF, as listed in Table 1. The iron and nickel contents are 18.48% and 1.81%, respectively. It has high SiO2 and MgO content. From the XRD analysis, as illustrated in Fig. 1, it can be observed that most iron and nickel are associated with lizardite ((Ni,Fe,Mg)3Si2O5(OH)4. Some goethite ((Fe,Ni)OOH) and chromium oxide (CrO2) are also found. From the Rietveld analysis using high score plus software, the composition of goethite, lizardite, and chromium oxide was 41.6 wt%, 49 wt%, and 9.3wt%, respectively.
Bituminous coal and sodium sulfate were used as reductant and additive, respectively. The proximate analysis of the reductant is listed in Table 2. Fluxes, such as CaO, MgO, SiO2, and Al2O3, were used to modify basicity from 0.1 to 1.0. Sodium sulfate and all fluxes were of analytical grade.
2.2 Methods
Saprolite ore and bituminous coal were ground into less than 149 µm. 50 g of saprolite, 1.9 g of bituminous coal, 5 g of sodium sulfate, and some fluxes were mixed homogeneously using a milling unit prior to pelletization into 10–15 mm diameter. Various amounts of fluxes were added into the pellets to modify ore basicity from 0.1 to 1.0. The saprolite ore basicity, according to Eqs. (9–11), is presented in Table 3.
The pellets were then placed in a tightly closed graphite crucible for the reduction process, carried out in a muffle furnace at 1150 °C for 60 min. After the reduction process, the pellets were quenched rapidly into the water to prevent them from re-oxidized. It was then dried and crushed into 95% less than 74 µm prior to the magnetic separation process using 500 Gausses of a magnetic field. It produced concentrates (magnetic) containing ferronickel and tailings (non-magnetic) containing impurities. The workflow diagram is illustrated in Fig. 2.
3 Results and Discussions
The effect of binary, ternary, and quaternary basicity on metal grade and recovery, phase transformation, and microstructure of ferronickel in the selective reduction process of saprolitic ore has been briefly discussed. The appropriate basicity types for the selective reduction process of saprolitic nickel ore will be determined from the optimum result of nickel grade in concentrate.
3.1 Effect of Binary Basicity (BB)
The effect of binary basicity (BB1 = CaO/SiO2 and BB2 = MgO/SiO2) on the selective reduction of saprolitic nickel ore was investigated clearly. The value of binary basicity was modified from 0.1 to 1.0 with the addition of CaO for BB1, while SiO2 and MgO for BB2. From Fig. 3a, the nickel grade increases to the optimum value, then decreases significantly with the increase of binary basicity. The optimum nickel grade is 14.12% and 13.29%, with nickel recovery of 37.02% and 26.94%, resulting from 0.2 of BB1 and 0.3 of BB2, respectively. Figure 3b and c shows that the nickel and iron recovery increase with the increase in the binary basicity value.
The addition of CaO, MgO, and SiO2 in the selective reduction process is very important for destroying the iron–nickel magnesium silicates structure in saprolitic nickel ore [10, 27]. Thus, iron and nickel will be released, then reduced into metallic iron and nickel. From Fig. 3, the concentrate with optimum nickel grade is obtained from a low binary basicity value. Nevertheless, It has low nickel recovery, which indicates that more iron and nickel go to the tailing. From XRD analysis in Fig. 4a and b, more iron and nickel are still entrapped in magnesium silicate structure, i.e., forsterite ((Fe,Ni,Mg)2SiO4) and enstatite ((Fe,Ni,Mg)2Si2O6) at low basicity. Therefore, the ferronickel peak is low, for both BB1 and BB2.
From the XRD analysis in Fig. 4a, it looks that the ferronickel peaks increase with the increase in the binary basicity (BB1). It was agreed with Li et al. [10] that the CaO addition in the reduction of saprolitic ore could destroy the iron–nickel–magnesium silicate structure and release the nickel and iron to form ferronickel. At 0.2 of BB1, the diopside is found. The addition of a small amount of CaO could replace the iron and nickel forsterite structure to form diopside ((Ca,Mg)2SiO6) and iron–nickel silicate ((Fe,Ni)2SiO4), as expressed in Reaction (12). Furthermore, the iron–nickel silicate will be reduced into metallic ferronickel by the following Reaction (4–5). The addition of more CaO, resulting in higher basicity, would transform the diopside into melilite (Ca,Na)2MgSi2O7, as expressed in Reaction (13). This reaction also generates iron–nickel oxide ((Fe,Ni)O), released from the silicate structure. Therefore, the recovery of nickel and iron increases significantly from 0.2 to 0.6 of basicity, as illustrated in Fig. 3. Nevertheless, the increase in iron recovery results in decreasing the nickel grade. At 0.6 basicity, monticellite ((Ca,Mg)SiO4) is observed. It is observed by the reaction of melilite with CaO, as expressed in Reaction (14). No more iron and nickel is extracted from silicate structure. Therefore, the iron recovery look steady at basicity 0.6 to 1.0.
Phase transformation of reduced saprolitic ore with various basicity is also analyzed using a ternary diagram as shown in Fig. 5. The result is almost similar to the XRD analysis. At 0.2 basicity, it shows the presence of pyroxene or diopside. Furthermore, with the increase in the basicity, the melilite and monticellite phases are formed. The phase transformation of saprolite in various binary basicity values (BB1 = CaO/SiO2), modified by CaO addition, is illustrated in Fig. 6.
Diopside, melilite, and monticellite have high melting point temperatures, i.e., 1391, 1454, and 1503 °C, respectively. It would contribute to a negative effect on the agglomeration of ferronickel particles. The presence of iron sulfide or troilite (FeS), which has a low melting point temperature, is very important in the selective reduction of nickel ore. Nevertheless, the troilite peaks seem to decrease with the increasing binary basicity (BB1) in this work, as shown in Fig. 4a. CaO could react with sulfur to form CaS. Therefore, the more the CaO is added, the less the FeS is formed.
The illustration of phase transformation of saprolite with SiO2 and MgO addition is given in Fig. 6. From XRD analysis, as shown in Fig. 4b, forsterite ((Fe,Ni,Mg)2SiO4), enstatite ((Fe,Ni,Mg)2Si2O6), quartz (SiO2), and small of ferronickel (FeNi) are found at low basicity. The reaction of forsterite with SiO2 will promote the formation of enstatite as expressed in Reaction (15). Thus, the iron and nickel recovery of saprolitic nickel ore at 0.2 of BB2 is low due to the remaining iron and nickel in the enstatite structure. However, no enstatite is found at 0.4 of BB2, which means that the MgO addition could suppress the enstatite. It has resulted in increasing the forsterite formation, as shown in the ternary diagram (Fig. 5). By adding MgO, the magnesium atom could replace the iron and nickel in the forsterite structure, as expressed in Reaction (16). Therefore, the ferronickel peaks starts to increase significantly at 0.5 of BB2. Nevertheless, the release of more iron from forsterite is very harmful. It decreases the nickel grade in ferronickel, as shown in Fig. 3.
From microstructure analysis of reduced ore, as illustrated in Fig. 7, the ferronickel (point 1) slightly grows large with the increasing binary basicity, for both BB1 and BB2. Due to the high melting point and less troilite phase, which inhibits the agglomeration of ferronickel particles, thus resulting a small ferronickel size. However, this particle growth indicates that more iron and nickel are released from the forsterite. The ferronickel in BB1 is larger than BB2, which means that CaO addition is more effective for releasing the iron and nickel from forsterite than MgO. The flux addition (CaO and MgO) also could release the iron from chromite (point 2). Figure 7 shows that the particle size of chromite (Fe–Cr–O) is getting smaller with the increase in the basicity.
3.2 Effect of Ternary Basicity (BT)
The effect of ternary basicity (BT = CaO + MgO/SiO2) in selective reduction of saprolitic nickel ore is discussed briefly in this section. The basicity value is modified from 0.1 to 0.4 with the addition of SiO2, while it is modified from 0.5 to 1.0 with the addition of CaO and MgO. The ternary basicity value modified from 0.1 to 1.0 with SiO2–CaO and SiO2–MgO addition are BT1 and BT2, respectively. From Fig. 8a, the nickel grade increases significantly from 0.1 to 0.2; then, it tends to steady until 0.4 of ternary basicity, for both BT1 and BT2. The nickel grade increases from 0.5 to 0.6, then decrease significantly to 1.0 of BT1. Nevertheless, the nickel grade tends to decrease significantly from 0.5 to 1.0 of BT2. From Fig. 8b and c, the nickel grade and iron recovery increase with increasing ternary basicity, for both BT1 and BT2. It means that SiO2, MgO, and CaO addition in ternary basicity also could destroy the iron–nickel–magnesium silicate structure, thus releasing iron and nickel from this complex structure. In this work, the optimal ternary basicity value is found at 0.6 of BT1 (with CaO addition), resulting in 16.11 wt% and 50.57% for nickel grade and recovery, respectively.
Figure 9a illustrates the phase transformation of reduced saprolitic nickel ore by modifying the ternary basicity of nickel laterite with SiO2–CaO addition (BT1). At low basicity, the reduced ore is dominated by forsterite and enstatite. A small peak of ferronickel is also found. Enstatite is formed due to the reaction of forsterite with SiO2, as expressed in Reaction (15). The enstatite and forsterite peaks decrease, while the ferronickel peaks enhance with the increase in the BT2 value. Melilite starts to form at 0.8 of BT1, and the ferronickel peak increases significantly. It means that most of the iron and nickel in forsterite decomposes into the ferronickel with the increase in the CaO addition. Furthermore, melilite is transformed to monticellite with more CaO addition at 1.0 of BT1. This calcium silicate formation is expressed in Reaction (12–14). The phase transformation of BT1 is almost similar with BB2 in Fig. 6. The lower monticellite peaks is of BT1 than BB1 due to the lower amount of CaO addition at 1.0 of basicity value. From Fig. 9a, the troilite peak seems to increase from the 0.2 to 0.6 basicity. It then decreases to 1.0 of BT1. No calcium silicate (diopside) structure in this basicity seems to affect this process positively. As explained before, the diopside has a higher melting point than troilite. Thus, the presence of diopside, as resulted in 0.6 of BB2, could inhibit the troilite formation. Troilite is very important for suppressing the metallization of iron and agglomeration of ferronickel size, thus enhancing the nickel grade in ferronickel [4]. It is indicated with the low peak of ferronickel in 0.2–0.6 of BT2. The addition of more CaO at a higher BT2 value results in more releasing iron from forsterite, which indicates the increase in the ferronickel peaks. Thus, low nickel grade is obtained, as shown in Fig. 9a. Nevertheless, it also suppresses the troilite due to the CaS formation.
Figure 9b shows the phase transformation of reduced ore in various values of BT2. The ferronickel peaks increase, with the increase in the ternary basicity, starting from 0.6 of BT2. It means that the addition of MgO will release the iron and nickel from the forsterite structure, then it transforms to ferronickel, as expressed in Reaction (16). A high peak of ferronickel indicates high iron content on it, thus lowering the nickel grade. The troilite phase decreases significantly with the increase in the ternary basicity. It is regarding the presence of more forsterite at high basicity along with the increase in the MgO addition. Forsterite has high melting point (1890 °C). Its presence surrounding the ferronickel phase will inhibit the reaction of iron with sulfur to form troilite.
Phase transformation analysis of ternary basicity in this work is also carried out using a ternary diagram, as shown in Fig. 10. The SiO2 is found at 0.2 to 0.4 of ternary basicity, for both BT1 and BT2. Furthermore, modified ternary basicity with CaO addition (BT1) results in pyroxene (diopside) and melilite at basicity 0.8 and 1.0. It is almost similar to XRD analysis (Fig. 9). Nevertheless, monticellite could appear due to its basicity point located on the melilite–monticellite boundary phase.
The microstructure analysis of reduced ore with various ternary basicity is illustrated in Fig. 11. It shows that the ferronickel size is growing large with increasing ternary basicity, for both BT1 and BT2. However, the ferronickel size of BT2 is larger than BT1 at the same value of basicity. It indicates that the addition of MgO is more effective than CaO in releasing iron and nickel from forsterite structure in the ternary basicity system. It is contrary different from the binary basicity. The amount of CaO addition in ternary basicity is lower than binary basicity. Thus it affects the releasing of iron and nickel from the magnesium silicate structure.
3.3 Effect of Quaternary Basicity (BQ)
In this section, the effect of quaternary basicity (CaO + SiO2/Al2O3 + MgO) value in selective reduction of saprolitic nickel ore on metal grade and recovery, phase transformation, and microstructure of ferronickel is described clearly. The nickel ore basicity value is modified with the addition of SiO2 decreasingly from 0.1 to 0.4 and CaO increasingly from 0.5 to 1.0 basicity, namely with BQ1. It is also modified with the addition of Al2O3 decreasingly from 0.1 to 0.4 and MgO increasingly from 0.5 to 1.0 basicity, namely with BQ2. Figure 12 shows that the nickel grade increases from 0.1 to 0.5 and then decreases from 0.6 to 1.0 of ternary basicity value, for both BQ1 and BQ2. However, the iron and nickel recovery increase with the increase in the ternary basicity. The optimum nickel grade is obtained from 0.5 of quaternary basicity value for BQ2, i.e., 14.62 wt.% Ni with 46.25% of nickel recovery.
Figure 13 illustrates the phase transformation of reduced saprolitic ore with various quaternary basicity values, for both BQ1 and BQ2. From Fig. 13a, where the value of basicities are modified with the addition of SiO2 and CaO, the forsterite decreases with the increasing of SiO2 and CaO addition. At low basicity (0.2 of BQ1), the reduced ore is dominated by forsterite and enstatite. However, the enstatite also decreases with the increase in the basicity until BQ1 = 0.4, which is modified by decreasing the SiO2 addition. From Reaction (14), the enstatite is formed when forsterite is reacted with SiO2. The troilite seems to increase with the decreasing SiO2 addition from 0.1 to 0.4 of BQ1, which means that the SiO2 addition will suppress the troilite formation. Nevertheless, the troilite decreases with the increase in the CaO addition from 0.5 to 1.0 of BQ2. It is due to the fact that CaO could be reacted with sulfur, which could inhibit the troilite formation. At high basicity (0.8 of BQ1), the melilite is formed. More CaO addition could transform the melilite into monticellite, which is found at 1.0 of BQ1. It is similar to the binary and quaternary basicity modified by CaO addition, as expressed in Reaction (12–14).
Figure 13b, where the quaternary basicity is modified with Al2O3 and MgO addition, shows that the forsterite and troilite increase, while the ferronickel decreases from 0.1 to 0.4 basicity, which is modified by decreasing Al2O3 addition. It seems that Al2O3 could replace the iron and nickel in the magnesium silicate structure to promote the formation of ferronickel. It also has a similar role with SiO2 and MgO to suppress the formation of troilite. As shown in Fig. 13b, the forsterite increases with the increase in the MgO addition from 0.5 to 1.0. Li et al. [11] reported that the forsterite will cover the iron and nickel oxide structure. Thus, it will inhibit the reaction of iron with sulfur to form troilite. From Reaction (16), the more the MgO addition, the more the iron and the nickel released from a silicate structure to form ferronickel.
Phase transformation analysis is also carried out by using a ternary diagram, as shown in Fig. 14. Pyroxene (diopside) and forsterite dominate at 0.4 to 0.8 of BQ1 due to the basicity point located on its boundary. At 1.0 of BQ2, the basicity point is located on the boundary phase of melilite and forsterite. However, from the XRD analysis (Fig. 13a), monticellite is also observed. For BQ2, it is observed that the forsterite is more dominant with the increase in the basicity, which is modified by increasing the MgO addition.
The effect of quaternary basicity (BQ1 and BQ2) on the microstructure of reduced ore is illustrated in Fig. 15. Similar to the previous discussion, the ferronickel particle (point 2) is growing large with the increase in the quaternary basicity value, for both BQ1 and BQ2. It indicates that CaO, SiO2, Al2O3, and MgO effectively release iron and nickel from the forsterite structure.
3.4 The Appropriate Basicity Type for Selective Reduction of Saprolitic Nickel Ore
Saprolitic nickel ore consists of high MgO and SiO2 content. It also has lower iron and higher nickel content than limonite. Most iron and nickel are associated with magnesium silicate to form lizardite. Furthermore, the lizardite will be hydroxylated into forsterite at 600 °C. Iron and nickel in forsterite are more difficult to reduce than goethite. Therefore, it is important to break the magnesium silicate structure of forsterite to release the iron and nickel to form ferronickel. From the previous discussion, iron and nickel could be released from the forsterite structure by modifying the ore basicity with the addition of CaO, MgO, Al2O3, and SiO2. It is indicated by the increase in the iron and nickel recovery with the increase in the basicity. The optimum nickel grade is obtained from reducing saprolitic nickel ore using 0.6 of ternary basicity (modified with CaO addition), which results in the concentrate of ferronickel containing 16.11 wt.% Ni grade with nickel recovery of 50.57%. This optimum basicity type for saprolitic nickel ore is different from the limonitic nickel ore from the previous work [18], which is obtained from the 0.1 of binary basicity (CaO/SiO2). It might be because the limonite consists of high SiO2 and a small amount of MgO. Thus, the MgO does not play an important role in the basicity calculation. In this work, the CaO is more effective than MgO, Al2O3, and SiO2 for releasing iron and nickel from forsterite because it could break the structure and reacts with magnesium silicate structure to form calcium–magnesium silicate structure, i.e., melilite and monticellite.
4 Conclusion
Binary (CaO/SiO2 or MgO/SiO2), ternary (CaO + MgO/SiO2), and quaternary (CaO + MgO/Al2O3 + SiO2) basicity, which is expressed as the ratio of impurities oxide, are common parameters used in pyrometallurgy to obtain the characteristic of slag/impurities. In the selective reduction process of saprolitic nickel ore, the basicity has influenced the metal grade and recovery of ferronickel, phase transformation, and microstructure. The nickel grade is decreased with the increase in the basicity. Nevertheless, the iron and nickel recovery increase with the increase in the basicity. The addition of CaO, MgO, Al2O3, and SiO2 for modifying the basicity values has played an important role in the breakage of forsterite structure, thus releasing the iron and nickel to form ferronickel. However, more iron released will suppress the nickel grade to concentrate. The CaO, MgO, SiO2, and Al2O3 will replace the iron and nickel in the forsterite structure. The addition of more CaO at high basicity will transform the forsterite into melilite and monticellite. The forsterite structure does not change with the addition of MgO, Al2O3, and SiO2 unless it contains less iron and nickel. More forsterite will be found at high basicity with MgO addition.
The ferronickel particle is growing large with the increase in the basicity. However, the modification basicity with CaO addition results in a larger ferronickel size than MgO addition, which indicates that the CaO addition is more effective for releasing the iron and nickel from the forsterite structure.
In this work, the optimum basicity value is found from the 0.6 of ternary basicity (CaO + MgO/SiO2), modified by CaO addition, resulting in the highest nickel grade, i.e., 16.11% wt. Ni with nickel recovery is 50.57%. Thus, this is the appropriate basicity for the selective reduction process of saprolitic nickel ore.
References
Chen S, Guo S, Jiang L, Xu Y, Ding W. Transactions of Nonferrous Metals Society of China. 2015; 25; 3133.
Chuang H, Hwang W, and Liu S. Materials Transactions. 2009; 50; 1448.
Dalvi AD, Bacon WG, and Osborne RC. in Proceedings of PDAC 2004 International Convention, Trade Show & Investor Exchange, Canada (2004), p. 1.
Dong J, Wei Y, Zhou S, Li B, Yang Y, and Mclean A. JOM. 2018; 70; 2365.
Elliott R, Pickles CA, and Peacey J. Minerals Engineering. 2017; 100; 166.
Farrokhpay S, Filiipov L, Fornasiero D. Minerals Engineering. 2019; 141; 1.
Harjanto S and Rhamdhani MA. Minerals. 2019; 9; 1-21.
Jiang M, Sun T, Liu Z, Kou J, Liu N, Zhang S. International Journal of Mineral Processing. 2013; 123; 32.
Jiang X, He L, Wang L, Xiang D, An H, and Shen F. Metallurgical and materials transactions B. 2020; 51; 2653.
Li G, Luo J, Peng Z, Zhang Y, Rao M, and Jiang T. ISIJ International. 2015; 55; 1828.
Li G, Shi T, Rao M, Jiang T, Zhang Y. Minerals Engineering. 2012; 32; 19.
Li Q, Cui Y, Zhu D, Zhu J, Pan J, Zhang H, and Zheng G. in Proceedings of XXV International Mineral Processing Congress (IMPC), (ed) Pocock K, The Australasian Institute of Mining and Metallurgy, Australia (2010), p. 1549.
Li Y, Sun Y, Han Y, and Gao P. Transactions of Nonferrous Metals Society of China. 2013; 23; 3428.
Liu W, Li X, Hu Q, Wang Z, Gu K, Li J, Zhang L. Transactions of Nonferrous Metals Society of China. 2010; 20; s82.
Liu Z, Sun T, Wang X, and Gao E. International journal of Minerals, Metallurgy and Materials. 2015; 22; 901.
Nakajima K, Nansai K, Matsubae K, Tomita M, Takayanagi W, and Nagasaka T. Science of the total environment. 2017; 586; 730.
Nurjaman F, Astuti W, Bahfie F, and Suharno B. Materials Today: Proceedings. 2021; 44; 1488.
Nurjaman F, Saekhan K, Bahfie F, Astuti W, and Suharno B. Periodico di Mineralogia. 2021; 90(2); 91.
Pan J, Zheng G, Zhu D, Zhou X. Transactions of Nonferrous Metals Society of China. 2013; 23; 3421.
Rao M, Li G, Zhang X, Luo J, Peng Z, and Jiang T. Separation Science and Technology. 2016; 51; 1408.
Setiawan I, Triana T, Febriana E, and Firdiyono F. AIP Conference Proceedings. 2020; 2232; 1.
Suharno B, Nurjaman F, Ramadini C, and Shofi A. Mining, Metallurgy & Exploration (in press).
Sun Y, Zhu X, Han Y, Li Y, Gao P. Journal of Cleaner Production. 2020; 261; 1.
Swinburne D R. Mineral Processing and Extractive Metallurgy. 2014; 123; 127.
Tian H, Pan J, Zhu D, Yang C, Guo Z, Xue Y. Journal of Materials Research and Technology. 2020; 9; 2578.
Wang X, Sun T, Chen C, and Kou J. International Journal of Minerals, Metallurgy and Materials. 2018; 25; 383.
Xueming L, Jie Q, Meng L, Mei L, and Xuewei L. in Proceedings of The Fourteenth International Ferroalloys Congress, (ed) Paton B, Ukraine (2015), p. 561.
Zhang F, Zhu D, Pan J, Guo Z, Yang C. Powder Technology. 2019; 342; 409.
Zhang J, Gao L, He Z, Hou X, Zhan W, Pang Q. Journal of Materials Research and Technology. 2020; 9; 12223.
Zhou S, Dong J, Lu C, Li B, Li F, Zhang B, Wang H, and Wei Y. Materials Transactions. 2017; 58; 790.
Zhou S, Wei Y, Li B, Wang H, Ma B and Wang C. Scientifif Reports. 2016; 6; 1.
Zhu D, Pan L, Guo Z, Pan J, Zhang F. Advanced Powder Technology. 2019; 30; 451.
Acknowledgements
The authors would like to thank the Ministry of Research and Technology/National Research and Innovation Agency for the doctoral dissertation grant with Contract No. NKB-326/UN2.RST/HKP.05.00/2021 for funding this research, Universitas Indonesia for supporting this work and LIPI's science services for research laboratories.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nurjaman, F., Sari, Y., Manurung, P. et al. Study of Binary, Ternary, and Quaternary Basicity in Reduction of Saprolitic Nickel Ore. Trans Indian Inst Met 74, 3249–3263 (2021). https://doi.org/10.1007/s12666-021-02391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-021-02391-7