Abstract
Organic farming systems are significantly challenged by the invasive Halyomorpha halys (Stål) and native stink bug species that injure vegetable crops. This two-year study evaluated a polyculture trap crop composed of sunflower and sorghum for organic pepper production at 11 sites in 8 mid-Atlantic and Southeastern states. Stink bug densities in the trap crop and peppers were recorded weekly (mid-June through September), and stink bug fruit injury was compared for trap crop-protected and unprotected control peppers. The trap crop was highly attractive, harboring 5–50× more stink bugs per m2 than the peppers and providing an 8-week attraction period coinciding with peak stink bug activity. Despite this attractiveness, the trap crop was not effective at diverting adult stink bugs away from the pepper crop during the early fruiting period at most sites. However, the average density of stink bug nymphs in pepper plots surrounded by trap crops was 4× lower than controls 5 weeks after planting for pooled sites. Trap crop-protected peppers also had significantly less injury compared to control peppers pooled across sites and years. However, the resulting reduction in pepper damage was insufficient to be economically viable. Overall, results provide evidence that a polyculture trap crop was most effective during the latter weeks of the pepper crop cycle. Future research should address spatial arrangement of the trap crop or integration of complimentary management tactics within the trap crop earlier in the growing season to target the initial colonizing adult stink bugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Organic agriculture lacks non-pesticide alternatives for managing the invasive brown marmorated stink bug.
-
Our multi-state study evaluated a trap crop of sunflower and sorghum for reducing stink bug injury in peppers.
-
The trap crop was highly attractive to stink bugs, reduced their densities in peppers during the later fruiting period, and slightly reduced (~2%) associated injury to peppers.
-
Future research should address additional management tactics with the trap crop to target colonizing adult stink bugs.
Introduction
Organic farming systems rely on biological control, non-synthetic inputs and cultural control tactics for pest management (Zehnder et al. 2007) and are significantly challenged by the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), whose key natural enemies are not established in the USA (Lee et al. 2013). Although Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae), an Asian egg parasitoid of H. halys in its native range, has been detected in the USA, population numbers are currently insufficient in the mid-Atlantic USA to provide effective biological control of H. halys in commercial agroecosystems (Talamas et al. 2015). Some parasitoids native to the USA may recognize and oviposit in eggs of H. halys, but the host is not suitable for complete development of their offspring (Haye et al. 2015). Options for managing H. halys organically are currently limited, with inadequate availability of efficacious alternatives to synthetic insecticides (Lee et al. 2014). A small number of organically approved insecticides (e.g., spinosad and pyrethrins) have shown promise against H. halys in laboratory assays (Lee et al. 2014), but their efficacy has yet to be validated in the field. Furthermore, these products have varying levels of efficacy against native species of stink bug (Hemiptera: Pentatomidae) that also can cause damage to vegetable crops. Physical controls such as barrier screens are currently under investigation as a potential strategy for managing stink bugs organically (Dobson et al. 2016). Infestations of H. halys and native stink bug pests and their resulting crop injury can vary considerably depending on site and year. As the growing season progresses, H. halys populations tend to increase dramatically with high densities occurring in late July to mid-August, depending on crop and location (Nielsen and Hamilton 2009). For example, peak abundances of adult H. halys in bell peppers in Maryland occurred during mid-July and late August (Zobel et al. 2016). Bell pepper fruits are highly susceptible to injury from H. halys and other stink bugs, as they feed primarily on plant reproductive structures, causing corking or deformed fruit (Leskey et al. 2012). Zobel et al. (2016) reported injury rates ranging from 7.2 to 41.7% for bell peppers grown in highly diversified systems typical of the mid-Atlantic region.
Trap cropping, or planting a plant species known to attract pests and limit their spreading to cash crops (Hokkanen 1991), is one tactic that could be readily integrated into organic vegetable production and that is recognized as an acceptable pest management strategy within the USDA National Organic Standard (USDA 2016). Planting a highly attractive trap crop border in a perimeter around the cash crop (Shelton and Badenes-Perez 2006) could potentially exploit the strong perimeter-driven behavior of H. halys and native stink bugs such as Euschistus spp. (Tillman et al. 2009; Venugopal et al. 2014; Blaauw et al. 2016). A successful trap cropping system must both attract and retain stink bugs in order to protect the cash crop at stages vulnerable to insect injury (Holden et al. 2012). The majority of trap cropping studies have used a single host plant. For instance, Soergel et al. (2015) studied a monoculture trap crop of sunflower to protect bell peppers from stink bugs in Pennsylvania and found higher H. halys densities in sunflowers than peppers, but no reduction in fruit injury to peppers surrounded by the trap crop as compared to control plots. By combining host plants with slightly offset phenologies, it may be possible to devise a polyculture trap crop that optimizes both attraction and retention over time. In Florida, Mizell et al. (2008) identified triticale, sorghum and sunflower as potential trap crops against Euschistus servus (Say), Chinavia hilaris (Say) and Nezara viridula (L.) (Hemiptera: Pentatomidae), indicating that these could serve as trap crops throughout most of the Eastern USA. A recent study conducted in four mid-Atlantic states evaluated pearl millet (Pennisetum glaucum L.), okra (Abelmoschus esculentus Moench), field pea (Pisum sativum subsp. arvense L.), sorghum (Sorghum bicolor L. Moench) and sunflower (Helianthus annuus L.) as potential trap crop species and concluded that sunflower and sorghum had the longest period of stink bug (H. halys and native pentatomids) attraction temporally and harbored the highest seasonal densities of stink bugs (Nielsen et al. 2016).
The goal of this study was to evaluate a polyculture perimeter trap crop composed of sunflower and sorghum for reducing stink bug injury in small-scale plots of bell peppers under highly diversified production systems typical of organic farms in the mid-Atlantic and Southeastern regions. We also evaluated the attraction of the trap cropping system for H. halys and phytophagous native pentatomids (i.e., E. servus, Euschistus tristigmus (Say), Euschistus variolarius (Palisot de Beauvois) and C. hilaris), as it is nearly impossible to distinguish stink bug feeding injury by causative species. By combining two highly attractive host plants into a trap crop perimeter, our goal was to optimize the period of attractiveness to stink bugs, coinciding with the fruiting period for bell peppers. In order to determine the breadth of applicability of the trap cropping scheme, we tested this tactic under a range of site-specific conditions in 11 sites within the mid-Atlantic and Southeastern USA. We also examined the seasonal dynamics of H. halys and other stink bug pests by location.
Methods
Plot establishment
In 2014 and 2015, plots were established on USDA-certified organic or transitional land in eight states, either as a single replicate or in a randomized block design with four replicates. Pooled across all field sites, there were 28 replicates in 2014 and 20 replicates in 2015 (Table 1). The field sites were: University of Maryland Clarksville Research and Education Center, Clarksville, MD (‘UMD’); Cane Creek Valley Farm, Fletcher, NC (‘Cane’); Rutgers Agricultural Research and Extension Center, Bridgetown, NJ (‘RAREC’); Muth Farm, Williamstown, NJ (‘Muth’); Stratford Farm, OH (‘Stratford’); Bridgeman Farm, OH (‘Bridgeman’); the Rodale Institute, Kutztown, PA (‘Rodale’); University of Tennessee East Tennessee AgResearch and Education Center, Knoxville, TN (‘UT’); Sunnyside Farm, VA (‘Sunnyside’), Redbud Farm, LLC, Inwood, WV (‘Redbud’); and West Virginia University, Morgantown, WV (‘WVU’). Each replicate contained two plots of bell peppers (untreated seed of var. Aristotle, Capsicum annuum L.; Seminis Vegetable Seeds, St. Louis, MO) spaced a minimum of 10 m apart, with one plot surrounded by a trap crop border (Fig. 1, ‘Trap Crop’) and one plot without trap crop (‘Control’). Crop rows (7.62 m long, 1.5 m on center, 5 rows/plot) were prepared with black plastic film in April, and pepper seedlings (20 plants/row, 38.1–45.7 cm apart) were transplanted after last frost date (ranging from 5/29 to 6/27, 2014; 5/14 to 7/2, 2015). No chemical pest control inputs were applied, and weed management consisted of regular cultural control (hand weeding and tillage) between crop rows. The area between plots contained either orchard grass or winter wheat mowed regularly or a non-host crop (e.g., basil, cucumber, summer squash, winter squash, statice, bachelor’s buttons, or watermelon depending on the site) planted into black plastic and maintained as above. Experimental plots were spaced a minimum of 10 m from other crops.
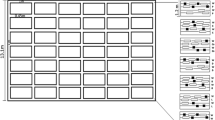
Schematic depicting one experimental field plot containing five rows of ‘Aristotle’ bell peppers (100 plants/plot) in black plastic surrounded by polyculture trap crop border comprised of an interior sorghum (Sorghum bicolor L. Moench planted in two rows at a seeding rate of 56 kg ha−1) strip and an exterior sunflower (Helianthus annuus L. planted in two rows at a seeding rate of 11.2 kg ha−1) strip. The aisles between crop rows were maintained open for ease of access and equipment usage for mowing
To schedule trap crop and pepper plantings such that trap crop stages attractive to stink bugs (i.e., flowering or seed head, see Nielsen et al. 2016) coincided with pepper fruiting, the trap crop borders were direct-seeded ~2 weeks before peppers (ranging from 5/16 to 6–27, 2014; 5/15 to 7/7, 2015). A polyculture trap crop border (1.21 m wide) was planted around the perimeter of the pepper plot, excluding aisles (Fig. 1). The border consisted of an exterior 0.61-m-wide strip of open-pollinated organic sunflower seed mix (Johnny’s Seed #2160SG.36, Helianthus annuus L.; Johnny’s Select Seed, Winslow, ME) planted in two rows at a seeding rate of 11.2 kg ha−1 (seeds spaced 15.2 cm apart within the row). In 2015, the outside sunflower row was direct-seeded ~10 to 21 days after the first row, to increase the period of attractiveness to stink bugs. Adjacent to the sunflowers, an interior 0.61-m-wide strip of sorghum (var. 65B3cnv, Sorghum bicolor L. Moench; Blue River Hybrids, Ames, IA) was planted in two rows at a seeding rate of 56 kg ha−1 (seeds spaced 7.6 cm apart).
Stink bug densities
Pentatomids (eggs, nymphs, adults) were assessed weekly on peppers and trap crops by whole-plant counts beginning one week after pepper planting. Pentatomid observations were conducted between 0700 and 1000 each day. In pepper plots, a linear sample containing 10 pepper plants was randomly selected within each row (five samples/plot/w) and thoroughly examined to record the number of stink bugs and their life stage by species. On each side of the trap crop border, two 1-m linear sections of each trap crop species were randomly sampled with two people simultaneously assessing alternate sides of the border for a minimum of 3 min per sample. Stink bug densities were recorded on a total of eight samples per trap crop species (two samples per cardinal direction of border). Generalized plant phenological stage (vegetative, flowering, seed head/pod, senescence) was recorded by trap crop species for each sample.
Pepper injury
Beginning 3 weeks after pepper planting and continuing weekly through the growing season (i.e., 8 consecutive weeks), all 100 pepper plants of each plot were examined (after insect sampling), and all mature fruits (8 cm or > in diameter) were harvested. Each fruit was assessed for stink bug feeding injury (as described in Zobel et al. 2016). The total number of peppers harvested and the number of fruit with injury classified as minor (limited to two or fewer feeding clusters of light spots indicative of stink bug feeding via proboscis) or major (three or more feeding clusters affecting multiple portions of the fruit surface) were recorded. The percentages of minor, major and total stink bug injury per plot were calculated.
Statistical analyses
All sampling dates for each field site within each year were adjusted to reflect weeks after pepper planting in order to align stink bug data to a common pepper phenology. The weekly densities in peppers of pentatomid adults, nymphs and both life stages combined (H. halys and native stink bugs) were summed for weeks 3–10 after planting each year (2014 and 2015). This 8-week period coincided with the main fruiting time for peppers and the time of the growing season when stink bugs were at high densities. All analyses were performed using the mixed-model procedure in SAS Version 9.3 (SAS Institute 2013). To satisfy the assumptions of ANOVA, sites with <10 total stink bugs in peppers across the season were not included in the analysis (Bridgeman, Muth, Cane 2014, Sunnyside 2014, Stratford, and WVU 2014). The data from the 2015 Sunnyside site in VA was not included either due to an unavoidable late planting that delayed sampling until September (which was well outside the period of stink bug activity representative of the other sites). Separate ANOVAs using weekly data from all other sites were performed to test for main and interaction effects of trap crop over time on the density of adults, nymphs and total stink bugs (adults and nymphs combined). In each model, site and year were treated as random factors, sampling week was treated as a fixed factor, and the repeated measures option was used to correct for autocorrelation among sampling weeks. Data per row were averaged for each week before analysis, and each replicate set of treatment plots within sites was considered an experimental unit. Before each analysis, data were evaluated for normality and homogeneity of variances by examining residual plots and Shapiro–Wilk statistic. The log10 transformation was used for data not meeting the assumptions of ANOVA. Interaction means were separated following a significant F test using Tukey’s HSD test (P < 0.05). To explore the local dynamics occurring at field sites with replicated plots during weeks 3–10, datasets from UMD, RAREC, UT, Redbud and WVU were individually analyzed by mixed-model ANOVAs with repeated measures.
The pepper injury data for weeks 3–10 were pooled over both years from all field sites except Sunnyside, which did not collect harvest data until week 11. Data on the percentage of injured fruit (minor, major, total) were arcsine-transformed to meet the assumptions of ANOVA. Separate mixed-model repeated measures ANOVAs of each category of fruit injury (minor, major, total) tested for the main and interaction effects of the trap crop treatment over time, with year and site as random factors and sampling week as a fixed factor adjusted for repeated measures. Post hoc comparisons of mean differences were made with Tukey’s HSD (P ≤ 0.05). To explore the local dynamics occurring at field sites with replicated plots during weeks 3–10, fruit injury (total) datasets from UMD (both years), RAREC (2014), UT (both years), Redbud (2014) and WVU (2014) were individually analyzed by mixed-model ANOVAs with repeated measures. The data were treated the same as described above, and the same fixed and random factors were included in the models.
To examine the population trend and abundance of pentatomids (H. halys and native stink bugs) in the pepper crop relative to the trap crop habitats, sampling data on stink bugs (adults and nymphs combined) for all sites were converted to a per m2 basis for weeks 1–11 to standardize bug densities across habitats, and weekly means and standard errors were calculated for each year by crop type (sunflower, sorghum, ‘control’ peppers and ‘trap crop’ peppers). To compare the relative attractiveness of the trap crop growth stages, mean stink bug density per m2 for each phenological stage of each trap crop (vegetative, flowering, seed head/pod, senescence) was also calculated from data pooled over both years and all field sites. These means were compared to the mean densities of stink bugs in the two pepper crops (trap crop, control) computed over the same periods of each phenological trap crop stage.
Results
Stink bug densities
The pentatomids observed included Acrosternum hilare (Say), C. hilaris, E. servus, E. tristigmus, E. variolarius, H. halys, Murgantia histrionica (Hahn), N. viridula, Oebalus pugnax (F.) and Podisus maculiventrus (Say). Totaled over all field sites and years, H. halys accounted for 81.4% of the stink bugs observed; however, six of the 19 sites reported more native pentatomids, mainly E. servus. Across sampling weeks, 53.8–100% consisted of adults, with higher proportions of nymphal stages recorded during weeks 7–9. There was considerable variation in stink bug population densities over the growing season among field sites and between years. The field sites (and years) with high densities of adults and nymphs were UMD (both years), Redbud (2014), Cane (2015) and UT (both years), where mean density ranged from 0.21 to 2.03 per 10 pepper plants, 0.03 to 7.17 per m2 of sunflower border and 0.35 to 8.81 per m2 of sorghum border (Table 1). These density ranges did not include the 2015 Sunnyside site, which experienced very high densities of stink bugs in pepper and trap crops late in September. Eight of the 19 field sites experienced very low populations, with less than 10 total stink bugs observed over the entire sampling period, particularly at the PA and OH field sites (Table 1).
ANOVA results based on all sites and both years, excluding the 2015 Sunnyside site and those with sparse datasets, revealed no main or interaction effects of the trap crop treatment (P > 0.67) on the densities of adult stink bugs only or both adult and nymphs combined. However, the trap crop border significantly affected the average density of nymphs on pepper plants (F = 10.62, df = 1, 110, P = 0.001), resulting in fewer nymphs per 10 pepper plants in trap crop-protected plots (0.09 ± 0.02) compared to the density in control plots (0.22 ± 0.03). Although the interaction with sampling week was not significant, pepper plants surrounded by the trap crop harbored fewer stink bug nymphs starting at 5 weeks after planting and differences between treatments increased through the duration of the sampling period (Fig. 2b, weeks 5–10). The main effect of sampling week also was significant for all stink bugs (F = 4.64, df = 7, 393, P < 0.001), with densities increasing to a peak at week 5, followed by a steady decline during weeks 6 through 9, and then peaking again at week 10 (Fig. 2a).
Weekly densities of stink bugs (H. halys and native pentatomids) in organic bell peppers planted in plots surrounded by a trap crop border composed of sunflower and sorghum compared to pepper control plots. Plotted are means (±SEM) by week after pepper planting for: a nymphs and adults combined, b nymphs
The repeated measures analyses of replicated data from individual field sites produced variable results depending on the site and year (Table 2). At UMD and UT in 2014, there were significant interaction effects between trap crop and sample week, with these sites experiencing similar trends in stink bug densities over time. Densities of stink bugs were higher on peppers surrounded by trap crop during weeks 3–5, but then shifted to higher densities in the control peppers, particularly in weeks 8–10. Though stink bug abundance was low at WVU in 2015, the interaction effect was significant, indicating no treatment differences until the last sampling week when significantly higher numbers of stink bugs were recorded in the control plots compared to the trap crop-protected plots. The trap crop treatment also had a highly significant main effect at Redbud in 2014, with lower stink bug densities on peppers surrounded by trap crop compared to the control plots during every sample week. Analyses of the other replicated sites (RAREC in 2014; UMD and UT in 2015) revealed no significant main or interaction effects for the trap crop treatment (P values >0.200).
Abundance and seasonality of stink bugs in individual trap crop species compared to the pepper crop are shown in Fig. 3. In 2014, the trap crop border harbored 5–23× higher densities of stink bugs per m2 than densities on pepper plants through the entire growing season (Fig. 3a). Stink bug numbers were highest in the sunflower portion of the trap crop border from three to 6 weeks after pepper planting, a period that corresponded with the sunflowers reaching the reproductive stages (Fig. 3a, weeks 3–6), but then declined in sunflower as the seed heads senesced, and concurrently increased in the sorghum as flowers and seed heads developed (Fig. 3a, weeks 7–11). The sorghum portion of the trap crop attracted higher densities of stink bugs throughout the remainder of the season. In 2015, stink bug densities in both pepper plots and the trap crop border were initially low (<1 bug/m2) during the first 3 weeks (Fig. 3b). However, the trap crop border was infested with an average 6–50× more stink bugs per m2 than the pepper plants, starting at week 4 and continuing throughout the season. In contrast to 2014, the exterior sunflower row was planted ~10–21 days later in 2015 to extend the period of attractiveness to stink bugs, and, consequently, the trap crop border supported average stink bug densities of 1.5–2.7/m2 from 4 weeks after pepper planting through the remainder of the season (Fig. 3b, weeks 4–11).
Abundance and seasonality of stink bugs (H. halys and native pentatomids) in sunflower and sorghum trap crops, pepper surrounded by trap crop (Pepper-TC), and pepper in control plots (Pepper-C). Plotted are weekly mean densities (±SEM) of stink bugs (adults and nymphs) per m2 of each habitat pooled across all sites in eight states in a 2014 and b 2015
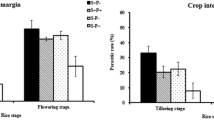
Overall mean densities of stink bugs differed significantly between the vegetative, flowering, seed head/pod and senescence stages of each trap crop (Fig. 4). Based on the number of stink bugs per m2 area of habitat, all growth stages of both trap crops were more attractive to stink bugs than the pepper crop during the same periods. Both trap crops contained comparatively lower densities of stink bugs during the vegetative and senescence stages compared to the flowering and seed head/pod stages. Sunflower was most attractive during flowering, when stink bug densities peaked, and then levels declined during the seed head and senescence stages (Fig. 4a). In contrast, stink bug densities in sorghum reached the highest levels during the flowering and seed head stages and attracted higher total numbers of stink bugs over the entire sampling period (Fig. 4b). Sorghum was a very attractive trap crop during the seed head stage, harboring 2.4× more stink bugs per m2 than sunflower during the same growth stage (Fig. 4b).
Abundance of H. halys and native pentatomids (primarily Euschistus spp.) at different phenological stages of the sunflower (a) and sorghum (b) trap crops. Plotted are overall mean densities (±SEM) per m2 of each trap crop, relative to the mean densities in the pepper crop of all plots, during each phenological stage. Data are pooled across years 2014–2015, field sites and sampling weeks
Pepper injury
The level of pepper injury caused by stink bugs in weeks 3–10 varied widely among field sites depending mainly on the overall level of the stink bug population (Table 1). The percentage of fruit injury pooled from all field sites over both years averaged 16.77 ± 0.95 in the control plots (ranged from 5.1 to 39.9%) compared to 14.91 ± 0.88 in plots surrounded by the trap crop border (ranged from 2.3 to 43.9; Fig. 5). There were no interaction effects for pepper injury, and the main effect of trap crop was not statistically significant for major fruit injury (P = 0.2045) or all injury combined (P = 0.059). However, peppers surrounded by the trap crop had significantly lower levels of minor stink bug injury compared to peppers in control plots (F = 4.37, df = 1, 408, P = 0.037). Analyzing each year separately revealed similar overall levels of fruit injury but different trends between treatments over the sampling period. In 2014, there was a significant interaction between sample week and trap crop for the overall percentage of pepper injury (F = 3.01, df = 7, 190, P = 0.005), with the highest injury levels (~20%) occurring in early sampling weeks and then generally decreasing over time (Fig. 6a). At 6 weeks after planting, peppers surrounded by the trap crop had significantly less injury than those of control plots, a trend that continued through the harvest season. In 2015, there were no significant effects, except for overall differences across sampling weeks (F = 4.46, df = 7, 197, P < 0.001). Stink bug injury was not evident until 4 weeks after planting (Fig. 6b), and although not statistically significant, a similar trend of lower injury on peppers in trap crop plots was evident during weeks 8–10 (Fig. 6b). The individual repeated measures ANOVAs performed for field sites with replicated treatment plots revealed significant main or interaction effects of the trap crop treatment for only UMD, Redbud and WVU. During weeks 3–5 at UMD (both years), pepper injury averaged 11.7 and 22.3% in the control plots and plots surrounded by the trap crop, respectively. However, this trend reversed in weeks 8–10 when the percentage of injured peppers was significantly higher in the control plots (F = 4.07, df = 7, 84, P < 0.001). In contrast, stink bug injury at Redbud in 2014 was consistently higher in the control plots throughout the sampling period, with an average of 13.6% ± 2.59 and 3.9% ± 1.19 of peppers injured in the control plots and plots surrounded by the trap crop, respectively (F = 34.24, df = 1, 33, P < 0.001). WVU in 2014 also reported higher levels of injured peppers in the control plots compared to peppers in trap crop plots (F = 10.61, df = 1, 15, P = 0.005).
Effects of a sunflower and sorghum trap crop border on the overall level of feeding injury on bell peppers by stink bugs (H. halys and native pentatomids). Plotted are means (±SEM) for the percentage of minor, major and overall damaged peppers, based on data pooled across years 2014–2015, field sites and sampling weeks 3–10 after pepper planting. The asterisk above mean comparisons indicates statistically significant difference between control and trap crop treatment (P < 0.05)
Weekly percentage of peppers damaged by stink bugs (H. halys and native pentatomids) in control plots compared to plots surrounded by a sunflower and sorghum trap crop border. Means (±SEM) are plotted for harvest weeks 3–10 after pepper planting, based on data from all field sites for 2014 (a) and 2015 (b)
Discussion
This study is the first to evaluate a polyculture trap crop specifically designed to manipulate the polyphagous behavior of the invasive H. halys. A viable trap cropping system should both attract and retain pests during critical growth periods of the cash crop (Shelton and Badenes-Perez 2006). Our results demonstrate that by combining two highly attractive host plants with slightly offset phenologies, it is possible to provide an 8-week period of attraction to H. halys and native stink bug species during the critical fruiting period of a pepper crop in the mid-Atlantic and Southeastern regions. The trap crop perimeter harbored on average about 85% more stink bugs per unit area than the pepper crop from mid-July (3 weeks after pepper planting) through the last harvest date, and this period of attraction coincided with peak activity of stink bugs in the pepper crop. Both trap crops were most attractive to stink bugs when fruiting structures were available for feeding. In other studies, H. halys and native pentatomids consistently exhibited a strong preference for feeding on flowering structures and developing seeds during the reproductive stages of plant growth (McPherson and McPherson 2000; Zobel et al. 2016). Our results further demonstrate that successive plantings of sunflower, in order to extend the period of attractiveness, can improve the polyculture trap crop tactic; however, additional research is needed to maximize its attractiveness throughout the crop cycle of the cash crop.
In many cases, deployment of a trap crop system may be highly attractive to the target pest but fail to reduce pest densities in the cash crop (Shelton and Badenes-Perez 2006). The polyculture trap crop in this study attracted 1.6–10.3× more stink bugs in sunflower and 4.3–13.9× more in sorghum than the average densities in either pepper crop (trap crop-protected or control plots). Despite its attractiveness throughout the growing season, the trap crop was not effective at diverting stink bugs (adults and nymphs combined) away from the pepper crop. A separate analysis of nymphs showed that their average densities were significantly lower in pepper plots surrounded by trap crops; however, nymphal stages comprised only 16% of the overall stink bug populations, and treatment differences in their numbers occurred later in the season in the pepper crop. The degree to which a trap crop retains the target pest, diverting them from the cash crop, is critical to its success (Holden et al. 2012). A parallel study investigating the behavioral mechanism of the sunflower and sorghum trap crop using mark recapture techniques indicated that our polyculture trap crop had high rates of H. halys retention during the week-long period studied, with minimal movement from the trap crop to the peppers. This may suggest that the trap crop was not acting as a source population, but rather arrested stink bug movement as designed (Blaauw et al. 2017).
The objective of this study was to evaluate the trap crop approach for organic pepper production across a range of localities, in order to assess the tactic’s general efficacy. It is interesting to note that we observed considerable variation in stink bug population levels (Table 1) and seasonal dynamics among field sites, even though cultural management and control tactics in the pepper plots were held constant across all sites, and experimental plots were spaced a minimum of 10 m from other crops that could serve as potential stink bug hosts. We attribute these differences in stink bug populations in part due to differences in the length of time since ‘invasion’ of H. halys at each site (Leskey et al. 2012; Haye et al. 2015), as well as regional landscape and management factors (Wallner et al. 2014). Although six of the 11 sites experienced very low stink bug populations with datasets too sparse to analyze separately, it is noteworthy that individual analyses of datasets from four field sites with established stink bug populations and replicated plots (Table 1: UMD 2014, UT 2014, Redbud 2014, and WVU 2015) revealed significant main or interaction effects for the trap crop treatment and sampling week. Redbud had significantly lower stink bug densities in pepper plots surrounded by the trap crop during the entire sampling period, whereas the trap crop treatment at the UMD, UT and WVU sites was effective only in reducing stink bug densities in peppers in the latter sampling weeks. The results at these sites provide evidence that a polyculture trap crop has potential as a management approach and suggest that integrating an additional control tactic with the trap crop earlier in the growing season (i.e., 3–5 weeks after pepper planting) could reduce numbers of the initial colonizing adult stink bugs. Many successful trap cropping systems require additional management tactics (e.g., vacuuming or pesticide application) applied directly to the trap crop (Holden et al. 2012). Nielsen et al. (2016) have shown that brief flaming directed to a sunflower trap crop is effective at killing H. halys nymphs. Flaming and other tactics such as vacuuming are acceptable in organic production and could be employed to remove stink bugs from the trap crop in the initial weeks after planting to reduce subsequent damage to the cash crop.
Our results showed that the combination of a sunflower and sorghum trap crop was highly attractive to stink bugs and reduced bug densities in the pepper plots at several sites. However, the bottom-line indicator of effectiveness for any management tactic is preventing economic levels of fruit injury. Given that action thresholds for H. halys in peppers have not been established (Kuhar et al. 2016), it is unclear how many stink bugs can be tolerated in organic peppers without resulting economic injury. Zobel et al. (2016) found that the best predictor of pepper injury in Maryland was the average density of H. halys adults and nymphs during the week prior to harvest. Although the level of pepper injury in our study varied widely (2.3–43.9%), we found that peppers surrounded by the trap crop had statistically significantly lower levels of minor stink bug injury, as compared to peppers of control plots. Minor fruit injury induced by stink bugs may have additional implications for growers, as it has been linked to fruit infection by decay-causing pathogens including anthracnose (Colletotrichum acutatum Simmonds) in tomato (Voshell 2015). However, the reduction in injury as a result of any trap crop effect was relatively small (only a difference of about 2% compared to control plots), and importantly, levels of major pepper injury (ranging 5.50–5.95%) did not significantly differ between treatments. Thus, similar to the findings of Soergel et al. (2015), despite the attractiveness to stink bugs throughout the growing season and evidence of reduced stink bug densities in nearby peppers, the trap crop perimeter alone did not provide a meaningful reduction in fruit injury across all field sites of this study. Although we did not observe any edge effects within the relatively small pepper plots of this study, edge effects have been reported for stink bug injury to field corn and soybeans (Venugopal et al. 2014), suggesting that future studies might benefit from evaluating larger pepper plots.
This study demonstrated that a trap crop composed of sorghum and sunflower is highly attractive to H. halys and native stink bug species and has potential for reducing the pest density and injury in a pepper cash crop over an extended period. However, because results indicated that the trap crop was most effective during the latter weeks of the pepper crop cycle, the commercial application of this tactic may require an additional stink bug removal tactic in either the trap crop or pepper crop to target early colonizing adults. Future research should address the integration of other management tactics in combination with the trap crop approach that are consistent with organic production methods. Additional studies should also focus on successive plantings of both trap crops to maximize their attractiveness throughout the most vulnerable period of the cash crop and examine different spatial arrangements of the trap crop to increase retention of stink bugs away from the cash crop.
Author contributions
CRM and ALN conceived and designed the research. All authors conducted experiments. GD analyzed data. CRM wrote the manuscript. All authors read and approved the manuscript.
References
Blaauw BR, Jones VP, Nielsen AL (2016) Utilizing immunomarking techniques to track Halyomorpha halys (Hemiptera: Pentatomidae) movement and distribution within a peach orchard. PeerJ 4:e1997. doi:10.7717/peerj.1997
Blaauw BR, Morrison WR, Mathews CR, Leskey TC, Nielsen AL (2017) Measuring host plant selection and retention of Halyomorpha halys (Hemiptera: Pentatomidae) by a trap crop. Entomologia Experimentalis et Applicata. Accepted 23 Dec 2016
Dobson R, Rogers M, Moore J, Bessin R (2016) Exclusion of the brown marmorated stink bug from peppers using barrier screens. HortTechnology 26:191–198
Haye T, Gariepy T, Hoelmer K, Rossi JP, Streito JC, Tassus X, Desneux N (2015) Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: an increasing threat to field, fruit and vegetable crops worldwide. J Pest Sci 88:665–673
Hokkanen HMT (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138
Holden MH, Ellner SP, Lee D-H, Nyrop JP, Sanderson JP (2012) Designing an effective trap cropping strategy: the effects of attraction, retention and plant spatial distribution. J Appl Ecol 49:715–722
Kuhar T, Morrison R, Leskey T, Aigner J, Dively G, Zobel E, Brust J, Whalen J, Cissel B, Walgenbach J, Rice K, Fleischer S, Rondon S (2016) Integrated pest management for brown marmorated stink bug in vegetables. http://www.stopbmsb.org/stopBMSB/assets/File/BMSB-in-Vegetables-English.pdf. Accessed 22 Apr 2016
Lee D-H, Short BD, Bergh SJ, Leskey TC (2013) Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan and the Republic of Korea. Environ Entomol 42(4):627–641
Lee D-H, Short BD, Nielsen AL, Leskey TC (2014) Impact of organic insecticides on the survivorship and mobility of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in the laboratory. Fla Entomol 97(2):414–421
Leskey TC, Hamilton GC, Nielsen AL, Polk DF, Rodriguez-Sanoa C, Bergh CJ, Herbert DA, Kuhar TP, Pfeiffer DG, Dively GP, Hooks CRR, Raupp MJ, Shrewsbury PM, Krawczyk G, Shearer PW, Whalen J, Koplinka-Loehr C, Myers E, Inkley D, Hoelmer KA, Lee D-H, Wright SE (2012) Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag 23:218–226
McPherson JE, McPherson RM (2000) Stink bugs of economic importance in America North of Mexico. CRC Press LLC, Boca Raton
Mizell RF, Riddle TC, Blount AS (2008) Trap cropping system to suppress stink bugs in the Southern coastal plain. Proc Fla State Hort Soc 121:377–382
Nielsen AL, Hamilton GC (2009) Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Ann Entomol Soc Am 102:608–616
Nielsen AL, Dively GP, Pote JM, Zinati G, Mathews C (2016) Identifying a potential trap crop for a novel insect pest, Halyomorpha halys (Hemiptera: Pentatomidae), in organic farms. Environ Entomol 45:472–478
SAS Institute Inc (2013) The SAS system, Release 9.4. SAS Institute, Cary, NC
Shelton AM, Badenes-Perez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308
Soergel DC, Ostiguy N, Fleischer SJ, Troyer RR, Rajotte EG, Krawczyk G (2015) Sunflower as a potential trap crop of Halyomorpha halys (Hemiptera: Pentatomidae) in pepper fields. Environ Entomol 44(6):1581–1589
Talamas EJ, Herlihy MV, Dieckhoff C, Hoelmer KA, Buffington M, Bon MC, Weber DC (2015) Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J Hymenopt Res 43:119–128
Tillman PG, Northfield TD, Mizell RF, Riddle TC (2009) Spatiotemporal patterns and dispersal of stink bugs (Heteroptera: Pentatomidae) in peanut-cotton farmscapes. Environ Entomol 38:1038–1052
United States Department of Agriculture National Organic Standards (2016) ELECTRONIC CODE OF FEDERAL REGULATIONS Title 7, Subtitle B, Chapter I, Subchapter M, §205.206. http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&sid=3f34f4c22f9aa8e6d9864cc2683cea02&tpl=/ecfrbrowse/Title07/7cfr205_main_02.tpl. Accessed 22 Apr 2016
Venugopal PD, Coffey PL, Dively GP, Lamp WO (2014) Adjacent habitat influence on stink bug (Hemiptera: Pentatomidae) densities and the associated injury at field corn and soybean edges. PLoS ONE 9:e109917
Voshell R (2015) Interactions of brown marmorated stink bug, Colletotrichum acutatum and trap crops in organic tomato production. M.S. Thesis, West Virginia University
Wallner AM, Hamilton GC, Nielsen AL, Hahn N, Green EJ, Rodriguez-Saona CR (2014) Landscape factors facilitating the invasive dynamics and distribution of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), after arrival in the United States. PLoS ONE 9(5):e95691
Zehnder G, Gurr GM, Kühne S, Wade SR, Wratten SD, Wyss E (2007) Arthropod pest management in organic crops. Annu Rev Entomol 52:57–80
Zobel ES, Hooks CRR, Dively GP (2016) Seasonal abundance, host suitability, and feeding injury of the brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in selected vegetables. J Econ Entomol. doi:10.1093/jee/tow055
Acknowledgments
We thank the farmers who generously provided their land and field support to make this research possible and numerous student interns and volunteers in the Mathews, Dively, Nielsen and Wszelaki labs. This project was funded by United States Department of Agriculture National Institute of Food and Agriculture Grant #2012-51300-20097.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CM, BB, GD, JK, JM, TT, DP, EO, JW, CW, GZ and AN declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by T. Haye.
Special Issue: The brown marmorated stink bug Halyomorpha halys an emerging pest of global concern.
Rights and permissions
About this article
Cite this article
Mathews, C.R., Blaauw, B., Dively, G. et al. Evaluating a polyculture trap crop for organic management of Halyomorpha halys and native stink bugs in peppers. J Pest Sci 90, 1245–1255 (2017). https://doi.org/10.1007/s10340-017-0838-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0838-z