Abstract
The polyphagous larvae of click beetles (Coleoptera: Elateridae) are major pests of spring wheat in Montana, USA. Presently available insecticides are unable to provide control over wireworm populations, and the use of natural enemies has not been successful under field conditions. In this study, we examined the effect of seven trap crops: pea, lentil, canola, corn, durum, barley, and wheat, for their attractiveness to wireworms compared to spring wheat. Experimental plots were located in two commercial grain fields in Valier and Ledger, Montana, USA and the trials took place from May to August in 2015 and 2016. Wheat plants damaged by wireworms were recorded and their relative locations in wheat rows and adjacent trap crop rows within a plot were determined using destructive soil samples. In 2016, variable row spacing (0.25, 0.5, 0.75, and 1 m) between the trap crops (pea and lentil) and wheat was assessed. Shade house bioassays were conducted using potted pea, lentil, and wheat plants to support field trial results. Limonius californicus larvae, released at the center of each pot were sampled 4 and 10 days after sowing. Wheat intercropped with pea and lentil had significantly fewer damaged wheat plants. Wireworm numbers were lower in wheat intercropped with pea compared to the control for both locations and years. Shade house results corresponded with field results, with more wireworms collected from pea and lentil than wheat. In the spacing trials, wheat plant counts were also significantly higher when paired with pea and lentil, particularly at 0.5 m spacing. Regardless of inter-row spacing, significantly fewer wireworms were associated with wheat when intercropped with pea and lentil trap crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wireworms, the soil-dwelling larvae of various species of click beetles (Coleoptera: Elateridae) are one of the major pest groups attacking spring wheat in Montana, North Dakota, South Dakota, and Minnesota (Hermann et al. 2012). Montana is the third highest state for wheat and barley production in the US, with 5,265,000 harvested acres of wheat and 850,000 harvested acres of barley in 2015, valued at 0.25 and 0.93 billion US dollars, respectively (2015 State Agriculture Overview). Wireworm populations are found in a wide range of habitats including grasslands, cultivated land and forests (Traugott et al. 2015), and are the pest of many crops including carrot, sugarcane, strawberry, and potatoes. In soil, wireworm larvae move in search of food and feed on roots, seeds, seedlings of various plants, and decaying organic matter (Vernon et al. 2009). Larval feeding can facilitate secondary damage to crops from pathogens and snails (Keiser et al. 2012). Johnson (2009) reported 921 species of click beetles in 99 genera in North America. In Montana, 164 species in 43 genera were identified, and among these 23 wireworm species were found to be of potential economic importance (Seibert 1993). According to field surveys in 2011 and 2012, Limonius californicus (Mannerheim) and Hypnoidus bicolor (Eschscholtz) are the most important wireworm species damaging spring wheat in the Western Triangle Region of Montana (Morales-Rodriguez et al. 2014).
Wireworms can live a long time and undergo biannual vertical movement in the soil. In early May when the temperature is around 13–15 °C, wireworms are within 10 cm of the soil surface. As the temperature reaches 22–25 °C during June and July, larvae move deeper down and then in August to mid-October come towards the surface again to feed (Lafrance 1968). This vertical migration makes it difficult to sample wireworm populations or predict changes in their density. The high species diversity, long larval lifespan in the soil, poorly known taxonomy and life history, and cryptic habitats of wireworms all make them difficult to control (Traugott et al. 2015) resulting in stand thinning, poor growth, cosmetic damage, and reduced yield of crops they infest (Parker and Howard 2001; Barsics et al. 2013). Poor species-specific knowledge due to difficulty in identifying larvae to species is one factor that impedes the development of Integrated Pest Management tactics for wireworms.
Wireworms are becoming more common with the increasing use of no-till farming practices and the disappearance of residues of older pesticides (Jedlicka and Frouz 2007). Neonicotinoid seed treatment is the most common approach for managing wireworms. Zhang et al. (2017) reported that seed treated with fipronil (40 g AI 100 kg−1 seed), thiamethoxam (360 g AI 100 kg−1 seed), thiamethoxam + fipronil and clothianidin + fipronil (360 + 40 g AI 100 kg−1 seed) significantly decreased wireworm damage and population densities of Pleonomus canaliculatus (Faldermann) in winter wheat fields. Moreover, additive interactions were observed by the authors between thiamethoxam and fipronil, suggesting that this combination would be the most effective at controlling wireworm populations in winter wheat. Van Herk and Vernon (2007) reported that wireworms feeding on neonicotinoid-treated seeds/seedlings would experience toxic effect but would often survive, with the time of recovery depending on the concentration of insecticide and wireworm weight. This inadequate toxicity has the effect of increasing wireworm resistance to insecticides. Entomopathogenic fungi have been used to control wireworms in Canada (Kabaluk et al. 2001), Italy, the UK (Parker and Howard 2001), and Switzerland (Keller and Schweizer 2001). The principal species examined have been Metarhizium brunneum F52 (formerly M. anisopliae F52), (Ascomycota: Hypocreales), and Beauveria bassiana (Ascomycota: Hypocreales). Brandl et al. (2017) reported that M. brunneum was able to reduce wireworm damage to potato by 37–75% relative to the untreated control and the process was enhanced by an attract-and-kill approach using baker's yeast (Saccharomyces cerevisiae Meyen ex Hansen) as a source of carbon dioxide. In addition, our previous study (Reddy et al. 2014) indicated that three insect pathogenic fungi (M. brunneum F52, B. bassiana GHA, and M. robertsii DWR 346) applied as seed treatments were effective in controlling L. californicus and H. bicolor in spring wheat in Montana. However, changes in relative humidity and temperature, the high cost of production, and the need for high application rates have hindered the practical use of these biological agents to control wireworms (Bourassa et al. 2001; Jaronski 2010). Given this reality, trap crops have been proposed as a cost effective method for controlling wireworms (Staudacher et al. 2013). A trap crop is defined as plant stands that are, per se or via manipulation, deployed to attract, divert, intercept, or retain targeted insects or pathogens they vector in order to reduce damage to the main crop (Shelton and Badenes-Perez 2006). Hokkanen (1991) describes approximately forty successful cases of trap crops being used. Crop preferences of wireworms at close row spacing depends on the specific plant chemicals (Horton 2007) used by wireworms to locate their host plants. Trap crops have been shown to be effective in trapping and concentrating wireworms in strawberry (Miles and Petherbridge 1927; Vernon et al. 2000) and sugar beets (Petherbridge 1938). The intercropping of wheat in strawberry fields reduced wireworm damage to about 30% of that in strawberry monoculture in autumn (Vernon et al. 2000). Wheat and corn planted in sweet potato fields as trap crops were found to reduce tuber damage from wireworms (Seal et al. 1992). Peas grown in potato plots were reported to attract more wireworms than wheat or oilseed crops, and was recommended as a potential trap crop for managing wireworms in potato in Austria (Landl and Glauninger 2011).
So far, no studies have examined the use of trap crops to control and manage wireworms in spring wheat. Based on previous findings, we chose pea, lentil, canola, durum, barley, and corn to intercrop with spring wheat. The objectives of this study were (1) to compare the ability of these different trap crops to manage wireworms in spring wheat, (2) to examine the effect of trap crop and row spacing on wireworm infestation in spring wheat, and (3) to determine the effects of trap crop on the distribution of wireworms.
Materials and methods
Trials sites
Field trials were conducted at two sites: Ledger (48.2583°N, 111.8257°W) and Valier (48.3078°N, 112.2498°W) in the Golden Triangle region of Montana, from May to August in both 2015 and 2016. Both fields have a history of wireworm infestation. Soil at the Valier site was sandy loam while that at the Ledger site was silt and clay, rich in humus. Both fields have central pivot irrigation and spring wheat was the main crop grown in these fields.
Effectiveness of Trap Crops in Field Trials
Experimental Design
At each location, a study area measuring 36 × 13.1 m2 was established and divided into 42 experimental units of 1.2 × 4.8 m2. A complete randomized block design with seven treatments and six replications was used (Fig. 1). The blocks were separated by 1 m buffers and the two plots within each block were separated by 0.45 m. Each experimental plot had four rows separated by 0.3 m. The six trap crop treatments were Montech pea (T1), Hyeless 955 canola (T2), sweet corn (T3), Montrail durum (T4), Metcalfe barley (T5), and Green Land lentil (T6). All these seeds are commercially available. The main crop (Duclair spring wheat) and the treatment trap crop were planted in alternating rows in each experimental plot. A plot with four rows of Duclair wheat served as a no-choice control (T0). The plots were maintained under minimal tillage and seeding was done with a four-row experimental drill (Fabro Ltd., Swift Current, Saskatchewan, Canada). Seeding was done on the first week of May at both sites. Seeding rates were those used by growers: 72, 85, 85, and 78 seeds/meter for wheat, lentil, barley, and durum, respectively. Spacing for corn and pea was 7 and 53 seeds/m, respectively. There was no irrigation at the time of sowing. The herbicide ammonium sulfate (AMS) was broadcast at planting at 2.24 kg/ha as were fertilizers at an N, P, and K ratio of 224.2, 0, and 22.4 kg/ha, respectively.
The diagram on the left shows the allocation of the 46 trial plots in the field sites. Each column represents each block where the seven treatments (W Wheat (control), P Pea, C Canola, Co Corn, D Durum, B Barley, and L Lentil) were intercropped with the main crop of spring wheat. The diagram on the right shows a block with seven plots, each having four rows. The crops were sown in the plots as represented by the letter. The black square box represents the destructive soil samples and the rectangular box represents the 1 m line intercept used for wheat counts
Sampling for plant damage and wireworm density
To determine the level of crop damage from wireworms, the number of seedlings in each plot was counted along a randomly selected 1 m line intercept (Canfield 1941; Jonasson 1988). Within each experimental plot, wheat plant counts were recorded on three randomly selected 1 m line intercepts. Wheat plants were then categorized as damaged or not damaged. Wireworms have been found to be attracted to the root systems of barley (Barsics et al. 2016), but they will also feed on germinating seeds or young seedlings. In wheat plants damaged by wireworms, the central leaves die and the damaged plant exhibits wilting, yellowing, or stunted growth (Vernon et al. 2009). The same three random 1 m intercepts was used for each sampling. In both years and both locations, the first observation was 2 weeks after seeding, and sampling was conducted eight times each year. The first five observations was taken at weekly intervals from the last week of May to the last week of June, while the remaining three recordings were done at 2 week intervals, on the second and fourth weeks of July and second week of August.
A destructive soil sampling method was used to determine wireworm larval density. A square metal tool measuring 0.15 m on each side (creating a 3375 cm3 soil sample) was used for sampling. Within each experimental treatment plot, two random soil samples from the wheat rows and two random samples from the trap crop rows were collected. Soil samples were taken after plant counts. A total of eight soil samples were collected from each site in each year. Samples were placed in labeled plastic bags and brought to the laboratory where they were manually processed. Each bag of soil was emptied into a plastic tray (30 × 15 × 6 cm) and the number of wireworms in each sample were recorded. Wireworms from samples were kept in small plastic cups 8 cm in diameter and filled with 5 cm of sphagnum peat moss and soil from the soil samples and stored in a refrigerator at 8 °C for later identification and use in shade house experiments. Wireworms were identified using taxonomic keys described by Etzler (2013).
Determining optimal row spacing for trap crops
Location and experimental design
Trials to determine the optimal row spacing for traps crops were conducted in 2016 at both Valier and Ledger. A complete randomized block design was used, with row spacing treatments of 0.25, 0.5, 0.75, and 1 m between alternating rows of spring wheat and pea and lentil trap crops. Each treatment was replicated four times (Fig. 2) and spring wheat alone served as a no-choice control. Row spacing was used to define experimental blocks and trap crop treatments were randomly assigned to plots within each block. With four spacings, three crops (including the control), and four replications, there were 48 plots in total, each measuring 4 m2. Blocks and plots were separated from each other by a 1.5 m buffer. Montech pea, Green Land lentil, and Duclair wheat were manually planted at the same rate used for the first field trials. Due to spring weather conditions, plots were planted late; in Ledger, on the third week in May and in Valier, on last week in May.
The diagram shows the blocking in spacing trials. Each column from left to right is a block of intercropping row spacing of 1, 0.75, 0.50, and 0.25 m treatment, in which three trap crops treatments pea (asterisk), lentil (plus), and wheat (solid line) are intercropped in alternate rows as shown. The black square box represents the destructive soil samples and the rectangular box represents the 1 m line intercept used for wheat counts
Sampling for wheat plant counts and wireworm densities
Wheat plant counts were taken using the 1 m line intercept method described above in randomly selected wheat rows. For each counting event, the same random 1 m line of wheat was used. A total of six plant counts were taken at each location, the first four at Ledger on the second, third, and fourth week of June and the first week of July, while at Valier the first four were on the third and fourth weeks of June and the first and second weeks of July. The last two counting events were then conducted at 2 week intervals at both sites.
Six soil sampling events were conducted, in which two destructive soil samples were taken at random from each experimental plot, one from a randomly selected wheat row, and one from a trap crop row. Soil samples were taken immediately after plant counts. Soil samples were labeled, brought to the laboratory, and the number of wireworms recorded before wireworms were then stored and identified as described previously.
Shade house bioassay
Shade house bioassays were conducted at the Western Triangle Agriculture Research Center in Conrad, Montana during August 2016. An average room temperature of 18–22 °C was maintained throughout the experiment. Assay units were square plastic flower pots (10.5 cm square and 9.2 cm deep) filled with a mixture of soil from the soil sample collected from Valier after wireworm processing, peat moss (Alaska Peat Moss Inc.), and filtered sand at a 4:2:1 ratio. Two trap crops, Montech pea and Green Land lentil, were tested in two-choice assays with Duclair spring wheat. Each assay pot was divided into nine quadrants. Five grams of wheat seeds were planted in two opposite quadrants of the middle row and 5 g of treatment crop seeds were planted in the remaining two opposite quadrants of middle row perpendicular to the first (Fig. 3). The seeds were sown together at the center of these four opposite quadrants at a depth of 4–5 cm. Limonius californicus larvae collected from the field trials were used for the experiments. Larvae not less than 1.5 cm were kept in plastic pots (12 cm diameter by 8 cm deep) filled with peat moss (Alaska Peat Moss, Inc.) for a week before the experiments to starve them. Nine L. californicus larvae were then released in the central quadrant of each pot. A total of eight pots were created for each comparison. On days 4 and 10 after releasing the larvae, four pots each for treatment were destructively sampled by dividing the pot and soil into nine equal quadrants, comprising the four corners, the four sides, and the center. The number of wireworms in each quadrant was recorded.
The diagram in the left shows the upper view of the pot used for the shade house trails. The pot was divided into nine quadrants and the crops pea, lentil, and wheat (P pea, W wheat) were plants in the middle quadrant as shown in figure. In the Lentil treatment, lentil seeds were sown in the middle quadrant as with the peas. Limonius californicus larvae were released at the center of the center quadrant. The picture in the right is the soil sample from nine quadrants of the pot on day 4 after sowing
Statistical analysis
Analysis of variance (ANOVA) was used to analyze data in r-software version 3.1.3 (R Development Core Team 2012). For both studies, the effectiveness of trap crops, the row spacing of trap crops, and sampling date were considered fixed variables, while wheat plant damage, wheat plant counts, and row spacing were considered random variables. Data were pooled and analyzed using ANOVA. Tukey’s HSD post hoc test at 95% confidence interval was used for pair-wise comparison among treatments if the results for the treatment were determined by ANOVA to be significant (P < 0.05). For wireworm counts from wheat rows intercropped with trap crops, a paired t test at 95% confidence interval was employed to analyze differences and determine the best trap crops for attracting wireworms. In the spacing trials, ANOVA was used to analyze the number of wireworms recorded from the wheat rows intercropped with trap crops. If the results were significant, Tukey’s HSD post hoc test was used for pair-wise comparison at a 95% confidence interval. Chi-square tests of fitness were performed for the greenhouse bioassays and P-values <0.05 were considered significant. If the results were significant, a paired t test was used for comparison of wireworms number recorded from the quadrants of trap crops.
Results
Effect of trap crops on spring wheat damage in field trials
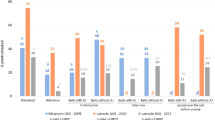
In both years at the Valier location, significant differences in the percentage of damaged spring wheat plants were found between the different trap crop treatments (F = 9.01, df = 6, and P < 0.01 in 2015; F = 15.54, df = 6, and P < 0.01 in 2016). In 2015, except for the barley (P > 0.05) and canola (P > 0.05) treatments, significant differences were found between tested trap crops and the wheat control (Fig. 4). Likewise, in 2016, damage in wheat plants intercropped with pea (P < 0.01), lentil (P < 0.01), and corn (P < 0.05) was significantly lower than in the control (Fig. 5). In Valier, pea (P < 0.01) and lentil (P < 0.01) treatments showed the lowest damage to wheat plants in both years.
Similarly, in both years at the Ledger location, significant differences were observed in damage (%) of spring wheat intercropped with trap crops (F = 59.49, df = 6, and P ≤ 0.01 in 2015; F = 13.68, df = 6, and P < 0.01 in 2016). In 2015, except for the barley (P > 0.05) trap crop, significant differences were detected in damage % of spring wheat plants intercropped with trap crops (P < 0.05) compared to the control. Damage to wheat plants was significantly lower in pea (P < 0.01) and lentil (P < 0.01) treatments than in other trap crops (P < 0.05) (Fig. 4). In 2016, except for the durum (P > 0.05) trap crop, significant differences were found between trap crops and the control (P < 0.05). Moreover, significantly lower damage in wheat plants intercropped with pea (P < 0.01) and lentil (P < 0.01) was observed compared to other trap crops (P < 0.05) (Figs. 4, 5).
Effect of trap crops on wireworm densities in spring wheat
At the Valier location, significantly lower numbers of wireworms were recorded in both years from wheat rows intercropped with pea (t = 3.41, P < 0.05 in 2015; t = 3.24, P < 0.05 in 2016) compared with the control and other trap crop treatments (Table 1). However, when we compared wireworm numbers from different trap crops with those in the control wheat rows we found significant lower numbers of wireworms in corn (P < 0.05 in both years) and canola (P < 0.05 in 2016).
At the Ledger location, significant lower wireworm numbers were found in both years in wheat rows intercropped with pea (t = 3.993, P < 0.05 in 2015; t = 3.16, P < 0.05 in 2016) compared to control wheat rows (Table 1). Meanwhile, comparing wireworm numbers from the different trap crop rows with control wheat rows we found significantly lower numbers of wireworms in corn rows (P < 0.05 in both years) than in control trap wheat rows.
In 2015, we collected a total of 693 and 380 wireworms at Valier and Ledger, respectively. In 2016, we recorded 301 wireworms in Valier, of which 25 were Aeolus mellillus, 117 H. bicolor, and 159 L. californicus. That same year in Ledger, 262 wireworms were found, among which 15 were A. mellillus, 125 H. bicolor, and 124 L. californicus (Fig. 6 for 2015 and Fig. 7 for 2016).
Effect of row spacing and trap crops on wheat plant density
At Valier, wheat plant counts showed significant differences between trap crop treatments (df = 2, F = 158.8, P < 0.01) and between different row spacings (df = 3, F = 58, P < 0.01). Significantly (P < 0.01) more wheat plants per meter were recorded in plots intercropped with pea and lentil compared to control wheat plots (Fig. 8). In addition, intercropping spacings of 0.25 and 0.5 m between the trap crops and spring wheat had significantly more wheat plants than in treatments with 0.75 and 1 m spacing (Fig. 9).
At the Ledger field location, significant differences in wheat plants counts were found among trap crops (df = 2, F = 33.66, P < 0.01) and between row spacings (df = 3, F = 4.36, P < 0.01). Wheat plant counts in plots intercropped with pea and lentil were significantly higher than in the control wheat plots (P < 0.01) (Fig. 8). Wheat plant counts were also significantly higher at 0.75 m row spacing than 0.25 m (P < 0.01), and no significant (P > 0.01) differences in wheat plant numbers were found between 0.75, 0.5, and 1 m row spacings (Fig. 9).
Effect of trap crops and row spacing on wireworm density
At the Valier location, the number of wireworms recorded from wheat rows intercropped with different trap crops showed significant differences (df = 5, F = 5.87, P < 0.01), but no differences in wireworm density were observed between the different row spacing levels used. Wireworms numbers in wheat rows intercropped with pea (P = 0.05) and lentil (P < 0.01) were significantly lower than in control wheat rows. We collected 209 wireworms at Valier, of which 11 were A. mellillus, 70 H. bicolor, and 128 L. californicus. At the Ledger location, there were no significant differences in the number of wireworms in wheat rows intercropped with different trap crops nor at different row spacing levels (P > 0.05 for both factors). We collected 178 wireworms at Ledger, of which 15 were A. mellillus, 73 H. bicolor, and 90 L. californicus.
Effect of trap crops on wireworm distribution in shade house bioassay
In the shade house bioassay, the distribution of wireworms within pots on the fourth day after sowing showed significant differences when wheat was planted with pea (P < 0.01) or lentil (P < 0.01) compared to the no-choice control (wheat alone). Pea and lentil had more wireworms within their regions/quadrants compared to these quadrants in the control wheat pots. Moreover, pea and lentil quadrants had more wireworms compared to the control wheat quadrants. Similar results were observed on the tenth day after planting, with both pea (P < 0.01) and lentil (P < 0.01) treatment. More wireworms were recorded in pea and lentil quadrants compared to wheat quadrants. On both day 4 and 10, the distribution of wireworms was not uniform in treatment pots, with one larva in each of the nine quadrants, while in the control pots the distribution was uniform (P > 0.05) (Tables 2, 3). However, paired t tests revealed that significantly more wireworms were present in pea quadrants compared to wheat quadrants on day 10. We never found wireworms evenly distributed across all nine sections of the pot, confirming that wireworms have either random or aggregated distribution but not uniform distribution in the soil.
Discussion
This study found pea and lentil to be the most effective trap crops for managing wireworms in spring wheat in Montana. When spring wheat was intercropped with either pea or lentil, damage to wheat from wireworms was reduced significantly and two to threefolds more wireworms were collected from the soil taken from pea or lentil rows than intercropped wheat rows. Both pea and lentil are legumes and fix nitrogen in soil, increasing the soil fertility. Pea is also resilient to both wireworm damage and to drought (Miles and Petherbridge 1927; Griffiths 1974). The Ledger location has a silt clay soil. Lower numbers of wireworms were collected there compared to the Valier location, which has a sandy loam soil, suggesting that soil composition might affect wireworm distribution. Although we were unable to preserve and identify all wireworms from the soil samples, H. bicolor was the dominant species in Ledger and L. californicus in Valier in 2015 (personal observations). Meanwhile, the ratio of these two species was almost equal in both sites in 2016. As the summer approaches L. californicus become more aggressive, feeding on roots and seeds of the plants (Milosavljević et al. 2016) which may be the reason for the higher number of this species collected from soil samples.
Wireworms have been found to follow carbon dioxide gradients associated with plant roots (Doane et al. 1975). According to Johnson and Nielsen (2012), soil-borne pests rely on such CO2 gradients to find hosts, and to a lesser extent use more specific volatile organic compounds (Hiltpold et al. 2013) and semio-chemicals found on the surface of roots (Barsics et al. 2012) to determine host suitability. Durum, barley, and wheat belong to the same family, Poaceae. Interestingly, the percentage of wheat plants damaged by wireworms within treatments in our study was almost the same across these three crops, and the number of wireworms associated with these trap plants were not significantly different. Vernon et al. (2000) reported wheat to be an effective trap crop, reducing wireworm damage in strawberries when planted between rows.
A number of studies have shown wireworms to be attracted to wheat (Miles and Petherbridge 1927; Kabanov 1975; Parker 1994). Rashed et al. (2017) reported that wheat appeared to be more susceptible than barley to L. californicus, showing greater reductions in emergence success and foliar biomass. In our study, corn intercropped with spring wheat was able to reduce the initial wireworm damage from 2 to 3 weeks immediately after sowing, although more wireworms were collected from the adjacent wheat rows, resulting in increased damaged to the wheat. Furlan et al. (2010) found wireworms to be attracted to corn and sorghum. The average temperature for both years in the month of May at the time of seed sowing was 10 °C (Reddy et al. 2015, 2016), but corn requires 18 °C for germination (Shaw 1988). Thus, some corn seeds failed to germinate in our field trials which may be a reason that wireworms moved towards adjacent wheat rows just a few weeks after sowing. Canola, which belongs to the Brassicaceae, was only able to reduce damage in wheat plants in low-density wireworm location in our study (Ledger). It has been reported that ionic thiocyanate (SCN−) present in brassica plants do not have any effect on wireworm feeding (McCaffrey et al. 1995).
Regardless of year or location, wheat intercropped with lentil or pea showed significantly lower damage compared to other tested combinations in our study. Possibly, this may be that besides carbon dioxide, both these crops provide easily accessible sugar (Miles and Petherbridge 1927; Griffiths 1974). Pea and lentil have nitrogen fixing bacteria which may be a major factor involve in wireworm attraction towards them, as these bacteria nodules are a source of nutrition. In addition, wireworms are polyphagous and legumes (both pea and lentil) are major source of protein, so it is possible that wireworms might prefer to feed on legumes even if other food sources are available. The nodule bacteria themselves are also a source of protein. However, we never found wireworms to be totally absent in wheat rows intercropped with pea or lentil, and furthermore it seems that the attractiveness of pea diminishes over time (Miles and Petherbridge 1927; Griffiths 1974). Thus, we hypothesize that wireworms move back and forth between wheat and pea or lentil but spend a greater amount of time on pea and lentil, which was the main reason why more wireworms were caught on these trap crops and which would explain the reduced level of damage to adjacent wheat plants. Pea has also been reported to be an effective trap crop in reducing wireworm damage and population levels in potato fields (Landl and Glauninger 2011).
In the spacing trial, wheat plant counts were significantly higher when intercropped with pea or lentil and at a spacings of 0.25 and 0.5 m in sandy soil (Valier) and at 1, 0.75, and 0.5 m in heavy clay (Ledger). It has been suggested that wireworms can move considerable horizontal distances (Langenbuch 1932), and under laboratory conditions, wireworms were found to move up to 20 cm to reach a CO2 source (Doane et al. 1975). Moreover, Vernon et al. (2000) found wireworms to be attracted towards wheat from 50 cm away. Under field conditions, it was determined that wireworms could travel 0.75 m (Landl and Glauninger 2011). Our results support these findings. Meanwhile, Miles and Petherbridge (1927) reported wireworms to be attracted to CO2 sources from 1.2 m away, and although we did find wireworm movement towards pea or lentil at a spacing of 1 m; these results were neither significant nor consistent. Wireworms numbers were significantly lower in wheat rows of pea and lentil treatments compared to control wheat rows in sandy soil with a high wireworm population pressure, suggesting that the distance from which wireworms can detect CO2 and VOCs depends on a combination of plant type and soil properties. Aeration and drainage in soil also influence its CO2 content (Russell 1936).
In the shade house choice bioassay, we found that wireworms were attracted towards lentil and pea when sown with wheat on both the fourth and tenth days after sowing. We also observed that released wireworms did not follow a uniform distribution pattern but rather were seen to have random or aggregate distribution when pea and lentil trap crops were present. Surprisingly, in the control we saw no evidence to support an aggregated distribution pattern, perhaps because in monocultures the wireworm populations (in this case L. californicus) follow a uniform distribution pattern (equal attraction). It is more likely that pea and lentil have some specific compounds in their roots that are highly attractive to wireworms. Barsics et al. (2016) found wireworms to be attracted to a synthetic blend of aldehydes, but live barley roots were more attractive than this synthetic blend of VOCs, confirming the role of CO2 as a strong attractant for wireworms.
Wireworm larvae are polyphagous (Parker and Howard 2001) and can persist in soils for prolonged periods, resulting in increasing damage to seeds and seedlings each year (Vernon et al. 2009). The cryptic habitat (Hyslop 1916) of wireworms also makes them difficult to control. Neonicotinoids are available but are not consistently effective at controlling wireworms (Van Herk and Vernon 2007). Under such conditions, trap crops may be a better method (Staudacher et al. 2013). Trap crops confine wireworm movement, concentrating them in their root zones (Schallhart et al. 2011) thereby protecting the main crop. The VOCs to which wireworms are attracted vary widely with cropping conditions. Diffusion properties in the air and water phases of a soil can play significant roles in wireworm detection of CO2 and VOCs through olfaction, and the distance between wireworms and the source is equally important (Barsics et al. 2016). Moreover, the chirality of the compounds affects the resulting behavior (Svensson et al. 2004). Further work is needed to determine the specific compounds in the roots of peas and lentil that attract wireworms. These compounds could be used as baits to trap and kill wireworm populations in the field. Studies on agronomic practices such as irrigation and fertilization should also be carried out to examine the role of cropping conditions on trap crops and wireworm densities. In addition, cost-benefit ratio analyses of trap cropping to control wireworms are needed to persuade growers to adopt these cultural practices.
References
Barsics F, Latine F, Gfeller A, Laloux M, Lognay G, Wathelet JP, Haubruge E, Verheggen FJ (2012) Do root-emitted volatile organic compounds attract wireworm. Commun Agric Appl Biol Sci 77:561–565
Barsics F, Haubruge E, Verheggen FJ (2013) Wireworms’ management: an overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 4:117–152
Barsics F, Delory BM, Delaplace P, Francis F, Fauconnier ML, Haubruge E, Verheggen FJ (2016) Foraging wireworms are attracted to root-produced volatile aldehydes. J Pest Sci 90:69–76
Bourassa C, Vincent C, Lomer CJ, Borgemeister C, Mauffette Y (2001) Effects of entomopathogenic hyphomycetes against the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae), and its predator, Teretriosoma nigrescens Lewis (Coleoptera: Histeridae). J Invertebr Pathol 77:75–77
Brandl MA, Schumann M, Przyklenk M, Patel A, Vidal S (2017) Wireworm damage reduction in potatoes with an attract-and-kill strategy using Metarhizium brunneum. J Pest Sci 90:479–493
Canfield RH (1941) Application of the line interception method in sampling range vegetation. J For 39:388–394
Doane J, Lee Y, Klingler J, Westcott N (1975) The orientation response of Ctemcera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and to carbon dioxide. Can Entomol 107:1233–1252
Etzler FE (2013) Identification of economic wireworms using traditional and molecular methods. M.S. thesis dissertation, Montana State University, Bozeman, Montana
Furlan L, Bonetto C, Finotto A, Lazzeri L, Malaguti L, Patalano G, Parker W (2010) The efficacy of biofumigant meals and plants to control wireworm populations. Ind Crop Prod 31:245–254
Griffiths DC (1974) Susceptibility of plants to attack by wireworms (Agriots spp.). Ann Appl Biol 78:7–13
Hermann A, Brunner N, Hann P, Wrbka T, Kromp B (2012) Correlations between wireworm damages in potato fields and landscape structure at different scales. J Pest Sci 86:41–51
Hiltpold I, Bernklau E, Bjostad LB (2013) Nature, evolution and characterisation of rhizosphere chemical exudates affecting root herbivores. In: Johnson SN, Hiltpold I, Turlings TCJ (eds) Advances in insect physiology, vol 45. Academic, Oxford, pp 97–157
Hokkanen HMT (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138
Horton D (2007) How do wireworms find their hosts? Potato Prog VII 10:1–3
Hyslop JA (1916) Wireworms destructive to cereal and forage crops. US Dep Agric Farmers Bull 725:1–10
Jaronski ST (2010) Ecological factors in the inundative use of fungal entomopathogens. Biocontrol 55:159–185
Jedlička P, Frouz J (2007) Population dynamics of wireworms (Coleoptera, Elateridae) in arable land after abandonment. Biologia 62:103–111
Johnson PJ (2009) Classification of the Nearctic genera of Elateridae. www.sdstate.edu/ps/Severin-McDaniel/project
Johnson SN, Nielsen UN (2012) Foraging in the dark—chemically mediated host plant location by belowground insect herbivores. J Chem Ecol 38:604–614
Jonasson S (1988) Evaluation of the point intercept method for the estimation of plant biomass. Oikos 52:101–106
Kabaluk TJ, Goettel M, Vernon RS, Noronha (2001) Evaluation of Metarhizium anisopliae as a biological control for wireworms. Organic Agriculture Centre of Canada. http://www.agbio.ca/ResearchDatabase/res_biol_ctrl_wireworms.asp
Kabanov VA (1975) Uber Vorkommen und Entwicklung von Agriotes lineatus (Coleoptera, Elateridae) im europaischen Teil der UdSSR. Pedobiology 15:98–105
Keiser A, Häberli M, Stamp P (2012) Dry core appears to result from an interaction between Rhizoctonia solani and wireworm (Agriotes ssp.)—evidence from a 3-year field survey. Potato Res 55:59–67
Keller S, Schweizer C (2001) Ist das Drahtwurm-Problem ein Pilz-Problem. Agrarforschung 7:248–251
Lafrance J (1968) The seasonal movements of wireworms (Coleoptera: Elateridae) in relation to soil moisture and temperature in the organic soils of southwestern Quebec. Can Entomol 100:801–807
Landl M, Glauninger J (2011) Preliminary investigations into the use of trap crops to control Agriotes spp. (Coleoptera: Elateridae) in potato crops. J Pest Sci 86:85–90
Langenbuch R (1932) Beiträge zur Kenntnis der Biologie von Agriotes lineatus L. and Agriotes obscurus L. Z Ang Entomol 19:278–300
McCaffrey J, Williams L, Borek V, Brown P, Morra M (1995) Toxicity of ionic thiocyanate-amended soil to the wireworm Limonius californicus (Coleoptera: Elateridae). J Econ Entomol 88:793–797
Miles HW, Petherbridge RFR (1927) Investigations on the control of wireworms. Ann Appl Biol 14:359–387
Milosavljević I, Esser AD, Crowder DW (2016) Seasonal population dynamics of wireworms in wheat crops in the Pacific Northwestern United States. J Pest Sci 90:77–86
Morales-Rodriguez A, O’Neill RP, Wanner KW (2014) A survey of wireworm (Coleoptera: Elateridae) species infesting cereal crops in Montana. Pan-Pacific Entomol 90:116–125
Parker WE (1994) Evaluation of the use of food baits for detecting wireworms (Agriotes spp., Coleoptera: Elateridae) in fields intended for arable crop production. Crop Prot 13:271–276
Parker WE, Howard JJ (2001) The biology and management of wireworms (Agriotes spp.) on potato with particular reference to the UK. Agric Forest Entomol 3:85–98
Petherbridge FR (1938) Wireworms and sugar-beet. J Minist Agric Lond 45:23–70
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Rashed A, Rogers CW, Rashidi M, Marshall JM (2017) Sugar beet wireworm Limonius californicus damage to wheat and barley: evaluations of plant damage with respect to soil media, seeding depth, and diatomaceous earth application. Arthropod-Plant Interact 11:147–154
Reddy GVP, Tangtrakulwanich K, Wu S, Miller JH, Ophus VL, Prewett J, Jaronski ST (2014) Evaluation of the effectiveness of entomopathogens for the management of wireworms (Coleoptera: Elateridae) on spring wheat. J Invertebr Pathol 120:43–49
Reddy GVP, Adhikari A, Frank A, Miller JH (2015) Evalaution of trap crops of the management of wireworms on spring wheat. Annual report. Western Triangle Agricultural Research Center, Conrad, pp 60–66
Reddy GVP, Adhikari A, Frank A, Miller JH, Prewett J (2016) Evaluation of trap crops for the management of wireowrms in spring wheat in Montana. Annual Report. Western Triangle Agricultural Research Center, Conrad, pp 110–124
Russell EJ (1936) Boden und Pflanze, Verlag von Theodor Steinkopff, Th. Steinkopff, Dresden und Leipzig
Schallhart N, Tusch M, Staudacher K, Wallinger C, Traugott M (2011) Stable isotope analysis reveals whether soil-living elaterid larvae move between agricultural crops. Soil Biol Biochem 43:1612–1614
Seal DR, Chalfant RB, Hall MR (1992) Effectiveness of different seed baits and baiting methods for wireworms (Coleoptera: Elateridae) in sweetpotato. Environ Entomol 21:957–963
Seibert CE (1993) A faunal survey of the Elateridea of Montana. Montana State University, Department of Plant Science and Plant Pathology. MSc Thesis. Bozeman
Shaw RH (1988) Climate requirement. Corn and corn improvement cornandcornimpr: 609–638
Shelton A, Badenes-Perez F (2006) Concepts and applications of trap cropping in pest management. Ann Rev Entomol 51:285–308
Staudacher K, Schallhart N, Thalinger B, Wallinger C, Juen A, Traugott M (2013) Plant diversity affects behavior of generalist root herbivores, reduces crop damage, and enhances crop yield. Ecol Appl 23:1135–1145
Svensson GP, Larsson MC, Hedin J (2004) Attraction of the larvalpredator Elater ferrugineus to the sex pheromone of its prey, Osmoderma eremita, and its implication for conservation biology. J Chem Ecol 30:353–363
Traugott M, Benefer CM, Blackshaw RP, van Herk WG, Vernon RS (2015) Biology, ecology, and control of elaterid beetles in agricultural land. Annu Rev Entomol 60:313–334
Van Herk WG, Vernon RS (2007) Morbidity and recovery of the Pacific Coast wireworm, Limonius canus, following contact with tefluthrin-treated wheat seeds. Entomol Exp Appl 125:111–117
Vernon RS, Kabaluk T, Behringer A (2000) Movement of Agriotes obscurus (Coleoptera: Elateridae) in strawberry (Rosaceae) plantings with wheat (Gramineae) as a trap crop. Can Entomol 132:231–241
Vernon RS, Van Herk WG, Clodius M, Harding C (2009) Wireworm management I: stand protection versus wireworm mortality with wheat seed treatments. J Econ Entomol 102:2126–2136
Zhang Z, Zhang X, Zhao Y, Mu W, Liu F (2017) Efficacy of insecticidal seed treatments against the wireworm Pleonomus canaliculatus Coleoptera: Elateridae) in China. Crop Prot 92:134–142
Acknowledgements
This project is supported by the Montana Wheat and Barley Committee. This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch under award Accn# 1009746. We would like to thank Dr. Bob Vernon, Agriculture and Agri-Food Canada, Dr. Kevin Wanner and Dr. Mike Ivie from Montana State University, Bozeman for their valuable suggestions, and summer interns Javan Caroll and Gaby Drishinski for their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Heikki Hokkanen.
Rights and permissions
About this article
Cite this article
Adhikari, A., Reddy, G.V.P. Evaluation of trap crops for the management of wireworms in spring wheat in Montana. Arthropod-Plant Interactions 11, 755–766 (2017). https://doi.org/10.1007/s11829-017-9533-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-017-9533-5