Abstract
The annual bluegrass weevil (ABW), Listronotus maculicollis Kirby, is an economically important pest of short cut turfgrass. Annual bluegrass, Poa annua L., is the most preferred and suitable host for ABW oviposition, larval survival and development. We investigated the involvement of grass volatiles in ABW host plant preference under laboratory and field conditions. First, ovipositional and feeding preferences of ABW adults were studied in a sensory deprivation experiment. Clear evidence of involvement of olfaction in host recognition by ABW was demonstrated. Poa annua was preferred for oviposition over three bentgrasses, Agrostis spp., but weevils with blocked antennae did not exhibit significant preferences. ABW behavioral responses to volatiles emitted by Agrostis spp. and P. annua were examined in Y-tube olfactometer assays. Poa annua was attractive to ABW females and preferred to Agrostis spp. cultivars in Y-tube assays. Headspace volatiles emitted by P. annua and four cultivars of Agrostis stolonifera L. and two each of A. capillaris L. and A. canina L. were extracted, identified and compared. No P. annua specific volatiles were found, but Agrostis spp. tended to have larger quantities of terpenoids than P. annua. (Z)-3-hexenyl acetate, phenyl ethyl alcohol and their combination were the most attractive compounds to ABW females in laboratory Y-tube assays. The combination of these compounds as a trap bait in field experiments attracted adults during the spring migration, but was ineffective once the adults were on the short-mown turfgrass. Hence, their usefulness for monitoring weevil populations needs further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The annual bluegrass weevil (ABW), Listronotus maculicollis Kirby, is a highly destructive and expanding pest of short-mown golf turf in the Northeast and Mid-Atlantic regions of the United States and in southeastern Canada (Vittum 2012). Overuse of synthetic insecticides for ABW management due to the lack of effective management alternatives combined with high turfgrass quality expectations has led to widespread development of resistance to pyrethroids (Ramoutar et al. 2009; Kostromytska et al. 2018) and insecticides from several other chemical classes (Koppenhöfer et al. 2012; Kostromytska et al. 2018; McGraw and Koppenhöfer 2017). Reducing the use of and reliance on chemical control is crucial for the development of more sustainable approaches for ABW management. Cost-effective, reliable, and consistent methods for monitoring ABW are critical to estimate timing and densities of populations thereupon to base management activities. However, presently available ABW monitoring methods are ineffective (cutting out turf pieces with a knife) or impractical and/or labor intensive (salt- or heat extraction of ABW stages from turf cores, vacuum sampling) (McGraw and Koppenhöfer 2009) and hence rarely used.
Semiochemical attractants (plant volatiles, pheromones) are widely used for monitoring of many insect pests. Various weevil species have been shown to respond to host-plant volatiles (e.g., Szendrei et al. 2009). McGraw et al. (2011) suggested that volatiles emitted by the preferred host, annual bluegrass (Poa annua L.), might play a role in ABW host recognition, but it remains unknown whether specific compounds are strong enough attractants to be used as a monitoring tool. Host plant volatiles in many cases synergize with pheromones, and they are used together as powerful lures (Borden et al. 2008; Gries et al. 1994; Saïd et al. 2011).
ABW is well adapted to the golf course environment. The adults overwinter off the short-mown turf in sheltered areas of the golf course (Diaz and Peck 2007; OSK, pers. observation). In early spring, they migrate back on to the short-mown areas to feed, mate, and oviposit (McGraw and Koppenhöfer 2007; Vittum 2012). Larvae initially feed inside the grass stem, older larvae (fourth and fifth instars) feed externally on the grass crowns. Pupation occurs near the soil surface, and new adults appear on the turf surface in late spring to early summer. ABW goes through 2–3 generations per year.
Most damage caused by ABW larvae is observed in areas with a high percentage of P. annua. Even though ABW populations have been observed in pure creeping bentgrass, Agrostis stolonifera L. (McGraw and Koppenhöfer 2017; AMK pers. observations), damage in bentgrass is usually less severe (Kostromytska and Koppenhöfer 2014, 2016). In a series of laboratory, greenhouse and field experiments it was clearly demonstrated that P. annua is also a superior host for ABW (Kostromytska and Koppenhöfer 2014) compared to bentgrasses. ABW females prefer P. annua for oviposition to any out of three bentgrass species (A. stolonifera; colonial bentgrass, A. capillaris L.; and velvet bentgrass, A. canina L.) in choice or no-choice tests, although no distinct preferences in adults feeding were observed. Poa annua is the most suitable host for larval survival, growth and development. Studies to elucidate the mechanisms of bentgrass resistance suggested that silicon and fiber contents in grass stem and leaf tissue cannot explain the observed grass resistance (Kostromytska and Koppenhöfer 2016).
McGraw et al. (2011) demonstrated a clear attraction of ABW females to volatiles emitted by intact P. annua. The volatiles emitted by intact plants and clippings were extracted, identified and compared. According to this study, volatiles (+)-β-pinene, (E)-2-hexenal, 6-methyl-5-hepten-2-one, (Z)-3-hexenyl acetate, decanal, hexanal, nonanal, octanal, and phenyl ethyl alcohol elicited significant electrophysiological responses in ABW adults, but the behavioral significance of the observed EAG responses to these compounds was not studied. However, understanding the behavioral responses to these compounds may help with the development of improved monitoring methods, traps and lures, to overcome the limitations of the existing methods. In addition, comparing volatiles produced by P. annua (suitable host) with the volatiles produced by bentgrasses (inferior hosts) may help to pinpoint attractants and elucidate the mechanisms involved in the increased bentgrass resistance. This knowledge is critical for the implementation of host plant resistance in ABW management and for the development of new management strategies.
Our ultimate goal is to identify attractants for the development of effective traps and lures in ABW monitoring and management. The current study investigated the involvement of host plant volatiles in ABW host plant preference and focuses on ABW olfactory responses to different grass species. In particular, it investigated 1) the role of olfaction in ABW host recognition in sensory deprivation and Y-tube assays; 2) the behavioral significance of volatiles emitted by P. annua extracted and identified in McGraw et al. (2011) and our studies in laboratory and field settings; and 3) the extraction, identification and comparison of headspace volatiles from bentgrasses and P. annua.
Methods and Materials
Grass Propagation
Cultivars of three bentgrass species, A. stolonifera (cvs ‘L93’ and ‘Penncross’, older widely known cultivars; ‘007’ and ‘Declaration’, newer high quality cultivars), A. capillaris (cv ‘Capri’), and A. canina (cvs ‘Greenwich’ and ‘Villa’), and wild type P. annua were used in our experiment.
Bentgrasses were seeded at rates recommended by Seed Research of Oregon (2008) (A. stolonifera, 6.25 g m−2; A. capillaris, 8.75 g m−2; A. canina, 4.75 g m−2). They were propagated in the greenhouse for 2 months prior to experiments (14 h light at 28 °C: 10 h dark at 18 °C; natural light supplemented with 400 watt high pressure sodium lamps). Poa annua plugs (10 cm diameter) were taken using a golf hole cup cutter from uniform established fields at Rutgers Horticultural Farm 2 (North Brunswick, NJ, USA) and their roots washed free of soil before planting. Seeded grass and plugs were grown in 540-ml deli-cups (Fabri-Kal®, Kalamazoo, MI, USA) with drainage holes, filled with a mixture of a pasteurized (3 h at 72 °C) sandy loam (61% sand, 27% silt, 12% clay; 2.3% organic matter, pH 5.9) and sand (3:1 ratio). Grasses were fertilized weekly (20–20-20 NPK, The Scotts Miracle-Gro Co., Marysville, OH, USA), watered as necessary and clipped 2×/week (1.3 cm height).
Insects
Overwintering generation adults were collected from overwintering sites at Pine Brook Golf Course (Manalapan, NJ, USA). They were sexed and kept in 840 ml plastic containers filled with moist (5% w/w) sand for 2–6 months in an incubator simulating overwintering conditions (10 h light at 6 °C: 14 h dark at 4 °C) to break their reproductive diapause (Wu et al. 2017). Prior to bioassays, adults were extracted and kept in groups of 60 in ventilated clear plastic containers on moist sand with black cutworm, Agrotis ipsilon (Hufnagel), diet (Bio-Serv, Frenchtown, NJ, USA) and organic wheat sprouts as food in an incubator simulating spring conditions (14 h light at 21 °C: 10 h dark at 14 °C) for 14 days to allow them to sexually mature after diapause termination (Wu et al. 2017).
Spring generation adults were handpicked from P. annua fields (mowing height 2.5 mm) at Rutgers Horticultural Farm 2 in late June. Adults were held in plastic containers with moist sand and food (14 h light at 21 °C: 10 h dark at 14 °C) for at least 1 week prior to the experiments.
Sensory Deprivation Experiment
To determine the role of host-plant chemical cues on ABW oviposition preference, a choice experiment with blocked antennae was conducted. In each experimental run, ABW adults were given a choice between three different grass species in two arrangements: 1) one core each of P. annua, cvs. ‘L-93’, ‘007’ (both A. stolonifera), and ‘Greenwich’ (A. canina) (set I), and 2) one core each of P. annua, cvs. ‘Penncross’, ‘Declaration’ (both A. stolonifera), and cv ‘Capri’ (A. capillaris) (set II). To create an oviposition arena, one turf core (4.5 cm diameter, 7.5 cm depth) of each of the specified greenhouse-grown grasses was fit into a 120-ml plastic vial and placed on the corner of a central quadrate (10 × 10 cm) in a rectangular clear plastic container (36 × 27 × 16 cm). Pasteurized sandy loam (61% sand, 27% silt, 12% clay; 2.3% organic matter, pH 5.9) amended with 10% (vol/vol) peat moss was added to the level of the core’s soil surface.
In each experimental replication, three different weevil treatments for each previously described set were arranged: a) sensory deprived weevils, b) weevils with painted elytra, and c) unmodified control weevils. To create sensory deprived weevils, the adults’ antennae were painted with Testors® modeling enamel (#1150 flat red rouge mat) (The Testor Corp., Rockford, IL, USA) using a number 0 artist’s brush under a dissection microscope (Pureswaran and Poland 2009). To ensure that the paint itself did not affect the weevil’s oviposition behavior, a similar amount of enamel was applied on the elytra of adults in the painted control (treatment “b” above). For each treatment, ten spring generation male–female pairs were introduced centrally on the soil surface of the arena. Arenas were held in environmental chambers for 1 week at L14 (21 °C): D10 (14 °C). Plugs were then removed from the arenas and examined under a compound microscope. The number of eggs per plug was counted. In addition, we counted the number of feeding scars to determine host-plant effects on weevil feeding behavior. For oviposition, females chew round notches in the grass stems before oviposition and then deposit eggs in the opening (Kostromytska and Koppenhöfer 2014). Often females create oviposition probes but do not deposit eggs in them. Feeding scars differ from oviposition probes by their irregular shape and location on the top part of leaf blades. A feeding score was determined on a 0 to 9 scale that took into account the number and size of feeding scars, with 0 being assigned when no feeding was observed and 9 if more than 135 feeding scars were found (Kostromytska and Koppenhöfer 2014).
Y-Tube Olfactometer Assays: Growing Plants
The olfactory responses of spring generation ABW adults to six bentgrass cultivars (‘L-93’, ‘Penncross’, ‘Declaration’, ‘007’, ‘Capri’ and ‘Villa’) and P. annua were tested using a Y-tube olfactometer (10-cm arms and 14-cm stem, 2 cm cross section, Analytical Research Systems Inc., Gainesville, FL, USA). Air filtered through activated charcoal was split into two 1.57 L/min air streams and delivered through the 10-cm arm via an odor source in a glass chamber. Odor sources consisted of a turf core (4.5 cm diam × 7.5 cm depth including soil/root zone) taken from greenhouse-grown grass or a soil core without grass as a negative control placed in a 500 ml flask (Kimax) and connected to the olfactometer odor adapters. Adults were confined individually in the bottom of the Y-tube in a glass inlet fit with a screen and observed for 20 min. A positive response was recorded if weevils moved to the end of an arm of the olfactometer (8-cm mark) and remained in the selected arm for at least 30 s. A bioassay was terminated if a choice was recorded. Weevils failing to make a decision were recorded as non-responding. Individuals were used only once. The experiment was conducted until 35 responding weevils of each sex were observed per treatment.
Between replicates, the arms holding the odor source were switched to exclude directional bias. Before each bioassay, purified air was passed through the Y-tube for 10 min to purge the system. For each assay, fresh odor sources were used for no longer than 30 min. After each observation, the Y-tube and all glass connections (with the exception of the odor sources) were rinsed with ethanol, then acetone, and oven dried (80 °C, 5 min) to avoid contamination. Assays were performed at 25 ± 2 °C and 260 lx. Two sets of experiments were conducted: in the first set, adults were given a choice between one of the grasses and the control; in the second set, adults were given a choice between one of the bentgrasses and P. annua.
Volatile Collection and Identification
Headspace volatiles of six bentgrass cultivars (‘L-93’, ‘Penncross’, ‘Declaration’, ‘OO7’, ‘Capri’ and ‘Villa’) and P. annua were collected using a push–pull volatile collection system (Rodriguez-Saona et al. 2009; Tholl and Röse 2006) arranged in a greenhouse (average temperature 22 °C day, 9 °C night). Each pot with dense grass cover (86.6 cm2) was covered by a 4-L glass chamber (Analytical Research Systems, Inc.). In each collection run, three different bentgrass cultivars and P. annua were used, grasses were cut 24 h prior to the start of volatile collection. Clean air passed through each chamber at 2 L min−1 and pulled at 1 L min−1 through a filter trap containing 30 mg of a Super-Q adsorbent (Analytical Research Systems, Inc.). Volatiles were collected for 72 h.
Filter traps were eluted with 150 μl of dichloromethane. An internal standard of 400 ng of n-octane was added to the extract. To quantify volatiles, a 1-μl aliquot of each extract was injected in splitless mode onto a Hewlett Packard 6890 GC equipped with a 10 m × 0.53 mm × 2.65 μm HP-1 column (Agilent Technologies) and a flame ionization detector (FID) as described in Rodriguez-Saona et al. (2009, 2011). Helium was the carrier gas at a 5 mL min−1 flow rate. The temperature program began at 40 °C for 1 min and ramped at 14 °C min−1 up to 180 °C, where it was held for 2 min, then ramped again at 40 °C min−1 up to 200 °C, where it was held for 2 min. The approximate total amount of each compound in each sample was calculated by relating its peak area to that of the internal standard. Volatile emissions were calculated in ng h−1. The identity of compounds detected was determined by GC-MS (Instruments 7890 and 5975 C; Agilent, Palo Alto, CA, USA) in both EI and CI (isobutane) modes by using a 30 m × 0.25 mm i.d. × 0.25 μm film thickness medium polar DB35 (35%-Phenyl-methylpolysiloxane) and non-polar DB-1 (100% Dimethylpolysiloxane) capillary column (Agilent). Samples were introduced with cold on-column injection into a 1 m deactivated fused silica retention gap between injector and analytical column. For both columns, the oven temperature was kept at 30 °C for 2.5 min after injection and then temperature programmed 20 °C min−1 to 90 °C and then 2 °C min−1 to 125 °C followed by 20 °C min−1 to 240 °C. The carrier gas flow rate was 30 cm sec−1 (constant flow), and the MS transfer line temperature was 250 °C. The ion source temperature was 220 °C in EI mode and 250 °C in CI mode. Volatile identity was confirmed by analyzing synthetic standards, when available, as well as mass spectral data libraries (NIST 11, National Institute of Standards and Technology), Adams2 (Allured Publishing Corporation), and a floral scent library (Department of Chemical Ecology, University of Göteborg, Sweden).
Y-Tube Olfactometer Assays: Individual Volatiles
Poa annua-emitted volatiles described by McGraw et al. (2011) and several of the volatiles extracted and identified in the current study (Table 1) were tested to determine their potential as ABW attractants using the same method as in the Y-tube experiment with different grasses. The following compounds were tested in the assay: (E)-2-hexenal (95% purity, SAFC, Sigma-Aldrich Corp., St. Louis, MO, USA), (+)-β-Pinene (98.5% purity, Sigma Aldrich, Buch, Switzerland); 6-Methyl-5-hepten-2-one (98% purity, Fluka, Buch, Switzerland); phenyl ethyl alcohol (99% purity SAFC, Sigma-Aldrich Corp., St. Louis, MO, USA), (Z)-3-hexenyl acetate (98% purity, Aldrich Chemical company, Milwaukee, WI, USA); octanal (92% purity, Aldrich Chemical company, Milwaukee, WI, USA), limonene (96% purity, Acros Organics, Thermo Fisher Scientific, New Jersey, USA), nonanal (95% purity, Fluka), decanal (98%, Sigma-Aldrich Corp., St. Louis, MO, USA), and linalool (97%, Sigma-Aldrich Corp., St. Louis, MO, USA). Four concentrations (0.02, 0.2, 2, and 20 mg) of each compound were diluted in hexane before testing in Y-tube bioassays. 10 μL of each dilution was applied on a filter paper strip (1 × 10 cm) that served as odor source. Filter papers were air dried for 10 min before using in the experiment to allow the solvent to evaporate. Each dilution series was tested against the control (filter paper with hexane only). The experiment was conducted until 30 responding weevils of each sex were observed per treatment. Insects were used in the tests only once.
Further on, chemical blends of the most attractive volatiles determined above were tested for their effect on weevil behavior in the same Y-tube assay set up. Chemicals were diluted in hexane in equal proportions, so the total concentration of compounds was 0.02 mg.
Field Testing of Host-Plant Attractants
The combination of the two most attractive compounds, (Z)-3-hexenyl acetate and phenyl ethyl alcohol, was field-tested during spring migration of the overwintering generation adults (March – May 2012) and when the spring generation adults became active (June – July 2012). In spring, two trap designs [linear pitfall (custom made) and dome trap (ISCA Technologies, Inc., Riverside, CA)] were used. Linear pitfall traps were constructed from 1 m PVC pipe sections (10 cm diam) with a longitudinal slit. One side of the section were capped and the other was connected to the receptacle (840 ml cup with pasteurized sand). Traps were placed in the taller rough grass between the fairway edge about 3 m from a tree line and about 1–2 m from the fairway edge with about 10 m between traps. Trapping was conducted at Pine Brook Golf Course and Bel-Aire Golf Course (Wall Township, NJ). The combination of these two compounds was distributed in 2-ml plastic vials which served as lures. Vials were placed in lure receptacles (ISCA Technologies, Inc., Riverside, CA) that were placed on the wires hanging above the midsection of linear pitfall traps and at the receptacle of the dome trap. Six blocks were arranged and each block had one baited and one non-baited trap of each design (N = 6 traps per treatment for each design). Traps were checked weekly and the bait assignments were randomly changed to avoid bias. At each collection date, six 10-s vacuum samples were taken from each block to assure that high enough adult population densities were present at given locations. Samples were examined immediately after taking them, and ABW adults were counted and returned to the sampled area.
Because the dome traps were ineffective in the spring, we used only pitfall traps in the following early summer experiment. The traps were placed into the rough at the edge of two different fairways with a history of high ABW population at Pine Brook GC. Twelve blocks were arranged with four traps per block (two baited and two non-baited) (N = 24 traps per treatment). Treatments were assigned randomly within block and changed weekly to avoid bias.
Statistical Analysis
To determine the significance of the ABW preferences in the deprivation oviposition and Y-tube experiments, two tailed G-test was used. Amount of volatiles emitted was calculated based on standard and analyzed using the MANOVA procedure, followed by the univariate analysis of variances with the Tukey-Kramer test for mean separation. Principal component analysis (PCA) was conducted to separate cultivars (SAS Institute Inc. 2011). Repeated measure analysis of variance was used (Proc Mixed) to determine overtime differences in number of ABW adults collected in the pitfall traps with and without lures.
Results
Sensory Deprivation Experiment
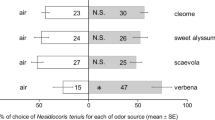
Poa annua was preferred to all bentgrasses tested in both cultivar arrangements by unmodified ABW females (G ≥ 18.3, df = 3, P < 0.01) and females with painted elytra (G ≥ 38.4, df = 3, P < 0.01) with on average 70% of all eggs laid in P. annua plugs (Fig. 1), similarly to the oviposition preference experiment with the same methodology conducted in the previous study using unmodified females only (Kostromytska and Koppenhöfer 2014). There were no significant differences among bentgrass cultivars. Adults with blocked antennae did not exhibit significant oviposition preferences in either cultivar arrangement (G ≥ 4.6, df = 3, P = 0.1) even though weevils with blocked antennae laid on average 40% of all eggs in P. annua (Fig. 1). The total number of eggs laid per arena did not differ among adult treatments (χ2 = 0.82; df = 2; P = 0.66), which suggests that paint itself did not significantly affect oviposition. No significant feeding preferrence (based on feeding scars) for any treatment was observed (G ≤ 0.001, df = 3, P = 1.0).
Ovipositional preferences of sensory deprived Listronotus maculicollis (±SE) compared to control adults in 4-choice test comparing Poa annua to bentgrass cultivars. Poa annua was preferred by ABW females in control (G = 55.1, df 3, P < 0.01 (a) and G = 38.4, df 3, P < 0.01 (b)), and painted control (G = 18.3, df 3, P < 0.01 (a) and G = 40.3, df 3, P < 0.01 (b)). No significant preference was observed when antennae were blocked (G = 5.8, df 3, P = 0.07 (a) and G = 4.6, df 3, P = 0.1 (b))
Y-Tube Olfactometer Assays: Growing Plants
Olfactometer assays demonstrated that, if given a choice between control and grass plugs, ABW females preferred P. annua (G = 6.5, df = 1, P < 0.01) (Fig. 2) and oriented away from the odor of cvs. ‘Villa’ (G = 4.9, df = 1, P < 0.01) and ‘Declaration’ (G = 3.5, df = 1, P = 0.03). Males did not exhibit any preference or repellency in this experiment. If given a choice between P. annua and bentgrass cultivars, females preferred P. annua to all tested cultivars (Fig. 3) (G ≥ 3.5, df = 1, P ≤ 0.03) except for ‘Capri’ (G = 0.3, df = 1, P = 0.3), whereas males exhibited no significant preferences (Fig. 3).
Behavioral responses of Listronotus maculicollis adults in two choice Y-tube olfactometer assays if given a choice between a plug with Poa annua or bentgrass cultivars and a grass free control plug. Percent of nonresponsive adults per treatment is presented at the right side of each bar. Asterisk marks statistically significant preference or repellency
Behavioral responses of Listronotus maculicollis adults in two choice Y-tube olfactometer assays if given a choice between a plug with bentgrass cultivars and a plug with Poa annua. Percent of nonresponsive adults per treatment is presented at the right side of each bar. Asterisk marks statistically significant preference or repellency
Volatile Profiles of P. annua and Bentgrasses
Overall 50 different peaks were observed in the headspace volatile blends of P. annua and six bentgrass cultivars. Among these, 24 were major compounds, comprising up to 83.3% of total emissions and were identified as mostly monoterpenes and sesquiterpenes, (Table 1). None of the identified volatiles were produced exclusively by P. annua. However, several compounds produced by bentgrasses were not found in the headspace of P. annua (α-pinene, myrcene, Z-β-ocimene, terpinolene, camphor, borneol, and E-α-bergamotene). In addition, the relative quantity of compounds differed significantly for the tested grasses (F = 3.2; df = 25, 114; P < 0.01). The compounds that were produced in the highest amounts by P. annua were (Z)-3-hexenyl acetate and linalool. The newer creeping bentgrass cvs. ‘Declaration’ and ‘007’ emitted the highest amounts of ɑ-pinene, camphene, myrcene, sabinene, terpinolene, limonene, camphor, and borneol and produced more Z-β-ocimene than P. annua. The highest amount of E-ɑ-bergamotene was emitted by the velvet bentgrass ‘Villa’. In PCA analysis, the score plot of PC1 versus PC2 showed that the volatile blend composition of P. annua and bentgrasses slightly overlapped, and that P. annua was most distant from creeping bentgrass cvs ‘007’ and ‘Declaration’.
Y-Tube Laboratory Assays: Individual Volatiles
At the highest rates (20 mg), all tested compounds except for octanal were repellent and males and females chose the control over the treatments. At the other concentrations (2.0, 0.2, 0.02 mg), most compounds had no significant effect. However, phenyl ethyl alcohol was marginally attractive to females at the two lowest concentration (G = 3.3, df = 1, P = 0.07), and (Z)-3-hexenyl acetate was attractive to females at the lowest concentration (G = 4.8, df = 1, P = 0.03) (Table 2). Males did not demonstrate any significant preference for any of the compounds tested. However, phenyl ethyl alcohol for females and octanal for males caused increased non-directional locomotion, where weevils walked in the Y-tube more and faster than without the compound, even though the movement not always resulted in a specific choice. In addition, at the lowest concentration of phenyl alcohol weevils made their choice faster than for most of the tested volatiles with exception of (Z)-3-hexenyl acetate, nonanal and hexanal (χ2 = 50.6; df = 10, 319; P < 0.01).
Among the tested host plant volatile blends only the blend of phenyl ethyl alcohol and (Z)-3-hexenyl acetate attracted females (G = 6.7, df = 1, P < 0.01) (Table 3). Therefore, it was used in the field experiments. Males did not exhibit preferences for any of the blends tested.
Field Testing of Host-Plant Attractants
In the spring field experiment, only pitfall traps effectively collected adult ABWs; therefore dome trap data were not included in the analysis and not used in the summer field tests. The total and relative differences in number of collected weevils between baited and non-baited pitfall traps varied over the time (F = 2.25, df = 8, 80; P = 0.03) but for the first 2 weeks of the experiment more adults were consistently collected in baited than in unbaited traps (Fig. 4). Later in the season, weevil numbers were too low and differences no longer observed (Fig. 4).
Discussion
This study provided clear evidence for the involvement of olfaction in host recognition by the annual bluegrass weevil (ABW). Poa annua has previously been shown to be preferred over bentgrasses for oviposition and larval development (Kostromytska and Koppenhöfer 2014, 2016). In this investigation, we showed that plant volatiles contribute to making P. annua more attractive than bentgrasses to ABW females. The effects of the grass-emitted volatiles are likely complex, and may involve attractiveness of P. annua emitted compounds combined with possible repellent/deterrent effects of secondary metabolites (mostly terpenoids) produced by less preferred bentgrass species. However, while our study indicated that weevils oriented away from two of the tested bentgrass cultivars ('Declaration' and 'Villa'), the repellency of any of the secondary metabolites found in these cultivars has yet to be shown experimentally. In the laboratory tests, clear oviposition preferences for P. annua plants were observed with non-modified control and “painted control” (painted elytra) weevils. These results were similar to those in choice assays conducted previously that used unmodified weevils only (Kostromytska and Koppenhöfer 2014). Weevils with blocked antennae, however, did not exhibit distinct oviposition preferences for P. annua. Most of the olfactory sensilla responsible for perceiving long distance chemical stimuli in insects are located on the antennae (Keil 1999), which are considered the main olfactory organs in insects. However, other sensillum types can be also present on insect antennae, suggesting multiple functions of the antennae. In addition, chemoreceptors are not confined to the insect antennae (Keil 1999). In other insect species including other Curculionidae, olfactory and gustatory sensilla have been found on the mouthparts, tarsi and ovipositor (Bland 1984; Keil 1999; Salama and Abdel Aziz 2001), and chemosensory input is known to be paramount in the host selection for the oviposition by many insect species (Städler 1994).
Our observations show that reducing the antennal input decreases the ABW oviposition preference for P. annua, suggesting that chemoreception plays at least a partial role in host recognition and acceptance by ovipositing ABW females. As in previous studies (Kostromytska and Koppenhöfer 2014), ABW adults demonstrated generalist feeding habits and fed equally on all grasses tested, suggesting that preferences are limited to egg-laying behavior. Moreover, Cameron and Johnson (1971) observed ABW adults feed on a number of other plant species including some grasses (Kentucky bluegrass, Poa pratensis L.; fescue (species on specified)) and non-grasses (annual clover, Trifolium sp.; mulberry, Morus rubra L.; dandelion, Taraxacum sp.; plantain, Plantago sp.) even though they tended to feed more on P. annua in choice tests.
The Y-tube olfactometer assays provided more evidence of olfaction involvement in host recognition by ABW. Females were attracted to P. annua if given a choice between plants and control and preferred P. annua to bentgrasses; however, males did not exhibit any preferences. Similarly, McGraw et al. (2011) found that female but not male ABW responded behaviorally to P. annua clippings and whole plant-emitted volatiles, even though physiologically (via electro-antennography) both sexes responded to P. annua volatiles. These observations are consistent with our observations in the sensory deprivation experiment and in previous studies (Kostromytska and Koppenhöfer 2014). ABW adults are generalist herbivores that feed and survive on several grass species whereas larval survival and development are affected by grass species with P. annua being a superior host (Kostromytska and Koppenhöfer 2014). Because ABW immature stages have limited mobility (Cameron 1970), host selection by ovipositing females is a key factor for offspring survival. All findings to date suggest that chemical cues are important for host recognition only for oviposition by females. McGraw et al. (2011) described ABW behavioral responses to damaged and not damaged P. annua, and demonstrated in Y-tube laboratory assays that ABW females preferred intact plants, but were repelled by clippings. In contrast, the main goal of the presented study was to compare ABW behavioral responses to P. annua (preferred host) to ABW behavioral responses to cultivars of Agrostis spp. (less suitable hosts), and to determine whether olfactory/chemoreceptory cues play a role in the host plant preferences. We emphasized the natural scenario of host plant growing conditions on the golf courses. Therefore, all grasses used in the Y-tube and deprivation bioassays and for volatile collections were maintained in the greenhouse mimicking maintenance conditions of the golf course. Composition and quantities of P. annua volatiles observed in our study and McGraw et al. (2011) were different. This may be explained by growing conditions of the plant material used. McGraw et al. (2011) used 5 g of intact plant material with thatch or clippings. In contrast, in our study actively growing P. annua and Agrostis spp. plants (in 10.5 cm-diameter pots), grown from cores taken from an established field, were used for volatile collection, and growing turf plugs were used for behavioral assays.
Studying and comparing the headspace volatiles revealed no P. annua specific volatiles and showed (Z)-3-hexenyl acetate and phenyl ethyl alcohol to be produced in larger amounts by P. annua. These two compounds, alone and in combination, were attractive to the weevils in Y-tube assays, which suggest that they might be responsible for P. annua attractiveness to ABW. However, this most attractive blend of volatiles identified in the laboratory, was not a strong enough attractant in the field for use as an effective ABW monitoring tool. (Z)-3-hexenyl acetate is a green leaf volatile, known to be emitted by many plants in response to mechanical damage and other stresses (Scala et al. 2013) and thus may therefore not be a reliable host finding cue. In the field, (Z)-3-hexenyl acetate and phenyl ethyl alcohol attracted weevils during the spring migration period but not once weevils were mostly on the fairways in late spring and early summer. This may be explained by the properties of the stimuli, physiological state of the weevils, and the surrounding conditions. Particularly, chemical stimuli might be active only over a short range and thus only attract weevils that pass in close proximity of the traps as they migrate from the overwintering sites to the fairways. In addition, because P. annua is more abundant and mown more often in the fairways than in the roughs, the lures are likely outcompeted by naturally present stimuli in the fairways. Moreover, it also has been demonstrated that attraction to host plant volatiles could be interrupted by the presence of non-host plant volatiles and background odors (Thiery and Visser 1986; Xu et al. 2017).
Headspace collections from bentgrasses, especially newer, more disease resistant cultivars, were richer in terpenoids than those from P. annua. This suggests that in ABW host plant recognition not only P. annua attractiveness is important but that defensive compounds in bentgrasses may also play a role as ABW repellents/deterrents. Production and maintenance of terpenoids, which often play an important role in plant defenses, is metabolically expensive (Gershenzon 1994). However, P. annua is an opportunistic species with prolific seed production (Hutchinson and Seymour 1982) and might therefore be less protected against ABW and other pests than other grass species. Bentgrass species have stoloniferous growing habits with less seed production. The cultivars tested in our study were specifically bred to improve tolerance to diseases and abiotic factors (Brilman 2003; Ruemmele 2003; Warnke 2003). In our study, we observed that bentgrasses produced a higher variety of compounds, mostly terpenoids, which were present in low quantity or not present at all in P. annua volatile profile. Chemical oviposition repellents/deterrents, as well as attractive compounds, apparently play an important role in the location and acceptance of plants as hosts by ovipositing ABW females.
Field observations and previous studies (Kostromytska and Koppenhöfer 2014) clearly demonstrated that P. annua is the most preferred ABW host. The current study determined that volatiles emitted by different grass species are at least partially involved in the host preference by ABW. While the plant volatiles tested in this study were not sufficiently attractive to be used for improving ABW trapping and monitoring on golf courses, combinations of these plant volatiles with insect pheromones may have a synergistic effect on insect attraction. In fact, (Z)-3-hexenyl acetate is reported to have such an effect on the attractiveness of insect produced pheromones (Light et al. 1993; Reddy and Guerrero 2004). Additional research is needed to investigate whether (Z)-3-hexenyl acetate and phenyl ethyl alcohol can be used as possible synergists to yet-to-be-identified ABW pheromones and whether such combinations could to be used for effective species monitoring.
References
Bland RG (1984) Mouthpart sensilla and mandibles of the adult alfalfa weevil Hypera postica and the Egyptian alfalfa weevil H. brunneipennis (Coleoptera: Curculionidae). Ann Entomol Soc Am 77:720–724
Borden JH, Pureswaran DS, Lafontaine JP (2008) Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). J Econ Entomol 101:1266–1275
Brilman L (2003) Velvet bentgrass. In: Casler M, Duncan RR (eds) Turfgrass biology, genetics, and breeding. Wiley, Hoboken, pp 201–206
Cameron RS (1970) Biology and control of a species of Hyperodes (Coleoptera: Curculionidae), a pest of turfgrass in New York. M.S. thesis. Cornell University
Cameron RS, Johnson NE (1971) Biology of a species of Hyperodes (Coleoptera: Curculionidae): a pest of turfgrass. Search Agric 1:1–31
Diaz MDC, Peck DC (2007) Overwintering of annual bluegrass weevils, Listronotus maculicollis, in the golf course landscape. Entomol Exp Appl 125:259–268
Gershenzon J (1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20:1281–1328
Gries G, Gries R, Perez AL, Gonzales LM, Pierce HD, Cameron Oehlschlager A, Kouame B (1994) Ethyl propionate: synergistic kairomone for african palm weevil, Rhynchophorus phoenicis L. (Coleoptera: Curculionidae). J Chem Ecol 20:89–897. https://doi.org/10.1007/bf02059585
Hutchinson C, Seymour G (1982) Poa Annua L. J Ecol 70:887–901. https://doi.org/10.2307/2260111
Keil TA (1999) Morphology and development of the peripheral olfactory organs. In: Hansson BS (ed) Insect olfaction. Springer-Verlag, Berlin, pp 6–45
Koppenhöfer AM, Alm SR, Cowles RA, McGraw BA, Swier S, Vittum PJ (2012) Controlling annual bluegrass weevil: optimal timing and rates. Golf Course Management, March 2012, pp 98–104
Kostromytska OS, Koppenhöfer AM (2014) Ovipositional preferences and larval survival of annual bluegrass weevil, Listronotus maculicollis, on Poa annua and selected bentgrasses (Agrostis spp.). Entomol Exp Appl 152:108–119
Kostromytska OS, Koppenhöfer AM (2016) Responses of Poa annua and three bentgrass species (Agrostis spp.) to adult and larval feeding of annual bluegrass weevil, Listronotus maculicollis (Coleoptera: Curculionidae). Bull Entomol Res 29:729–739
Kostromytska OS, Wu S, Koppenhöfer AM (2018) Cross-resistance patterns to insecticides of several chemical classes among Listronotus maculicollis (Coleoptera: Curculionidae) populations with different levels of resistance to pyrethroids. J Econ Entomol 111:391–392
Light DM, Flath RA, Buttery RG, Zalom FG, Rice RE, Dickens JC, Jang EB (1993) Host-plant green volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 4:145–152
McGraw BA, Koppenhöfer AM (2007) Biology and management of the annual bluegrass weevil, Listronotus maculicollis (Coleoptera: Curculionidae). In: Pessarakli M (ed) Handbook of Turfgrass Management and Physiology. CRC Press, Boca Raton, pp 335–350
McGraw BA, Koppenhöfer AM (2009) Development of binomial sequential sampling plans for forecasting Listronotus maculicollis (Coleoptera: Curculionidae) larvae based on the relationship to adult counts and turfgrass damage. J Econ Entomol 102:1325–1335. https://doi.org/10.1603/029.102.0360
McGraw BA, Koppenhöfer AM (2017) A survey of regional trends in annual bluegrass weevil (Coleoptera: Curculionidae) management on golf courses in Eastern North America. J Integrated Pest Manag 8:1–11
McGraw BA, Holdcraft R, Szendrei Z, Rodriguez-Saona C, Koppenhöfer AM (2011) Behavioral and electrophysiological responses of Listronotus maculicollis (Coleoptera: Curculionidae) to volatiles released from intact and mechanically damaged annual bluegrass. Environ Entomol 40:412–419
Pureswaran DS, Poland TM (2009) The role of olfaction in short-range mate finding by the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). J Insect Behav 22:205–216
Ramoutar D, Alm SR, Cowles RS (2009) Pyrethroid resistance in populations of Listronotus maculicollis (Col.: Curculionidae) from southern New England golf courses. J Econ Entomol 102:388–392
Reddy GVP, Guerrero A (2004) Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci 9:253–261
Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ (2009) Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. J Chem Ecol 35:163–175
Rodriguez-Saona C, Vorsa N, Singh A, Johnson-Cicalese J, Szendrei Z, Mescher M, Frost CJ (2011) Tracing the history of plant traits under domestication in cranberries: potential consequences on anti-herbivore defenses. J Exp Bot 62:2633–2644
Ruemmele BA (2003) Colonial bentgrass. In: Casler MD, Duncan RR (eds) Turfgrass biology, genetics, and breeding. Wiley, Hoboken, pp 187–200
Saïd I, Kaabi B, Rochat D (2011) Evaluation and modeling of synergy to pheromone and plant kairomone in American palm weevil. Chem Central J 5:14. https://doi.org/10.1186/1752-153x-5-14
Salama H, Abdel Aziz S (2001) Distribution of the sensillae of the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Coleoptera: Curculionidae). Insect Sci Appl 21:179–188. https://doi.org/10.1017/S1742758400020233
SAS Institue Inc. (2011) SAS/STAT 9.3 User's guide, Cary, NC
Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC (2013) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int J Mol Sci 14:17781–17811
Seed Research of Oregon (2008) Seed specification. http://www.sroseed.com/technical-specs/seeding-specifications.aspx
Städler E (1994) Oviposition behavior of insects influenced by chemoreceptors. In: Kurihara K, Suzuki N, Ogawa H (eds) Olfaction and Taste XI. Springer, Tokyo, pp 821–826
Szendrei Z, Malo E, Stelinski L, Rodriguez-Saona C (2009) Response of cranberry weevil (Coleoptera: Curculionidae) to host plant volatiles. Environ Entomol 38:861–869
Thiery D, Visser JH (1986) Masking of host plant odour in the olfactory orientation of the Colorado potato beetle. Entomol Exp Appl 41:65–172
Tholl D, Röse USR (2006) Detection and identification of floral scent compounds. In: Dudareva N, Pechersky E (eds) Biology of floral scent. Taylor and Francis, Boca Raton, pp 3–25
Vittum PJ (2012) Annual bluegrass weevil. In: Brandenburg RL, Freeman CP (eds) Handbook of turfgrass insect, 2nd edn. Entomological Society of America, Lanham, pp 9–11
Warnke SE (2003) Creeping bentgrass. In: Casler D, Duncan RR (eds) Turfgrass biology, genetics, and breeding. Wiley, Hoboken, pp 175–186
Wu S, Kostromytska OS, Xue F, Koppenhöfer AM (2017) Chilling effect on termination of reproductive diapause in Listronotus maculicollis (Coleoptera: Curculionidae). J Insect Physiol 104:25–32. https://doi.org/10.1016/j.jinsphys.2017.11.005
Xu X, Cai X, Bian L, Luo Z, Li Z, Chen Z (2017) Does background odor in tea gardens mask attractants? Screening and application of attractants for Empoasca onukii Matsuda. J Econ Entomol 110:2357–2363. https://doi.org/10.1093/jee/tox269
Acknowledgements
We appreciate the technical assistance of Eric Weibel and the staff of Rutgers Horticulture Farm 2. We are grateful for expertise and assistance provided by Dr. Stacy Bonos with grass propagation and maintenance. This research was supported by grants from the Golf Course Superintendents Assn. of America and supporting Chapters (GCSA of New Jersey, Hudson Valley GCSA, Keystone AGCS, Long Island GCSA, Metropolitan GCSAA, New Jersey Turfgrass Assn., Pocono Turfgrass Assn.), the US Golf Assn., the O.J. Noer Research Foundation, the Tri-state Turf Research Foundation, the Rutgers Center for Turfgrass Science, and by the USDA National Institute of Food and Agriculture Hatch Multistate project 0206130 through the New Jersey Agricultural Experiment Station, and Hatch Multistate projects NJ08295 and NJ08192.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kostromytska, O.S., Rodriguez-Saona, C., Alborn, H.T. et al. Role of Plant Volatiles in Host Plant Recognition by Listronotus maculicollis (Coleoptera: Curculionidae). J Chem Ecol 44, 580–590 (2018). https://doi.org/10.1007/s10886-018-0964-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-0964-y