Abstract
Artemisinin is effective against both chloroquine-resistant and -sensitive strains of Plasmodium species. However, the low yield of artemisinin from cultivated and wild plants is a serious limitation to the commercialization of this drug. Optimization of artemisinin yield either in vivo or in vitro is therefore highly desirable. To this end, we have overexpressed the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGR) gene (hmgr) from Catharanthus roseus L. in Artemisia annua L. and analyzed its influence on artemisinin content. PCR and Southern blot analyses revealed that the transgenic plants showed stable integration of the foreign hmgr gene. The reverse transcriptase-PCR results suggested that the hmgr was expressed at the transcriptional level in transgenic lines of Artemisia annua L., while the high-performance liquid chromatography analysis showed that artemisinin content was significantly increased in a number of the transgenic lines. Artemisinin content in one of the A. annua transgenic lines was 38.9% higher than that in non-transgenic plants, and HMGR enzyme activity in transgenic A. annua L. was also higher than that in the non-transgenic lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
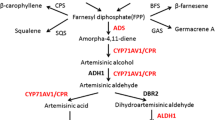
Artemisinin, a sesquiterpene-lactone isolated from the aerial parts of Artemisia annua L. plants, is currently the best therapeutic agent against both drug-resistant and cerebral malaria-causing strains of Plasmodium sp. (Newton and White 1999). It is also effective against other infectious diseases, such as schistosomiasis, human immunodeficiency virus (HIV), hepatitis B, and leishmaniasis (Jung and Schinazi 1994; Borrmann et al. 2001; Utzinger et al. 2001; Romero et al. 2005; Sen et al. 2007), and has recently been shown to be effective against a variety of cancer cell lines, including breast cancer, human leukemia, colon cancer, and small cell-lung carcinomas (Efferth et al. 2001; Singh and Lai 2001). Due to its current use in artemisinin based-combination therapy (ACT), the global demand of artemisinin is continuously increasing, but the relatively low yield of artemisinin from the A. annua L. plant (0.01–1.1%) is a serious limitation to its commercialization (Laughlin 1994; Van Agtmael et al. 1999). This has led to worldwide efforts to enhance its biosynthesis through biochemical and molecular approaches (Abdin et al. 2003; Weathers et al. 2005). Genetic engineering is thought to be one of the most reasonable approaches to enhance the production of artemisinin. By applying this technology, the enzymes catalyzing the rate-limiting steps of artemisinin biosynthesis can be overexpressed or the enzymes involved in other pathways competing for its precursors can be inhibited, resulting in transgenic A. annua L. plants that can accumulated high concentrations of artemisinin.
Mevalonate (MVA) is the primary building block for isoprenoid biosynthesis in higher plants. The final step in MVA production is catalyzed by the branch point enzyme, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMGR, EC 1.1.1.34), which shunts HMG-CoA into the isoprenoid pathway, leading to the synthesis of various intermediates. These intermediates are ultimately used for the biosynthesis of various terpenes, such as mono, di-, tri-, and sesquiterpenes, including artemisinin (Akhila et al. 1987; Kudakasseril et al. 1987; Towler and Weathers 2007). Hence, there is a strong competition among the various pathways of terpene synthesis for MVA, the product of the HMGR-catalyzed reaction. Previous studies showing constitutive overexpression of the HMGR gene (hmgr) and high HMGR activity in plants have also reported an increased accumulation of phytoalexins, sterols, shikonin, and lycopene, suggesting that the MVA pathway contributes C5 units to the synthesis of these terpenoids (Stermer and Bostock 1987; Gondet et al. 1992; Lange et al. 1998; Concepcion and Gruissem, 1999). The validity of this proposed mechanism has been further strengthened by the results of studies carried out recently by our group, in which we have shown that the MVA pathway is the major contributor of carbon (approx. 80%) to artemisinin biosynthesis; consequently, the MVA pathway limits the accumulation of artemisinin in A. annua L. plants.
Vergauwe et al. (1996) developed the first transgenic plant of A. annua L. with the aim of optimizing the commercial production and clinical utilization of artemisinin. Several transgenic plants of Artemisia annua L. have since been developed in which overexpression of enzymes in the early steps of terpene biosynthesis lead to a slightly higher production of artemisinin. These include plants that overexpress isopentenyl diphosphate (Sa et al. 2001) and farnesyl diphosphate (Chen et al. 2000; Han et al. 2006). Zhang et al. (2009) reported an increase in the artemisinin content of A. annua following suppression the expression of squalene synthase (SQS), a key enzyme of the sterol pathway (a competitive pathway of the artemisinin biosynthetic pathway) through loop-mediated RNAi, indicating that the sterol pathway can be manipulated to obtain higher production of artemisinin.
Here, we report the transformation of a high artemisinin-yielding strain of A. annua L (approx. 1.0% artemisinin in plants grown under field conditions) with Catharanthus roseous (L.) G. Don hmgr (accession No. AY623812) tagged with the 35S promoter using Agrobacterium tumefaciens strain LBA4404 and its impact on the artemisinin content.
Materials and methods
Chemicals and plant material
The radioactive chemicals and artemisinin used in this study were procured from Perkin Elmer (Life Science (Boston, MA) and Sigma–Aldrich (St. Louis, MO). All other chemicals were of analytical and molecular grades and procured from other companies, such as SD-Fine, Himedia laboratories, India, and Merck, India. The seeds of a high-yielding strain of A. annua were procured from the IPCA laboratory (Ratlam, MP, India) and sown in the experimental field of Jamia Hamdard, New Delhi, India.
Genetic transformation of A. annua L.
Agrobacterium tumefaciens strain LBA4404 harboring the binary vector pBinAR was used for transformation. The plasmid construct contains the HMG-CoA reductase gene linked to the cauliflower mosaic virus (CaMV) 35S promoter and nopaline opine synthase (NOS) terminator and to the neomycin phosphotransferase II (nptII) gene under the control of a NOS promoter and terminator that was used as the selectable marker (Fig. 1). A. annua plants were regenerated and transformed according to Han et al. (2005) with few modifications. The leaves from 4-week-old regenerating shoot cultures were used as the source of explants. Fresh cultures of A. tumefaciens (1–5 ml) grown in liquid YEM medium (25 ml) with kanamycin (50 mg/l) and rifampicin (10 mg/l) were centrifuged at 5000 rpm for 10 min to obtain a pellet, which was then resuspended in 25 ml fresh MS (Murashige and Skoog 1962) liquid medium. This bacterial suspension (from fresh cultures of Agrobacterium) and leaf explants was used for co-cultivation. Leaf explants were immersed the Agrobacterium suspension in petri plates and gently shaken for 30 min at room temperature. Agrobacteriul suspension-treated explants were blot dried on sterile filter paper and transferred onto MS solid medium in the dark at 25°C for 72 h for co-cultivation. The controls consisted of explants on MS medium that had not been treated with Agrobacterium suspension.
The 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMGR) gene construct. The hmgr is tagged with the CaMV 35S promoter (P CaMV35S) in the pBinAR binary vector harboring neomycin phosphotransferase II (Npt II) as the selection marker. LB, RB Left, right border, respectively, Pnos nopaline opine synthase promoter, OCS octopine synthase gene, BamHI, EcoRI, SalI, HindIII restriction enzymes
Selection and regeneration of transformants
After co-cultivation, the leaf explants were washed with milliQ water twice (5 min each) and then washed with autoclaved milliQ water containing cefotaxime (500 mg/l) for 20 min. The explants were then blot dried and cultured in shoot-induction selection medium (SISM) containing MS salts + vitamins + 0.05 mg/l α-naphthalene acetic acid (NAA) + 1.5 mg/l 6-benzylaminopurine (6-BA) + 20 mg/l kanamycin, pH 5.8, with 500 mg/l cefotaxime added to inhibit further agro-bacterial growth. The explants were transferred to fresh medium at weekly intervals for 4 weeks. The induced shoots were transferred onto the same medium with a reduced concentration of cefotaxime (200 mg/l after 8 weeks; at 12 weeks, cefotaxime was completely removed from the medium. The shoots were then subcultured on shoot elongation medium (MS salts + vitamins + 3% sucrose) with kanamycin for 4 weeks. After attaining an acceptable shoot length, the shoots were transferred onto rooting medium containing MS salts + vitamins + 0.5 mg/l NAA + 3% (w/v) sucrose and 0.8% (w/v) agar, pH 5.8. All cultures were kept in a culture room at 25°C under a 16/8-h (light/dark) photoperiod with light suppled at an intensity of 50–60 μE m−2 s−1.
PCR and Southern blot analysis of putative transformants
Genomic DNA from kanamycin-resistant and untransformed shoots of A. annua. L was isolated by the cetyl trimethylammonium bromide (CTAB) method (Doyle and Doyle 1990). Integration of the transgene into the plant genome was screened by PCR using an nptII-specific primer pair: 5′-CAATCGGCTGCTCTGATGCCG-3′ (forward primer) and 5′-AGGCGATAGAAGGCGATGCGC-3′ (reverse primer). Each reaction was performed in a 50-μl reaction mixture (final volume) consisting of 10× reaction buffer, 10 mM dNTPs, 0.5 μg/μl concentration of each of sequence-specific oligonucleotide primers, 0.1 μg templates DNA, and 3 U/μl Taq DNA polymerase. The reaction was heated at 94°C for 4 min, followed by 32 cycles of 94°C for 1 min (denaturation), 55°C for 1 min (annealing), and 72°C for 2 min ( extension), with a final extension step for 4 min to extend any premature synthesis of DNA. Plasmid DNA containing the nptII gene was used as the positive control, and DNA from nontransgenic shoots of A. annua L. was used as the negative control. The PCR fragments were then fractioned on a 0.8% agarose gel.
For the Southern blot analysis, genomic DNA (10 μg) was digested with SaII, resolved on a 0.7% agarose gel, and blotted onto a nylon membrane (Roche Diagnostics, Indianapolis, IN). For hybridization of the DNA, a probe was prepared using the hmgr gene. The probe was labeled with digoxigenin (DIG High-Prime DNA labeling and Detection Starter kit (Roche Diagnostics), and the blot was probed with the labeled hmgr gene probe at 44°C. After 16 h of hybridization, the membrane was washed and the probe detected following the manufacturer’s instructions.
RNA extraction and reverse transcriptase-PCR analysis
Total RNA was isolated from transgenic and nontransgenic shoots of A. annua L. using the RNeasy Plant Mini kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). It was subjected to reverse transcriptase (RT)-PCR using the One-Step RT-PCR kit (Qiagen) with hmgr gene-specific primers. The PCR conditions were 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min (35 cycles). Amplified PCR products were separated on a 1.2% agarose gel, stained with ethidium bromide, and visualized in a gel documentation system (UVItech, Cambridge, UK).
HMG-CoA reductase assay
The HMGR assay developed by Russell (1985) and modified in our laboratory was used to determine HMG-CoA reductase activity in the leaves of A. annua L. plants (Mauji Ram et al. 2010). This assay is based on the conversion of RS 3-[4C]-HMG-CoA to [14C]mevalonate, which was separated from HMG-CoA on the thin layer chromatography plate. HMG-CoA reductase activity was expressed as nanomoles MVA formed per hour per milligram protein (nmol h−1 mg−1).
Preparation of microsomal enzyme suspension
Fresh leaves (2 g) were washed and chopped into small pieces (1–2 mm), homogenized in 5 volumes of ice-cold homogenizing buffer (100 mM K-phosphate, pH 7.2, 30 mM EDTA, 0.35 mM sucrose, 20 mM β-mercaptoethanol, and 0.3% bovine serum albumin). The crude homogenate was then squeezed through two layers of muslin cloth, and the filtered homogenate was centrifuged at 5000 g for 15 min at 4°C. The pellet was discarded, and the supernatant was again centrifuged at 16000 g for 20 min at 4°C; the resulting supernatant was subsequently centrifuged at 105000 g for 1 h at 4°C. The pellet that was obtained from the centrifugation at 105000 g was resuspended in 500 μl of suspension buffer (100 mM K-phosphate, pH 7.2 and 25 mM DTT). A 20-μl sample of enzyme suspension was taken for protein estimation by the method of Bradford (1976). Bovine serum albumin was used as the standard.
Assay procedure
The reaction mixture [100 μl; 42 μl 10 μM phosphate buffer, pH 7.2, 10 μl 10 μM DTT, 10 μl 0.40 μM NADPH2, 10 μl microsomal enzyme suspension (0.05–0.3 mg protein)] was prepared and stored on ice. The reaction mixture was kept for 5 min at room temperature and the reaction started by adding 10 nmol of 3-[14C]HMG-CoA (120 990 dpm/nmol). The blank reaction was prepared by inactivating the enzyme by heat treatment before starting the reaction. The tubes were then capped with Parafilm and incubated for 20 min at 30°C in a shaking water bath; the reaction was stopped by adding 10 μl 2 M MVA and 10 μl 6 M HCl with immediate mixing. After standing at room temperature for at least 15 min (for maximum lactonization of mevalonic acid), the mixture was centrifuged at 5000 rpm for 5 min to pellet the protein. The pellet was discarded and 60 μl of supernatant was streaked on a TLC plate. The plate was developed with benzene/acetone (2:3 v/v) and then visualized with iodine vapors using standard MVA as reference. The MVA lactone (R f = 0.9) was then scrapped into a scintillation vial for counting. A similar area of silica gel in a low background region was also scrapped into the vial for background counts; 10 ml of Triton-toluene scintillation fluid was poured into the vial and swirled to disperse the silica gel and dissolve the product. Counting was performed in a β-scintillation counter.
Detection of artemisinin
Arteisinin was estimated in 1 g dry leaf material using the method described by Zhao and Zeng (1986). Derivatized artemisinin was analyzed and quantified through reverse phase column (C18, 5 μm, 4.6 × 250 mm) using premix methanol:100 mM K-phosphate buffer (pH 6.5) in the ratio of 60:40 as the mobile phase at a constant flow rate of 1 ml min−1, with the detector set at 260 nm. Artemisinin was quantified using a standard curve prepared by high-performance liquid chromatography (HPLC). Artemisinin content was expressed as percentage and as well as in micrograms per gram dry weight (mg g−1 DW) of leaves.
Results
Artemisia annua transformation was performed essentially according to the method described by Han et al. (2005). The composition of the shoot-induction medium was optimized to obtain a high frequency of shoot-induction, with the combination of 0.05 mg/l NAA and 1.5 mg/l 6-BA resulting in the highest shoot-induction frequency (88%). A. annua shoot induction was very sensitive to kanamycin. When the leaf explants were cultured on shoot-induction medium supplemented with 20 mg/l kanamycin, the non-transgenic explants did not generate shoot clusters; instead, they withered, became yellow, and ultimately died. The putative transgenic leaf explants, however, regenerated into shoots, which were further elongated during a 3- to 4-week culture on shoot elongation medium devoid of kanamycin. Roots appeared within 2 weeks after the shoots were transferred onto rooting medium. A total of 350 explants were co-cultivated with A. tumefaciens, out of which only 12 produced shoots on shoot-induction selection medium (transformation frequency 3.42%). The transgenic status of kanamycin-resistant shoots was subsequently confirmed by PCR and Southern blot analyses. Of the 12 putative transgenic plants, seven transgenic lines showed 0.75-kb amplification products when amplified with nptII gene-specific primers, (Fig. 2a). No amplification was detected in the non-transgenic shoot lines of A. annua L. plants. The effective frequency of transformed shoots (T0) was therefore 2%. In the Southern hybridization, a digoxigenin (DIG)-labeled hmgr probe hybridized to SalI-digested genomic DNA of putative transformed A. annua plants. Single-cut plasmid DNA of pBinAR was used as the positive control; the DNA sample from the untransformed shoot served as the negative control and did not show any hybridization (Fig. 2b). The copy numbers of the HMGR gene varied among the different transgenic lines, and three transgenic lines (T4, T6, and T7) were found to contain single copies of the transgene. The bands in the seven transgenic lines were of various sizes, indicating that transgene integration occurred at different loci in the genome (Fig. 2b).
Molecular analysis of transgenic lines of Artemisia annua L. plants. a PCR analysis for the presence of the nptII gene in kanamycin-resistant A. annua L. transgenic lines. Lanes: M 1-kb ladder, +ve control pBinAR plasmid, −ve control non-transgenic control plant, T1–T12 kanamycin-resistant A. annua L. plants. b Southern blot analysis following digestion of genomic DNA with SalI and hybridization with an hmgr-specific gene probe. Lanes: P Single-cut plasmid DNA, W non-transgenic control plant, T1–T9 transgenic plants
To confirm whether the HMG-CoA reductase gene was expressed at the mRNA level as a result of gene integration, RT-PCR analysis was performed using total RNA isolated from leaves of transgenic and untransformed shoot lines of A. annua. RT-PCR analysis of the seven transgenic shoot lines suggested that the foreign gene in five lines was expressed at the transcriptional level (Fig. 3). The amplification was higher in the T4, T6, and T7 lines, indicating a higher expression level in these transformed shoot lines.
Each of the transgenic lines was subsequently grown and analyzed for HMGR activity and its potential effect on levels of artemisinin. The enzyme assay showed that HMGR activity was higher in transgenic A. annua plants than in non-transgenic shoot lines. The enzyme activity was higher in transgenic line T4, T6, and T7 (Fig. 4). Whether the higher enzyme activity would result in a corresponding increase in the artemisinin content in transgenic A. annua was determined by analyzing artemisinin content by HPLC. The artemisinin peak was detected at 5.2 min (retention time) using 100 mM phosphate buffer in methanol (4:6) as the solvent system. The results indicated that there was a maximum enhancement of artemisinin content in transgenic line T4 (followed by T6 and T7) of 38.9% (0.6 mg/g DW) relative to the artemisinin content in non-transgenic (0.37 mg/g DW) A. annua plants (Fig. 5).
Discussion
Evidence is accumulating that implicates HMGR as an important control point for MVA biosynthesis in plants (Bach 1986; Maurey et al. 1986; Stermer and Bostock 1987; Gondet et al. 1992; Mauji Ram et al. 2010). There are several studies showing a strong correlation between HMG-CoA reductase activity and the biosynthesis of isoprenoid compounds as HMG-CoA reductase regulates the carbon flux from primary to secondary metabolism, leading to the synthesis of these compounds (Chappell 1995; Bach 1986). A strong correlation between HMG-CoA reductase activity and artemisinin content has been observed, confirming the involvement of this enzyme in the MVA pathway in artemisinin biosynthesis (Abdin et al. 2003; Mauji Ram et al. 2010). It is most likely, therefore, that overexpression of the HMG-CoA reductase gene may channel more carbon towards the synthesis of artemisinin in genetically transformed A. annua L. plants. A considerable body of literature also exists on the transgenic alteration of HMGR in plants and the effects of these alterations on cytosolic isoprenoid production (sesquiterpene phytoalexins and phytosterols). As reported by Chappell (1995), overexpression of the hamster HMG-CoA reductase gene in tobacco led to a three- to sixfold and three- to tenfold increase in HMG-CoA reductase activity and sterol content, respectively. Transgenic roots of Solanum aviculare Forst. containing extra copies of an HMGR gene accumulated up to 4.2-fold more steroidal alkaloid solasodine (Argôlo et al. 2000). Ayora-Talavera et al. (2002) reported overexpression in Catharanthus roseus L. hairy roots of a truncated hamster HMGR gene. One transgenic hairy root (clone 19) with a low hybridization signal had high soluble HMGR activity and produced high levels of campesterol and five- to sevenfold more serpentine than the control.
In a recent study performed in our laboratory, hmgr from Catharanthus roseus (L) G. Don was overexpressed in a low artemisinin-yielding variety of A. annua (0.03% artemisinin; Aquil et al. 2009). There was a 22% increase in artemisinin content in the shoots of one of the transgenic lines when compared with the non-transgenic A. annua plants.
In this study, we transformed a high artemisinin-yielding variety of A. annua with the hmgr gene from C. roseus tagged with the 35S promoter and analyzed its transformation and regeneration potentials. We also studied the impact of the constitutively overexpressed hmgr gene in transgenic lines of A. annua L. on HMG-CoA reductase activity and artemisinin contents. Our results confirmed the higher transformation and regeneration potentials of the A. annua L. plants used in our study compared with the results obtained by Aquil et al. (2009) earlier. Further, the artemisinin content of different transgenic lines of A. annua L. was found to be higher than that of the transformed lines of A. annua L. developed by Aquil et al. (2009). The increment in artemisinin was up to 38.9% (0.6 mg/g DW) in one of the transgenic shoot lines (T4), possibly due to higher hmgr expression in the transgenic lines. This finding was in accordance with a recent study performed by Mauji Ram et al. (2010), in which they concluded that HMGR activity limits artemisinin biosynthesis and its accumulation in A. annua plants as HMGR is the key regulatory enzyme of the MVA pathway and regulates the flux of carbon to the artemisinin biosynthesis pathway. The seven different transgenic lines that we developed varied in their expression of hmgr at the transcriptional level. This may be due to positional effect or random integration of the transgene at nonspecific sites in the plant genome (Prols and Meyer 1992). In our study, transgenic lines T2 and T9 failed to show any significant increase in artemisinin content, while transgenic lines T1 and T5 showed only a little increase. This may be due to the insertion of multiple copies of the HMGR gene at non-specific sites in the plant genome. Multiple copies of T-DNA insertions result in gene silencing in transgenic plants often associated with DNA methylation in many plant species (Tang et al. 2007). In order to verify the putative hmgr gene that was isolated from the genomic DNA of C. roseaus (L.) G. Don, we sequenced and analyzed the DNA for its similarity with a cDNA sequence reported earlier (Maldonado et al. 1992). The genomic DNA sequence possesses only a few polymorphic sites in the coding region that affect the amino acid sequence reported earlier (Aquil et al. 2009). CLUSTAL W analysis revealed that there is very little nucleotide similarity between the C. roseous and A. annua L. hmgr gene; hence, no band was detected in the non-transgenic shoots of A. annua L. in the RT-PCR analysis.
The relatively low rate of effective transformation (2%) in our study, when compared with an earlier report of a 4–10% transformation frequency in fascicled shoots (Han et al. 2005), could be due to genetic differences in A. tumefaciens strains, vectors, and the A. annua L. variety used for transformation in these studies (Vergauwe et al. 1996).
However, our results clearly demonstrate that the genetic engineering strategy of over-expressing the hmgr gene in a high-yielding strain of A. annua is an effective and suitable means of increasing the artemisinin content of these plants. Consequently, the development of such plants will contribute to reducing the cost of artemisinin-based combination therapy, which may in turn ultimately lead to improved therapeutic programs against drug-resistant malaria in developing countries of the Indian subcontinent and Africa. Although the over-expression of hmgr in A. annua L. was been shown to enhance artemisinin content, it would be interesting to evaluate the up-regulation of downstream enzymes of artemisinin biosynthesis in A. annua L. strains overexpressing hmgr in order to evaluate its impact on artemisinin synthesis and accumulation. This approach could possibly be an effective genetic engineering strategy to develop new transgenic varieties of A. annua L. with yet higher artemisinin content.
Abbreviations
- 6-BA:
-
Benzylaminopurine
- HMGR:
-
3-Hydroxy-3-methyl-glutaryl coenzyme A reductase
- MVA:
-
Mevalonate
- NAA:
-
Naphthaleneacetic acid
- NOS:
-
Nopaline opine synthase
- NPT:
-
Neomycin phosphotransferase
- SISM:
-
Shoot-induction selection medium
- SQS:
-
Squalene synthase
References
Abdin MZ, Israr M, Rehman RU, Jain SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–293
Akhila A, Thakur RS, Popli SP (1987) Biosynthesis of artemisinin in Artemisia annua. Phytochem 16:1927–1930
Aquil S, Husaini AM, Abdin MZ, Rather GM (2009) Overexpression of the HMG-CoA reductase gene leads to enhanced artemisinin biosynthesis in transgenic Artemisia annua plants. Planta Med 75:1–6
Argolo ACC, Charlwood BV, Pletsch M (2000) The regulation of solasodine production by Agrobacterium rhizogenes-transformed roots of Solanum aviculare. Planta Med 66:448–451
Ayora-Talavera T, Chappell J, Lozoya-Gloria E, Loyola-Vargas VM (2002) Overexpression in Catharanthus roseus hairy roots of a truncated hamster 3-hydroxy-3-methylglutaryl-CoA reductase gene. Appl Biochem Biotechnol 97:135–145
Bach TJ (1986) Hydroxymethylglutaryl-CoA reductase, a key enzyme in phytosterol synthesis. Lipid 21:121–125
Borrmann S, Szlezak N, Faucher JF, Matsiegui PB, Neubauer R, Biner RK, Lell B, Kremsner PG (2001) Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double blind, randomized, placebo-controlled study. J Infect Dis 184:1363–1366
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Chappell J (1995) Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol 46:521–547
Chen D, Ye H, Li G (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Concepcion RM, Gruissem M (1999) Arachidonic acid alters tomato HMG expression and fruit growth and induces 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent lycopene accumulation. Plant Physiol 119:41–48
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The antimalarial artesunate is also active against cancer. Int J Oncol 18:767–773
Gondet L, Weber T, Maillot VP, Benveniste P, Bach TJ (1992) Regulatory role of microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase in a tobacco mutant that over produces sterols. Biochem Biophys Res Comm 186:888–893
Han JL, Wang H, Ye HC, Liu Y, Li ZQ, Zhang Y, Zhang YS, Yan F, Li GF (2005) High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci 168:73–80
Han JL, Liu BY, Ye HC, Wang H, Li ZQ, Li GF (2006) Effects of over expression of the endogenous farnesyl diphosphate synthase on the artemisinin content in Artemisia annua L. J Integr Plant Biol 48(4):482–487
Jung M, Schinazi RF (1994) Synthesis and in vitro anti-human immunodeficiency virus acivity of artemisinin (Qinghaousu) related trioxanes. Bioorg Med Chem Lett 4:934–941
Kudakasseril GJ, Lam L, Staba EJ (1987) Effect of sterol inhibitors on the incorporation of 14C-isopentenyl pyrophosphate into artemisinin by a cell-free system from Artemisia annua tissue cultures and plants. Planta Med 53:280–284
Lange BM, Wildung MK, MacCaskill D, Croteau R (1998) A family of transketolases that directs isoprenoid biosynthesis via mevalonate-independent pathway. Proc Nat Acad Sci USA 95:21000–22104
Laughlin JC (1994) Agricultural production of artemisinin: a review. Trans Royal Soc Trop Med Hyg 88(1):21–22
Maldonado MIE, Burnett RJ, Nessler CL (1992) Nucleotide sequence of a cDNA encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase from Catharanthus roseus. Plant Physiol 100:1613–1614
Mauji Ram, Khan MA, Jha P, Khan S, Kiran U, Ahmad MM, Javed S, Abdin MZ (2010) HMG-CoA reductase limits artemisinin biosynthesis and accumulation in Artemisia annua L. Plants. Acta Physiol Plant 32:859–866. doi:10.1007/s11738-010-0470-5
Maurey K, Wolf F, Golbeck J (1986) 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in Ochmmonac malhamensis. Plant Physiol 82:523–527
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Newton P, White N (1999) Malaria: new development in treatment and prevention. Annu Rev Med 50:179–192
Prols F, Meyer P (1992) The methylation patterns of chromosomal integration regions influence gene activity of transferred DNA in Petunia hybrida. Plant J 2:465–475
Romero MR, Efferth T, Serrano MA, Castano B, Macias RI, Briz O, Marin JJ (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an ‘in vitro’ system. Antiviral Res 68:75–83
Russell DW (1985) 3-Hydroxy-3-methylglutaryl-CoA reductases from pea seedlings. Methods Enzymol 110:26–40
Sa G, Mi M, He-Chun Y, Ben-Ye L, Guo-feng L, Kang C (2001) Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci 160:691–698
Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M (2007) Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol 56:1213–1218
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70(1):49–56
Stermer BA, Bostock MB (1987) Stermer BA, Bostock MB (1987) Involvement of 3-hydroxy-3-methylglutaryl-CoA reductase in the regulation of sesquiterpenois phytoalexin synthesis in potato. Plant Physiol 84:404–408
Tang W, Ronald J, Newton D, Weidner A (2007) Genetic transformation and gene silencing mediated by multiple copies of a transgene in eastern white pine. J Exp Bot 58(3):545–554
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep 26:2129–2136
Utzinger J, Xiao S, N’Goran EK, Berquist R, Tanner M (2001) The potential of artemether for the control of schistosomiasis. Int J Parasitol 31:1549–1562
Van Agtmael MA, Eggelte TA, Boxtel CJ (1999) Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci 20:199–205
Vergauwe A, Cammaert R, Vandenberghe D, Genetello C, Van Montagu M, Vanden Eeckhout E (1996) Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. and regeneration of transgenic plant. Plant Cell Rep 15:929–937
Weathers PJ, Bunk G, McCoy MC (2005) The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant 41:47–53
Zhang L, Fuyuan J, Fupeng L, Li M, Wang Y, Wang G, Sun X, Tang K (2009) Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA mediated gene silencing. Biotechnol Appl Biochem 52:199–207
Zhao SS, Zeng MY (1986) Determination of Qinghaosu in Artemisia annua L. by high performance liquid chromatography. Chin J Pharm Anal 6:3–5
Acknowledgments
Tazyeen N. is highly grateful to the Council of Scientific and Industrial Research, India, for the award of JRF and SRF fellowship for her Doctoral research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nafis, T., Akmal, M., Ram, M. et al. Enhancement of artemisinin content by constitutive expression of the HMG-CoA reductase gene in high-yielding strain of Artemisia annua L.. Plant Biotechnol Rep 5, 53–60 (2011). https://doi.org/10.1007/s11816-010-0156-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-010-0156-x