Abstract
Many herbivorous insects use olfactory cues for host location. Extracts from Brassica napus L. have been shown to elicit electrophysiological and behavioural responses in the cabbage seedpod weevil, Ceutorhynchus obstrictus (Marsham) (syn. C. assimilis (Paykull)) (Coleoptera: Curculionidae). These include volatile products of the hydrolysis of glucosinolates. Here we present results of a laboratory olfactometer study examining the attractiveness of odours from flowering racemes and foliage of Sinapis alba L. (an inappropriate host for larval development), B. napus (an excellent host for larval development) and lines derived from S. alba × B. napus selected from colonization studies to demonstrate resistance or susceptibility. Results of this study indicate differential attraction of C. obstrictus to the odours of resistant and susceptible lines and suggest the role of hydrolysis products of glucosinolates, particularly the attractive effects of 2-phenylethyl isothiocyanate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cabbage seedpod weevil, Ceutorhynchus obstrictus (Marsham) (syn. C. assimilis (Paykull)) (Coleoptera: Curculionidae) is a pest of brassicaceous oilseed crops in Europe and North America (Hill 1987; McCaffrey 1992; Buntin et al. 1995; Dosdall et al. 2001). Adult C. obstrictus overwinter in the ground below leaf litter and emerge in late spring to feed on brassicaceous plants near their overwintering sites (Dmoch 1965; Ulmer and Dosdall 2006). Mass migration into crops of canola (Brassica napus L. and Brassica rapa L.) occurs shortly after flowering (Ulmer and Dosdall 2006). Oviposition occurs in developing siliques of Brassica spp.; five to six seeds can be consumed during three larval instars (Dmoch 1965). Mature larvae chew holes in pod walls, emerge and drop to the soil where they pupate. Emergence of the next generation of adults occurs in mid August in western Canada (Dosdall and Moisey 2004) and in late July in much of its European range (Bonnemaison 1957). Losses associated with larval feeding are from 15 to 20% for North American spring canola (Dosdall et al. 2001) and 35% for winter rape (McCaffrey et al. 1986). Losses can exceed 18% in Europe (Alford et al. 2003).
Introgression of Sinapis alba L. to B. napus has produced several accessions that have proven resistant to C. obstrictus in field and laboratory experiments (Dosdall and Kott 2006; Ross et al. 2006; Shaw et al. 2009). Mechanisms of resistance include antixenosis and antibiosis in resistant genotypes, with fewer eggs deposited and increased development times of C. obstrictus larvae (McCaffrey et al. 1999; Dosdall and Kott 2006; Tansey 2009). Reduced apparency (as per Feeny 1976) of resistant lines has also been demonstrated; C. obstrictus adults are less responsive to visual cues associated with resistant genotypes (Tansey et al. 2009). Although C. obstrictus will oviposit and can complete development on marginally suitable host plants like S. alba (Kalischuk and Dosdall 2004; Dosdall and Kott 2006), given choices, these weevils discriminate among populations of potential hosts (for example, Moyes and Raybould 2001). In addition to differences in visual cues, mechanisms for discrimination among resistant and susceptible introgressed lines are likely related to variations in the volatile compounds they emit.
Visser (1986) noted that volatile chemical cues are used by many herbivorous insects for host location and suggested that glucosinolates and their hydrolysis products, which are compounds relatively specific to Brassicaceae, should act as host association cues to Brassicaceae specialists. Evans and Allen-Williams (1993) found that field traps baited with B. napus extracts were located by C. obstrictus from distances of 20 m. Adults orient toward odours of B. napus foliage and flowers and their extracts in olfactometer studies (Evans and Allen-Williams 1993; Bartlet et al. 1997; Cook et al. 2006a). Smart et al. (1997) found that mixes of 3-butenyl, 4-pentenyl and 2-phenylethyl isothiocyanates are attractive to C. obstrictus during its crop colonizing migration. Smart and Blight (1997) reported that each of these compounds was also individually attractive to C. obstrictus in a field trapping study. Moyes and Raybould (2001) detected a relationship between population-wide 3-butenyl content and C. obstrictus oviposition and suggested that glucosinolate hydrolysis products facilitate location and discrimination of host populations.
Shaw (2008) examined upper cauline leaves using high performance liquid chromatography (HPLC) analysis and detected a polymorphic peak that differed in height among resistant and susceptible lines derived from S. alba × B. napus. The peak was associated with an as yet unidentified compound that was determined, through myrosinase degradation, to be a glucosinolate. Peak height was inversely correlated with susceptibility to weevil attack as indicated by exit-hole frequencies (weevil infestation scores) in pods of susceptible and resistant lines. Peak height of the uncharacterised glucosinolate was, on average, 3.5 times larger in resistant than susceptible lines (Shaw et al. 2009). Shaw et al. (2009) also detected differences among resistant and susceptible lines derived from S. alba × B. napus in the amounts of another uncharacterised glucosinolate in seeds of immature pods; peak heights were correlated with weevil infestation scores and, on average, were 3.5 times greater in susceptible than resistant lines.
Here we present results of laboratory olfactometer assessments of the volatile cues associated with whole plants, flowering racemes and cauline leaves of C. obstrictus-resistant and -susceptible lines developed by S. alba × B. napus and the parental genotypes, B. napus and S. alba. Responses were assessed for both overwintered- and new-generation weevils. We also investigated potential identities of the uncharacterised glucosinolate investigated by Shaw et al. (2009). Potential effects of hydrolysis products of these glucosinolates were used to draw inferences regarding the influence of detected polymorphisms on attractiveness of volatile cues associated with specific host genotypes. Potential courses for resistance breeding and deployment strategies for novel germplasm are also addressed.
Materials and methods
Plants and insects
Seed was obtained from the University of Guelph germplasm collection; the S. alba × B. napus accessions were derived as described in Dosdall and Kott (2006). Genotypes evaluated included B. napus var. Q2 (hereafter referred to as Q2), S. alba var. AC Pennant (hereafter referred to as S. alba), and three C. obstrictus-resistant and two susceptible lines derived from S. alba × B. napus (Accessions 171S, 154S and 276R, 173R and 121R, respectively; ‘S’ denotes susceptible, ‘R’ denotes resistant). Plants were propagated in a soilless growth medium consisting of a modified Cornell mix based on the recipe of Boodly and Sheldrake (1982). Plants were grown in a greenhouse chamber at the Agriculture and Agri-Food Canada Research Centre, Lethbridge, Alberta and maintained at 16:8 (L:D) and 60% relative humidity. Plants were at growth stage 4.3 (many flowers open, lower pods elongating) (Harper and Berkenkamp 1975) when tested because this is when most C. obstrictus oviposition occurs (Dosdall and Moisey 2004). Ceutorhynchus obstrictus adults were swept from a commercial B. napus field near Lethbridge, Alberta (49° 41′ 39″ N, 112° 49′ 58.3″ W) in late May, late June and mid-August 2007; weevils were maintained on potted, flowering B. napus var. Q2 in mesh cages in the laboratory at 12:12 (L:D) and introduced to experiments within 2 weeks of capture.
Bioassay
A Y-tube olfactometer (Fig. 1) was used to assess behavioural responses of C. obstrictus to olfactory cues associated with test genotypes. All tests were conducted in the laboratory at 21°C and between 10:00 and 15:00 h. The olfactometer was placed squarely under ceiling fluorescent lighting (GE T8 F32T8/SPX41, General Electric, Fairfield, CT). Brightly coloured materials were kept out of sight. The apparatus consisted of a modified 145-mm diameter by 250-mm high glass bell jar with a 29 mm circular opening in its side and a 42.5 mm circular opening in its top. A curved (45o), 115-mm long section of 38-mm diameter glass tubing was attached to the top of the bell jar; another similarly curved piece was attached to this. A 160-mm glass Y-intersection (45o) (42.5 mm internal diameter) was next in line; each arm was attached to a 50-mm diameter by 200-mm long glass bell jar that tapered to a 6.9 mm opening at its distal end. All glass sections of the apparatus were connected using 33.5-mm diameter Tygon™ tubing. A machined plastic venturi with a 12.8-mm diameter distal opening was inserted into the last bell jar to allow weevils to move into the jar but restrict movement back into the Y-tube. The ends of each small bell jar attached to the Y-tube were connected to 950-mm long by 190-mm diameter sample containers (Plexiglas™ cylinders) using 15-mm diameter Tygon™ tubing. Compressed air was maintained at 0.24 l min−1 using an air-flow regulator, filtered using activated charcoal and bubbled through distilled water to maintain consistent humidity before being pumped into the apparatus. Relative humidity in the device was measured at 1 s intervals over 2 h and determined to be 48.98 ± 0.009%. Airflow was run through a splitter and to odour sources and controls. All parts of the apparatus were washed in dish soap and water, rinsed with 70% ethanol and dried between runs.
Olfactometer. Black arrows indicate direction of airflow; grey arrow indicates desired direction of insect movement. A Air flow regulator, B activated charcoal filter, C distilled water, D sample container with perforated plastic dish to allow movement of air past sample, E Tygon™ tubing, F glass bell jar, G glass Y-tube, H glass bell jar, I air outflow with mesh to prevent escape of insects, J rubber stopper (insects introduced here), K close-up of venturi used to prevent insects from moving backwards into the apparatus inside close-up of bell jar attached to Y-tube, L tapered tip of glass bell jar with mesh to prevent entry of insects into sample containers, M Tygon™ connection pieces
Responses of at least four replicate groups of weevils to each odour source at each time were assessed. Replicates were tested in random order over the course of the experiments. Preliminary analysis indicated no significant differences between the responses of 40 individual weevils (65%) and four groups of 20 randomly selected weevils (70%) to whole, flowering Q2 (χ2 = 0.31; df = 1; P = 0.5805), so randomly selected, mixed-sex groups of 20 C. obstrictus were tested in all evaluations of responses. Weevils were introduced through the opening in the side of the large glass bell jar; the opening was plugged with a rubber stopper after their introduction. Weevil positions in one arm or the other of the Y-tube were recorded after 20 min. Sides containing test materials and controls were switched after each group of weevils. Sexing by examination of the pygidium as per Cook et al. (2006b) was found to be unreliable; therefore weevils were removed, preserved in 70% ethanol and dissected. Proportions of C. obstrictus responding in the apparatus (captured on both control and treatment sides) and proportions of males or females associated with the treatment sides of the Y-tube olfactometer were compared by analysis with binomial generalized estimating equations (SAS proc GENMOD) (SAS Institute 2005). Pair-wise comparisons of the proportions of males and females among genotypes, between plant parts or preparation techniques and among sampling dates were made using Wald chi-square tests (LS MEANS statement with ‘diff’ option in SAS proc GENMOD) (SAS Institute 2005).
Comparing responses to whole plants and flowering racemes
Potted, whole plants were placed in Plexiglas™ cylinders and air was pumped past them and through the apparatus. Pots of soilless growth medium were used as a control for these tests. To test flowers, the top 20 cm of intact flowering plants were inserted into a 30-cm plastic cylinder through a 4-cm opening in its side. Parafilm™ was placed over the opening to prevent escape of pumped air. An empty chamber was used as a control for these tests. Responses were compared in mid June and mid August 2007 (overwintered and new-generation weevils, respectively).
Responses to flowering racemes at different points in the growing season
Responses to flowering racemes were assessed in a manner consistent with the previous section. An empty chamber was used as a control for these tests. Responses were compared in mid June, mid July (overwintered generation) and mid August 2007 (new generation).
Responses to cauline leaves by weevil generation
Cauline leaves are the bracts just below an inflorescence. Fresh cauline leaves, excised and macerated (with scissors), freeze-dried (0.1 g reconstituted with 0.1 ml distilled water) and freeze-dried with myrosinases denatured (0.1 g heated to 110°C for 45 min then reconstituted with 0.1 ml of distilled water) were introduced into a sealed 250-ml flask. Air was forced through a hole in the rubber stopper sealing the flask, out another hole in the stopper and through the apparatus. The control for these tests was an empty Plexiglas™ cylinder. Responses to macerated and excised cauline leaves were assessed in mid June and mid August 2008 (overwintered and new-generation weevils, respectively) and responses to freeze-dried cauline leaves were assessed in mid August 2008 (new-generation weevils).
Relationships of glucosinolate content and weevil responses
Relationships of the contents of uncharacterised glucosinolates evaluated for immature seeds and cauline leaves (Shaw et al. 2009) and the proportions of C. obstrictus responding to whole plants, flowering racemes and cauline leaves were assessed using linear regression analysis (SAS proc REG) (SAS Institute 2005).
Glucosinolate characterization
Estimates of the identities of uncharacterised glucosinolates from Shaw et al. (2009) associated with cauline leaves (inversely correlated with susceptibility to weevil attack) and seeds (correlated with susceptibility to weevil attack) were performed using retention time shifts corrected by linear interpolation (as per Gong et al. 2004). Results of High Performance Liquid Chromatography–Mass Spectrometry (HPLC–MS) analysis of glucosinolate calibration standards (Lee et al. 2006; Rochfort et al. 2008) were compared with the results of plant tissue analyses of Shaw et al. (2009). Correlation analysis (SAS proc CORR) (SAS Institute 2005) was conducted to assess the validity of interpolated retention times associated with known compounds and to make estimates of the identities of unknown compounds from Shaw (2008) and Shaw et al. (2009).
Results
Comparing responses to whole plants and flowering racemes
Proportions of weevils responding (the sum of both control and treatment sides of the olfactometer) to whole plants and flowering racemes did not differ (χ2 < 0.01; df = 1; P = 0.999). Responses differed by genotype (χ2 = 132.43; df = 6; P < 0.001). Responses to Q2, 171 S and 154 S were similar (P > 0.05) and greater than those to 276 R, 173 R, 121 R or S. alba (P < 0.05 for all).
Significant differences in the responses of female weevils of the overwintered generation to tested genotypes were apparent (χ2 = 63.82; df = 6; P < 0.001). Responses to the susceptible genotypes Q2, 154 S and 171 S were similar (P > 0.05 for all comparisons) and greater than to the resistant genotypes S. alba, 173R, 121 R, and 276 R (P < 0.007 for all comparisons). There were no significant differences in the responses to resistant genotypes (P > 0.05 for all comparisons). Significant differences in the responses of overwintered generation males to genotypes were also apparent (χ2 = 28.84; df = 6; P < 0.001). Responses were similar to those of females. Both males and females responded similarly to whole plants and flowering racemes (χ2 = 0.03; df = 1; P = 0.8613 and χ2 = 0.02; df = 1; P = 0.895, respectively). No interaction of plant tissue (flowering racemes or whole plant) and genotype was apparent for males or females (χ2 = 4.73; df = 6; P = 0.5792 and χ2 = 8.50; df = 6; P = 0.204, respectively).
Responses to flowering racemes at different points in the growing season
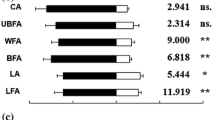
Proportions of weevils responding to flowering racemes differed by genotype (χ2 = 263.09; df = 6; P < 0.001). Responses to Q2, 171 S and 154 S were similar (P > 0.05) and greater than those to 276 R, 173 R, 121 R or S. alba (P < 0.05 for all) (Table 1).
Significant differences in the responses of female weevils to the tested genotypes were apparent (χ2 = 92.92; df = 6; P < 0.001). Responses to the susceptible genotypes Q2, 154 S and 171 S were similar (P > 0.05 for all comparisons) and greater than those associated with the resistant genotypes S. alba, 173R, 121 R, or 276 R (P < 0.01 for all comparisons) (Table 1). There were no significant differences in responses to resistant genotypes (P > 0.05 for all comparisons). The effect of date was significant (χ2 = 8.83; df = 2; P = 0.018). Responses were greater in June and August than July (χ2 = 5.72; df = 1; P = 0.017 and χ2 = 6.40; df = 1; P = 0.011, respectively). Differences between June and August were not significant (χ2 = 0.04; df = 1; P = 0.851). However, no significant interaction of testing date and genotype was apparent (χ2 = 6.42; df = 12; P = 0.893). Significant differences in the responses of male weevils to these genotypes were also apparent (χ2 = 71.72; df = 6; P < 0.001). Responses were similar to those of females (Table 1). No significant effects of testing date (χ2 = 2.55; df = 2; P = 0.279) or interaction of testing date and genotype (χ2 = 10.57; df = 12; P = 0.566) were detected.
Responses to cauline leaves by weevil generation
Proportions of weevils responding were greater to macerated than excised cauline leaves (χ2 = 13.68; df = 1; P < 0.001). Responses differed among genotypes (χ2 = 132.65; df = 6; P < 0.001). Responses to Q2, 171 S and 154 S were similar (P > 0.05) and greater than those to other genotypes (P < 0.05 for all). Responses to 276 R and 173 R were similar (P > 0.05) and greater than those to S. alba or 121 R (P < 0.05 for all). No interaction of preparation technique and genotype was detected (χ2 = 7.59; df = 6; P = 0.269).
Female weevils responded similarly to macerated and excised cauline leaves (χ2 = 0.95; df = 1; P = 0.329). A significant effect of genotype was apparent (χ2 = 64.55; df = 6; P < 0.001). Responses to the susceptible genotypes 154 S, 171 S and Q2 were similar (P > 0.05 for all comparisons) and significantly greater than those associated with S. alba; 154 S and 171 S were more attractive than 276 R, 173 R, or 121 R; 121 R was more attractive than S. alba (P < 0.01 for all comparisons) (Table 2). The effect of generation was also significant (χ2 = 6.55; df = 1; P = 0.011); responses were greater for overwintered than new generation female weevils. No interactions of generation by genotype, preparation technique by genotype, generation by preparation technique, or generation by preparation technique by genotype were apparent (P > 0.05 for all). Macerated cauline leaves were more attractive to male weevils than excised leaves (χ2 = 8.68; df = 2; P = 0.003). A significant genotype effect was also apparent (χ2 = 43.27; df = 6; P < 0.001). Responses to Q2, 171 S and 154 S were greater than those associated with S. alba, 121 R or 173 R; responses to Q2 and 154 S were greater than those to 276 R (P < 0.01) (Table 2). Responses did not differ by generation (χ2 = 0.14; df = 1; P = 0.707). No interactions of generation by genotype, preparation technique by genotype, generation by preparation technique, or generation by preparation technique by genotype were apparent (P > 0.05 for all).
Proportions of weevils responding to native and denatured odour sources were similar (χ2 = 2.10; df = 1; P = 0.148) and no interaction of heat treatment and genotype was detected (χ2 = 5.08; df = 4; P = 0.279). Differences in responses to genotypes without heat treatment were apparent: the susceptible 171S elicited greater responses than any other genotype; 154 S, 173 R and 121 R were similar (P > 0.05) and more attractive than S. alba (P < 0.05 for all). Differences in responses to genotypes with heat treatment were also apparent: 171S elicited similar responses as 154 S (P > 0.05) but greater responses than 121 R or S. alba (P < 0.05 for all) (Table 3).
Responses of C. obstrictus female weevils to reconstituted freeze-dried cauline leaves indicated differences among genotypes (χ2 = 18.30; df = 4; P = 0.001) (Table 3). No effects of heat treatment or significant interaction of heat treatment by genotype were apparent (χ2 = 0.01; df = 1; P = 0.912, and χ2 = 7.74; df = 4; P = 0.101, respectively). However, pair-wise comparisons indicated significant differences in responses to genotypes without heat treatment: 154 S and 171 S were more attractive than S. alba; 171S was also more attractive than 121R (P < 0.01 for all comparisons). No significant differences in responses to heat-treated leaves were detected (P > 0.05 for all comparisons) (Table 3). Responses of male weevils to reconstituted freeze-dried cauline leaves did not differ among genotypes (χ2 = 8.82; df = 4; P = 0.066) and no significant heat treatment effect was detected (χ2 = 1.88; df = 1; P = 0.170). However, a significant interaction of heat treatment and genotype was apparent (χ2 = 10.01; df = 4; P = 0.040) and pair-wise comparisons indicated that 154 S and 171 S without heat treatment were more attractive than S. alba or 173 R without heat treatment (P < 0.01 for all comparisons) (Table 3). No significant differences in responses of males to heat-treated leaves were detected (P > 0.05 for all comparisons) (Table 3).
Comparing responses to flowers and cauline leaves
Comparison of the responses of female weevils of C. obstrictus of the new generation to S. alba, 121 R, 173 R, 154 S and 171 S flowers, excised, macerated and freeze-fried cauline leaves indicated a significant genotype effect (χ2 = 43.13; df = 4; P < 0.001); susceptible genotypes attracted similar numbers of weevils (P > 0.05 for all comparisons) and significantly more than resistant genotypes (P < 0.01 for all comparisons). A significant effect of plant part or preparation technique was also apparent (χ2 = 43.83; df = 4; P < 0.001). No significant interactions of genotype and plant part or preparation technique were apparent (χ2 = 14.34; df = 16; P = 0.573). Flowering racemes attracted significantly more females than macerated, excised, freeze-dried and reconstituted or freeze-dried, heat-treated and reconstituted cauline leaves (P < 0.01 for all comparisons). Macerated and excised cauline leaves attracted similar proportions of females (χ2 = 0.01; df = 1; P = 0.841). Both treatments resulted in greater proportions attracted than for freeze-dried cauline leaves (P < 0.01 for all comparisons). Freeze-dried and reconstituted and freeze-dried, heat-treated and reconstituted cauline leaves attracted similar proportions of females (χ2 = 0.01; df = 1; P = 0.912).
Responses of males indicated a significant genotype effect (χ2 = 90.01; df = 4; P < 0.001); susceptible genotypes attracted similar numbers of weevils (P > 0.05 for all comparisons) and significantly more than resistant genotypes (P < 0.01 for all comparisons). A significant effect of plant part or preparation technique was also apparent (χ2 = 28.59; df = 4; P < 0.001), although no significant interaction of genotype and plant part or preparation technique was apparent (χ2 = 22.28; df = 16; P = 0.134). Flowering racemes attracted significantly more males than macerated, excised, freeze-dried and reconstituted and freeze-dried, heat-treated and reconstituted cauline leaves (P < 0.05 for all comparisons). Unlike females, males responded more strongly to macerated than excised cauline leaves (χ2 = 4.66; df = 1; P = 0.031). Males were also more attracted to excised than freeze-dried cauline leaves (P < 0.05 for both comparisons). Freeze-dried and freeze-dried and heat-treated cauline leaves attracted similar proportions of males (χ2 = 1.90; df = 1; P = 0.168).
Relationships of glucosinolate content and weevil responses
Significant negative relationships of female weevil responses to whole plants, flowers, macerated, excised and freeze-dried and reconstituted cauline leaves and mean peak heights associated with the uncharacterised cauline leaf glucosinolate (Shaw et al. 2009) were detected (P < 0.05 for all assessments). However, no relationship between female weevil responses to freeze-dried, heat-treated and reconstituted cauline leaves and this glucosinolate was detected (F 1,14 = 4.11; R 2 = 0.1718; P = 0.062). Similar trends were detected for males: responses to whole plants, flowers, macerated, excised and freeze-dried cauline leaves were strongly and negatively associated with this glucosinolate (P < 0.01 for all assessments). No relationship of male responses to heat-treated freeze-dried cauline leaves and this glucosinolate was detected (F 1,14 = 1.41; R 2 = 0.0918; P = 0.254). Relationships between female weevil responses and peak heights associated with the uncharacterised glucosinolate detected by Shaw et al. (2009) from seeds of these genotypes were demonstrated for whole plants and flowering racemes (F 1,21 = 13.08; R 2 = 0.3544; P = 0.002 and F 1,72 = 90.05; R 2 = 0.5495; P < 0.001, respectively). Similar relationships were demonstrated for males (F 1,21 = 11.26; R 2 = 0.3181; P = 0.003 and F 1,72 = 80.62; R 2 = 0.522; P < 0.0001, respectively).
Glucosinolate characterization
Comparisons of HPLC results from Shaw et al. (2009) and other studies (Lee et al. 2006; Rochfort et al. 2008) by linear interpolation indicate that the uncharacterised glucosinolate (retention time 20.5 ± 0.01 min) detected from seeds of immature pods and positively correlated with weevil infestation scores responses is likely 2-phenylethyl glucosinolate. Linear interpolation predicted the retention time of 2-phenylethyl glucosinolate at 20.6 min. Cauline leaves, mature foliage and seeds also exhibited a peak at 17 min. This second peak was likely associated with 3-butenyl glucosinolate and was relatively consistent among resistant and susceptible lines (Shaw et al. 2009). The peak associated with cauline leaves and negatively correlated with weevil infestation scores is likely 1-methoxy-3-indolylmethyl glucosinolate. Mean retention time associated with this compound was 21.4 ± 0.03 min; linear interpolation predicts a retention time of 21.4 min for 1-methoxy-3-indolylmethyl glucosinolate (Table 4). Correlation analysis of the results of retention time shifts of known standards from Lee et al. (2006) and Rochfort et al. (2008) corrected by linear interpolation and retention times associated with uncharacterised peaks from Shaw et al. (2009) indicated a significant relationship (R = 0.9998; P = 0.012).
Discussion
Results of this study indicate differences among the responses of C. obstrictus to olfactory cues associated with B. napus and S. alba and accessions obtained by crosses of these species. Odours from whole flowering plants, flowering racemes and cauline leaves of the susceptible genotypes B. napus Q2, 171 S and 154 S were significantly more attractive than resistant genotypes to both male and female weevils. Similarities in weevil responses among Q2, 171S and 154 S suggest similarities in the profiles of the volatile compounds that stimulate electrophysiological and/or behavioural activity in the weevil. Differences among responses of weevils to resistant and susceptible introgressed lines indicate chemical differences.
Ceutorhynchus obstrictus is attracted to volatile compounds associated with B. napus (Free and Williams 1978; Bartlet et al. 1993). Initial responses to host odours include positive anemotaxis (Evans and Allen-Williams 1998) consistent with olfactometer responses of weevils in the current study. Visser (1986) suggested that glucosinolates and their hydrolysis products should act as host association cues to crucifer specialists. Hydrolysis of glucosinolates in plants results from the action of specific myrosinases (β-thioglucosidases) that facilitate the irreversible hydrolysis of the thioglucosidic bond, liberating the D-glucose and aglycone moieties; unstable isothiocyanates (RN=C=S: R may include an alkyl, aryl or indolylmethyl substituent depending on the parent glucosinolate), epithionitriles and nitriles are the typical products (Cole 1976; Rask et al. 2000). Because myrosinase and glucosinolates are compartmentalized in the Capparales including the Brassicaceae, volatile hydrolysis products of glucosinolates are generally produced only when tissues, and thus myrosin and sulphur rich cells, are damaged (Rask et al. 2000; Andréasson et al. 2001). However, isothiocyanates have been detected emanating from undamaged B. napus (for example, Finch 1978).
Allyl, 3-butenyl, 4-pentenyl, and 2-phenylethyl isothiocyanates have been detected in headspace volatiles of cut flowering stems of B. napus and specific olfactory cells tuned to these compounds have been detected in C. obstrictus (Blight et al. 1995). Of these, only 2-phenylethyl isothiocyanate elicited strong responses in one study (Blight et al. 1995). In another study, 3-butenyl and 4-pentenyl isothiocyanate elicited the strongest electroantennogram responses (Evans and Allen-Williams 1992). Smart and Blight (1997) found that each of these compounds was attractive to C. obstrictus, and recommended baiting traps with 2-phenylethyl isothiocyanate for monitoring spring populations. This compound is also attractive to Ceutorhynchus napi (Gyllenhal) and C. pallidactylus (Marsham) (Walczak et al. 1998). Examination of foliar glucosinolates indicated no 3-butenyl, 4-pentenyl or 2-phenylethyl glucosinolate in S. alba although these compounds were detected in B. napus (McCloskey and Isman 1993). Shaw et al. (2009) determined that seeds from susceptible and resistant genotypes tested in the current study differed in the amounts of an uncharacterised glucosinolate. Peak height was correlated with weevil infestation scores and, on average, was 3.5 times greater in susceptible than resistant lines (Shaw et al. 2009). Genotype-specific peak heights associated with this compound were also correlated with male and female weevil olfactometer responses in the current study. Based on estimates of uncharacterised glucosinolates from Shaw et al. (2009) by retention time shifts corrected by linear interpolation (as per Gong et al. 2004), the identity of this compound is likely 2-phenylethyl glucosinolate.
Glucosinolate profiles can vary among plant tissues (for example, Porter et al. 1991). However, examination of results from Shaw (2008) indicates that the peak putatively associated with 2-phenylethyl glucosinolate is also present in mature foliage and is consistently greater in susceptible than resistant lines. Another peak corresponding to 3-butenyl glucosinolate was also associated with seeds and mature foliage for all genotypes except S. alba (Shaw 2008). The additive or synergistic effects of 3-butenyl and 2-phenylethyl isothiocyanates on C. obstrictus responses have been demonstrated in olfactometer studies (Bartlet et al. 1993). Smart and Blight (1997) also found that a mixture of 3-butenyl, 4-pentenyl, 2-phenylethyl, and allyl isothiocyanates were highly attractive to C. assimilis in trapping studies. In an examination of naturalized Brassica oleracea L. and Brassica nigra L. populations in England, Moyes and Raybould (2001) found greater C. obstrictus oviposition in plant populations that expressed higher levels of 3-butenyl glucosinolate. Greater seed and foliar expression of 2-phenylethyl and 3-butenyl glucosinolates by susceptible genotypes should contribute to differences in responses of C. obstrictus among susceptible and resistant genotypes detected in the current study.
We propose that the uncharacterised peak associated with cauline leaves and inversely correlated with weevil infestation scores and olfactometer responses was 1-methoxy-3-indolylmethyl glucosinolate. This compound has been shown by other researchers to increase in brassicaceous host plants in response to insect herbivore attack (Birch et al. 1992; Bodnaryk 1992, 1994; Doughty et al. 1995). Local increases in 1-methoxy-3-indolylmethyl glucosinolate concentration occur in B. napus in response to mechanical damage and Phyllotreta cruciferae (Goeze) (Coleoptera; Chrysomelidae) feeding on cotyledons (Bodnaryk 1992), and exogenous application of methyl jasmonate and jasmonic acid (Bodnaryk 1994; Doughty et al. 1995). Birch et al. (1992) reported that 1-methoxy-3-indolylmethyl glucosinolate content in the Brassicaceae they tested (these included a B. napus oilseed variety) increased systemically as much as 17-fold in response to Delia floralis (Fallén) (Diptera: Anthomyiidae) attack; this increase was the greatest of any individual glucosinolate.
Moyes and Raybould (2001) reported that 1-methoxy-3-indolylmethyl glucosinolate was present in all B. oleracea tested but that no relationship was evident between population-wide content of this compound and C. obstrictus oviposition. However, mean constitutive population-wide concentrations of 1-methoxy-3-indolylmethyl glucosinolate were significantly higher at one site than at the four other sites tested by Moyes et al. (2000). Plants from this site supported fewer C. obstrictus larvae than sites characterised by plants with lower levels of this glucosinolate in one study year (Moyes and Raybould 2001). Although Shaw et al. (2009) did not measure concentrations of individual compounds in the plant tissues tested in this study, putative 1-methoxy-3-indolylmethyl glucosinolate HPLC peaks that were, on average 3.5 times greater in resistant than susceptible lines derived from S. alba × B. napus indicate differences comparable to those detected by Moyes et al. (2000); their results indicated as much as 4.8-fold differences in plant population-wide concentrations of 1-methoxy-3-indolylmethyl glucosinolate content between sites.
Hydrolysis products of 1-methoxy-3-indolylmethyl glucosinolate include indole isothiocyanates, indole-cyanides, indolyl-3-carbinol, thiocyanate and possibly phytoalexins and auxins (Mithen 1992; Mewis et al. 2002). Indole isothiocyanates are unstable but the slightly volatile indole-cyanides are relatively stable and prevalent in B. rapa ssp. chinensis cvs. Joi Choi, Black Behi and Bai Tsai (Mewis et al. 2002). Indole glucosinolate hydrolysis products, particularly those of 1-methoxy-3-indolylmethyl glucosinolate, are suspected of contributing to reduced oviposition by the oligophagous butterfly Hellula undalis (Fabricius) (Lepidoptera: Pyralidae) (Mewis et al. 2002). Moreover, increased levels of indole glucosinolates and greater overall glucosinolate levels greatly reduced Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae) feeding on cotyledons of induced plants (Bartlet et al. 1999).
Cook et al. (2006a) found that B. rapa was more attractive to C. obstrictus than a high indolyl/ low alkenyl glucosinolate B. napus genotype, and in olfactometer trials responses to a high alkenyl-low indolyl genotype were similar to those of B. rapa. However, given the slight volatility of indole-cyanides, their role in long-distance olfactory responses of C. obstrictus seems unlikely. Although their effects in this study cannot be discounted, behavioural responses of weevils to 1-methoxy-3-indolylmethyl glucosinolate hydrolysis products are likely limited to more intimate ranges. Responses of C. obstrictus to volatile hydrolysis products of indolyl glucosinolates require more rigorous testing.
Responses of both male and female weevils to flowering racemes were greater than to cauline leaves. In addition to the glucosinolates associated with cauline leaves, flowering racemes also exude compounds such as (E,E,)-α-farnesene (Evans and Allen-Williams 1992); (E,E,)-α-farnesene is a major component of B. napus floral odour (Blight et al. 1995) but is exuded in much smaller amounts from S. alba (Tollsten and Bergstrom 1988). Evans and Allen-Williams (1992) found that it was the only volatile compound they detected from B. napus flowers that elicited strong electroantennogram responses from C. obstrictus at relatively low doses. Attractive glucosinolate hydrolysis products and (E,E,)-α-farnesene have also been suspected to have a synergistic attractive effect on C. obstrictus behaviour (Evans and Allen-Williams 1992, 1998). Omitting α-farnesene from artificial rape odour reduced C. obstrictus electroantennogram responses and attractiveness of odours in wind tunnel tests (Evans and Allen-Williams 1992, 1998).
Responses of both males and females were greater to fresh (macerated or excised) cauline leaf material than to freeze-dried and reconstituted material. Maceration, excision and processing cauline leaves into powder would damage tissues and allow release of glucosinolate hydrolysis products. Differences between preparation techniques are also likely associated with the presentation of lesser amounts of freeze-dried material. Although a significant effect of heat treatment after freeze-drying was not detected, samples without heat treatment elicited different behavioural responses and tissue from susceptible genotypes was more attractive. These effects were not seen with heat-treated samples. These results support the conclusion that differences in C. obstrictus responses to resistant and susceptible genotypes are associated with hydrolysis products of glucosinolates acting as attractive kairomones (as per McCaffrey et al. 1999). However, other enzymes and plant compounds may have also been influenced by heat treatment and affected weevil responses.
Males respond more strongly to macerated than intact cauline leaf material. Females respond similarly to these odour sources. The green leaf volatiles cis-3-hexen-l-ol and cis-3-hexenyl acetate have been detected from B. napus headspace and male weevils are more sensitive to these compounds than females (Evans and Allen-Williams 1992). These compounds comprise 90% of the odour from macerated B. napus leaves but less than 15% of crop odour (Evans and Allen-Williams 1992) and likely influenced male response in the current study.
Female response to flowering racemes from susceptible genotypes was greater in June than in July. Similar responses were detected in June and August. Bartlet et al. (1993) found that pre-diapause weevils (corresponding to August/new generation weevils in this study) were unresponsive to floral odours if they had been field-collected but responsive if reared from pods. Bartlet et al. (1993) suggested that the reduced response in field-collected specimens was associated with satiation of weevils that had fed in preparation for diapause. Responses of August females to host plant odours in this study suggest that these C. obstrictus had not yet completed pre-diapause feeding.
Differential responsiveness to olfactory cues may also be subject to other mechanisms. Weevils of both generations were held at 12:12 (L:D). Circadian rhythms influence Drosophila melanogaster L. (Diptera: Drosophilidae) juvenile hormone levels and responses to food odours (Krishnan et al. 1999). Acclimation of C. obstrictus to 12:12 (L:D) from the ambient 16:8 (L:D) photoperiod at time of capture may influence juvenile hormone levels and reduced receptiveness of overwintering generation weevils to olfactory cues. This hypothesis requires testing. In addition, reduced chemoreceptor sensitivity in insects is well documented and influenced by age; sensory input to the central nervous system may decrease as sensillae become inoperative (Schoonhoven 1969; Blaney et al. 1986). For instance, the sensitivity of boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae), chemoreceptors is greatest in the period coinciding with mating and host location (Dickens and Moorman 1990).
It should be noted that all weevils tested were collected and maintained on B. napus. The feeding experience of Lepidoptera larvae can influence food preference (Jermy et al. 1968). For example, orientation responses of third instar Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) to food odours increased following experience of the odour (Carlsson et al. 1999). However, unlike relatively sedentary Lepidoptera larvae, C. obstrictus is highly mobile and known to feed on a number of host plants, particularly shortly after emergence from overwintering. These include wild mustard (Sinapis arvensis L.), hoary cress (Lepidium draba L.), field pennycress (Thlaspi arvense L.), flixweed (Descurania sophia (L.) Webb), shepherd’s purse (Capsella bursa-pastoris (L.) Medik.), radish (Raphanus spp.) and volunteer canola (B. napus and B. rapa) (Dmoch 1965; Fox and Dosdall 2003; Dosdall and Moisey 2004). The influence of previous experiences of weevil adults and larvae on host selection is unknown and suggests a course for further study.
It should also be noted that, as these tests assessed the responses of mixed-sex groups of weevils, the possibility of interactions of host plant odours and conspecific cues exists. Spring generation female C. obstrictus have been reported to be attractive to conspecific males and females (Evans and Bergeron 1994). Interactions of generation and genotype were not detected in the current study. However, the possibility that these cues influenced results cannot be discounted.
Results of this study offer insights into the differences in susceptibilities of this novel canola germplasm and suggest potential strategies for resistance breeding. Restraining expression of attractive volatile cues associated with hydrolysis products of 3-butenyl and 2-phenylethyl glucosinolates and/or encouraging production of indole glucosinolates may facilitate production of canola genotypes that are less attractive to C. obstrictus. Deployment of less attractive germplasm coupled with a highly attractive trap crop such a B. rapa may prove to be an effective strategy for concentrating and controlling weevil populations (Cook et al. 2006a; Cárcamo et al. 2007). Examination of the responses of these weevils to current commercial cultivars that express varying proportions of alkenyl and indolyl glucosinolates is required and may allow this strategy to be used effectively with existing germplasm. Although olfactory cues are clearly important to C. obstrictus host location, it should be noted that visual cues are also essential to this interaction. Smart et al. (1997) found that traps baited with a mixture of allyl, 3-butenyl, 4-pentenyl and 2-phenylethyl isothiocyanate were more attractive if held vertically than at a 45° angle; traps baited with isothiocyanates alone were not attractive.
References
Alford DV, Nilsson C, Ulber B (2003) Insect pests of oilseed rape crops. In: Alford DV (ed) Biocontrol of oilseed rape pests. Blackwell, Oxford, pp 9–41
Andréasson E, Jorgensen LB, Hoglund AS, Rask L, Meijer J (2001) Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol 127:1750–1763
Bartlet E, Blight MM, Hick AJ, Williams IH (1993) The responses of the cabbage seed weevil (Ceutorhynchus assimilis) to the odour of oilseed rape (Brassica napus) and some volatile isothiocyanates. Entomol Exp Appl 68:295–302
Bartlet E, Blight MM, Lank P, Williams IH (1997) The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer. Entomol Exp Appl 85:257–262
Bartlet E, Kiddle G, Williams I, Wallsgrove R (1999) Wound induced increases in the glucosinolate content of oilseed rape and their effect on subsequent herbivory by a crucifer specialist. Entomol Exp Appl 91:163–167
Birch ANE, Griffiths DW, Hopkins RJ, Macfarlane Smith WH, McKinlay RG (1992) Glucosinolate responses of swede, kale, forage and oilseed rape to root damage by turnip root fly (Delia floralis) larvae. J Sci Food Agric 60:1–9
Blaney WM, Schoonhoven LM, Simmonds MSJ (1986) Sensitivity variations in insect chemoreceptors: a review. Cell Mol Life Sci 42:13–19
Blight MM, Pickett JA, Wadhams LJ, Woodcock CM (1995) Antennal perception of oilseed rape Brassica napus (Brassicaceae) volatiles by the cabbage seed weevil, Ceutorhynchus assimilis (Coleoptera: Curculionidae). J Chem Ecol 21:1649–1664
Bodnaryk RP (1992) Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochem 31:2671–2677
Bodnaryk RP (1994) Potent effect of jasmonates on indole glucosinolates in oilseed rape and mustard. Phytochem 35:301–305
Bonnemaison L (1957) Le charançon des siliques (Ceuthorhynchus assimilis Payk.), biologie et méthodes de lutte. Ann Épiphytes 4:387–543
Boodly JW, Sheldrake R (1982) Cornell peat-lite mixes for commercial plant growing. New York State College of Agriculture and Life Sciences, Cornell University, Ithaca
Buntin GD, McCaffrey JP, Raymer PL, Romero J (1995) Quality and germination of rapeseed and canola seed damaged by adult cabbage seedpod weevil, Ceutorhynchus assimilis (Paykull) [Coleoptera: Curculionidae]. Can J Plant Sci 75:539–541
Cárcamo HA, Dunn R, Dosdall LM, Olfert O (2007) Managing cabbage seedpod weevil in canola using a trap crop—a commercial field-scale study in western Canada. Crop Prot 26:1325–1334
Carlsson MA, Anderson P, Hartlieb E, Hansson BS (1999) Experience-dependent modification of orientational response to olfactory cues in larvae of Spodoptera littoralis. J Chem Ecol 25:2445–2454
Cole RA (1976) Isothiocyanates, nitriles, and thiocyanates as products of autolysis of glucosinolates in Cruciferae. Phytochem 15:759–762
Cook SM, Smart LE, Martin JL, Murray DA, Watts NP, Williams IH (2006a) Exploitation of host plant preferences in pest management strategies for oilseed rape (Brassica napus). Entomol Exp Appl 119:221–229
Cook SM, Watts NP, Castle LM, Williams IH (2006b) Determining the sex of insect pests of oilseed rape for behavioural bioassays. IOBC/WPRS Bull 29:207–213
Dickens JC, Moorman EE (1990) Maturation and maintenance of electroantennogram responses to pheromone and host odors in boll weevils fed their host plant or an artificial diet. Z Angew Entomol 109:470–480
Dmoch J (1965) The dynamics of a population of the cabbage seedpod weevil (Ceutorhynchus assimilis Payk.) and the development of winter rape. Part I. Ekologia Polska Ser A 13:249–287
Dosdall LM, Kott LS (2006) Introgression of resistance to cabbage seedpod weevil to canola from yellow mustard. Crop Sci 46:2437–2445
Dosdall LM, Moisey DWA (2004) Developmental biology of the cabbage seedpod weevil, Ceutorhynchus obstrictus (Coleoptera: Curculionidae), in spring canola, Brassica napus, in western Canada. Ann Entomol Soc Am 97:458–465
Dosdall LM, Moisey D, Cárcamo H, Dunn R (2001) Cabbage seedpod weevil factsheet. Alberta Agric Food Rural Dev Agdex 4:622–624
Doughty KJ, Kiddle GA, Pye BJ, Wallsgrove RM, Pickett JA (1995) Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry 38:347–350
Evans KA, Allen-Williams LJ (1992) Electroantennogram responses of the cabbage seed weevil, Ceutorhynchus assimilis, to oilseed rape, Brassica napus ssp. oleifera, volatiles. J Chem Ecol 18:1641–1659
Evans KA, Allen-Williams LJ (1993) Distant olfactory response of the cabbage seed weevil, Ceutorhynchus assimilis, to oilseed rape odour in the field. Physiol Entomol 18:251–256
Evans KA, Allen-Williams LJ (1998) Response of cabbage seed weevil (Ceutorhynchus assimilis) to baits of extracted and synthetic host-plant odor. J Chem Ecol 24:2101–2114
Evans KA, Bergeron J (1994) Behavioral and electrophysiological response of cabbage seed weevils (Ceutorhynchus assimilis) to conspecific odor. J Chem Ecol 20:979–989
Feeny PP (1976) Plant apparency and chemical defence. Recent Adv Phytochem 10:1–40
Finch S (1978) Volatile plant chemicals and their effect on host plant finding by the cabbage root fly (Delia brassicae). Entomol Exp Appl 24:150–159
Fox AS, Dosdall LM (2003) Reproductive biology of Ceutorhynchus obstrictus (Coleoptera: Curculionidae) on wild and cultivated Brassicaceae in southern Alberta. J Entomol Sci 38:365–376
Free JB, Williams IH (1978) The responses of the pollen beetle, Meligethes aeneus, and the seed weevil, Ceutorhynchus assimilis, to oilseed rape, Brassica napus, and other plants. J Appl Ecol 15:761–764
Gong F, Lian Y, Fung Y, Chau F (2004) Correction of retention time shifts for chromatographic fingerprints of herbal medicine. J Chromatogr A 1029:173–183
Harper FR, Berkenkamp B (1975) Revised growth-stage key for Brassica campestris and B. napus. Can J Plant Sci 55:657–658
Hill DS (1987) Agricultural insect pests of temperate regions and their control. Cambridge University Press, Cambridge
Jermy T, Hanson FE, Dethier VG (1968) Induction of specific food preference in lepidopterous larvae. Entomol Exp Appl 11:211–230
Kalischuk AR, Dosdall LM (2004) Susceptibilities of seven Brassicaceae species to infestation by the cabbage seedpod weevil (Coleoptera: Curculionidae). Can Entomol 136:265–276
Krishnan B, Dryer SE, Hardin PE (1999) Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400:375–378
Lee KC, Cheuk WM, Chan W, Lee AWM, Zhao ZZ, Jiang ZH, Cai Z (2006) Determination of glucosinolates in traditional Chinese herbs by high-performance liquid chromatography and electrospray ionization mass spectrometry. Anal Bioanal Chem 386:2225–2232
McCaffrey JP (1992) Review of the U.S. canola pest complex: cabbage seedpod weevil. In: Proceedings, 1992 U.S. Canola conference, 5–6 March 1992. American Pedigreed Seed Company, Memphis, TN, pp 140–143
McCaffrey JP, O’Keefe LE, Homan HW (1986) Cabbage seedpod weevil control in winter rapeseed. University of Idaho, College of Agriculture, Cooperative Extension Service, Agricultural Experiment Station, CIC Series No. 782
McCaffrey JP, Harmon BL, Brown J, Brown AP, Davis JB (1999) Assessment of Sinapis alba, Brassica napus and S. alba × B. napus hybrids for resistance to cabbage seedpod weevil, Ceutorhynchus assimilis (Coleoptera: Curculionidae). J Ag Sci 132:289–295
McCloskey C, Isman MB (1993) Influence of foliar glucosinolates in oilseed rape and mustard on feeding and growth of the bertha armyworm, Mamestra configurata Walker. J Chem Ecol 19:249–266
Mewis I, Ulrichs C, Schnitzler WH (2002) Possible role of glucosinolates and their hydrolysis products in oviposition and host-plant finding by cabbage webworm, Hellula undalis. Entomol Exp Appl 105:129–139
Mithen R (1992) Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 63:71–83
Moyes CL, Raybould AF (2001) The role of spatial scale and intraspecific variation in secondary chemistry in host-plant location by Ceutorhynchus assimilis (Coleoptera: Curculionidae). Proc R Soc Lond 268:1567–1573
Moyes CL, Collin HA, Britton G, Raybould AF (2000) Glucosinolates and differential herbivory in wild populations of Brassica oleracea. J Chem Ecol 26:2625–2641
Porter AJR, Morton AM, Kiddle G, Doughty KJ, Wallsgrove RM (1991) Variation in the glucosinolate content of oilseed rape (Brassica napus L.) leaves. I. Effect of leaf age and position. Ann Appl Biol 118:461–467
Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J (2000) Myrosinase: gene family evolution and herbivore defence in Brassicaceae. Plant Mol Biol 42:93–113
Rochfort SJ, Trenerry VC, Imsic M, Panozzo J, Jones R (2008) Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochem 69:1671–1679
Ross D, Brown J, McCaffrey J, Davis JB (2006) Cabbage seedpod weevil resistance in canola (Brassica napus L.), yellow mustard (Sinapis alba L.) and canola × yellow mustard hybrids. In: Proceedings of American Society Agronomy-Crop Science Society of America—Soil Science Society of America International Annual Meetings, Indianapolis. November 12–16, 2006
SAS Institute (2005) SAS, version 9.1. SAS Institute, Cary
Schoonhoven LM (1969) Sensitivity changes in some insect chemoreceptors and their effect on food selection behavior. Proc Sec Sci K Akad v Wetensch te Amst C 72:491–498
Shaw E (2008) The detection of biochemical markers for cabbage seedpod weevil (Ceutorhynchus obstrictus) resistance in Brassica napus L. × Sinapis alba L. Germplasm. M.Sc. thesis, University of Guelph, Guelph, 155 pp
Shaw EJ, Fletcher RS, Dosdall LL, Kott LS (2009) Biochemical markers for cabbage seedpod weevil (Ceutorhynchus obstrictus (Marsham)) resistance in canola (Brassica napus L.). Euphytica 170:297–308
Smart LE, Blight MM (1997) Field discrimination of oilseed rape, Brassica napus volatiles by cabbage seed weevil, Ceutorhynchus assimilis. J Chem Ecol 23:2555–2567
Smart LE, Blight MM, Hick AJ (1997) The effect of visual cues and a mixture of isothiocyanates on trap capture of cabbage seed weevil, Ceutorhynchus assimilis. J Chem Ecol 23:889–902
Tansey JA (2009) Mechanisms of cabbage seedpod weevil, Ceutorhynchus obstrictus, resistance associated with novel germplasm derived from Sinapis alba × Brassica napus. PhD thesis, University of Alberta, Edmonton, 325 pp
Tansey JA, Dosdall LM, Keddie BA, Noble SD (2009) Contributions of visual cues to cabbage seedpod weevil, Ceutorhynchus obstrictus (Marsham) (Coleoptera: Curculionidae), resistance in novel host genotypes. Crop Prot. doi:10.1016/j.cropro.2009.11.005
Tollsten L, Bergstrom G (1988) Headspace volatiles of whole plant and macerated plant parts of Brassica and Sinapis. Phytochemistry 27:4013–4018
Ulmer BJ, Dosdall LM (2006) Spring emergence biology of the cabbage seedpod weevil (Coleoptera: Curculionidae). J Appl Entomol 99:64–69
Visser JH (1986) Host odor perception in phytophagous insects. Ann Rev Entomol 31:121–144
Walczak B, Kelm M, Klukowski Z, Smart LE, Ferguson AW, Williams IH (1998) The effect of trap design and 2-phenylethyl isothiocyanate on catches of stem weevils (Ceutorhynchus pallidactylus Marsh and C. napi Gyll.) in winter oilseed rape. OILB WPRS Bull 21:141–146
Acknowledgments
We are most grateful to Ross Adams, Jordana Hudak, Mike Gretzinger, Analea Mauro and Christina Gretzinger for capable technical assistance and to Dr. Héctor Cárcamo of Agriculture and Agri-Food Canada, Lethbridge Research Centre for access to facilities. We would also like to thank Drs. Bob Lamb and Maya Evenden for invaluable comments on this manuscript. Funding for this project was provided by the Canola Council of Canada, University of Alberta and grants to LMD from the Natural Sciences and Engineering Research Council of Canada and the Alberta Agricultural Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sam Cook.
Rights and permissions
About this article
Cite this article
Tansey, J.A., Dosdall, L.M., Keddie, A. et al. Responses of Ceutorhynchus obstrictus (Marsham) (Coleoptera: Curculionidae) to olfactory cues associated with novel genotypes developed by Sinapis alba L. × Brassica napus L.. Arthropod-Plant Interactions 4, 95–106 (2010). https://doi.org/10.1007/s11829-010-9087-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-010-9087-2